Translate this page into:
Allergic bronchopulmonary aspergillosis
For correspondence: Dr Ritesh Agarwal, Department of Pulmonary Medicine, Postgraduate Institute of Medical Education & Research, Sector-12, Chandigarh 160 012, India e-mail: agarwal.ritesh@outlook.in
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Allergic bronchopulmonary aspergillosis (ABPA) is an inflammatory disease caused by immunologic reactions initiated against Aspergillus fumigatus colonizing the airways of patients with asthma and cystic fibrosis. The common manifestations include treatment-resistant asthma, transient and fleeting pulmonary opacities and bronchiectasis. It is believed that globally there are about five million cases of ABPA, with India alone accounting for about 1.4 million cases. The occurrence of ABPA among asthmatic patients in special clinics may be as high as 13 per cent. Thus, a high degree of suspicion for ABPA should be entertained while treating a patient with bronchial asthma, particularly in specialized clinics. Early diagnosis and appropriate treatment can delay (or even prevent) the onset of bronchiectasis, which suggests that all patients of bronchial asthma should be screened for ABPA, especially in chest clinics. The current review summarizes the recent advances in the pathogenesis, diagnosis and management of ABPA.
Keywords
Allergic bronchopulmonary aspergillosis
allergic bronchopulmonary mycosis
Aspergillus
asthma
azole
cystic fibrosis
glucocorticoids
Introduction
Respiratory disorders caused by fungi can be broadly classified as invasive, saprophytic or allergic1. Allergic respiratory mycosis represents the most severe expression of fungal allergy characterized by difficult-to-treat asthma, recurrent pulmonary opacities and bronchiectasis2. The most common fungus causing allergic pulmonary mycosis is Aspergillus fumigatus. Conventionally, allergic pulmonary mycoses is labelled as allergic bronchopulmonary aspergillosis (ABPA) when the causative agent is A. fumigatus and allergic bronchopulmonary mycosis (ABPM) when it is caused by fungi other than A. fumigatus3. ABPA commonly complicates the course of patients with asthma and cystic fibrosis (CF). The CF Foundation4 has provided guidance for the diagnosis of ABPA complicating CF. However, ABPA complicating asthma remains under-recognized, and a large number of cases (about 30%) are initially misdiagnosed as pulmonary tuberculosis, especially in developing countries56. The diagnostic delay can be as long as 10 yr from the occurrence of first symptoms7. The International Society for Human and Animal Mycology (ISHAM) has formed an ABPA Working Group to simplify the recognition of this disorder. This Working Group has proposed recommendations for the diagnosis and classification of ABPA complicating asthma3. In the last two decades, considerable progress has been made in the understanding of this enigmatic disorder8. The current review provides a summary of the advances made in the field of ABPA complicating asthma.
Epidemiology of ABPA
Aspergillus sensitization (AS) is defined by the presence of a type 1 reaction (immediate cutaneous hyper-reactivity) to A. fumigatus antigen injected intracutaneously or a raised immunoglobulin (Ig) E against A. fumigatus910. In a recent study, the recombinant Aspergillus antigens were found to provide a more accurate diagnosis of AS compared to crude antigens11. While patients with ABPA have poor lung function secondary to the underlying bronchiectasis, patients with AS without ABPA also have impaired lung function compared to patients with asthma without AS1213. Importantly, the prevalence of ABPA in Aspergillus-sensitized asthma can be as high as 40 per cent, highlighting the importance of recognizing AS14.
The population prevalence of AS and ABPA in patients with bronchial asthma remains obscure15. In the National Health and Nutrition Examination Survey conducted in the United States population, the prevalence of AS was noted to be 6.4 per cent using specific IgE against A. fumigatus16. However, the survey involved healthy individuals and not patients with bronchial asthma. In a scoping review, Denning et al17 estimated the global burden of ABPA complicating asthma to be at about five million (worldwide asthma population, 193 million), assuming the prevalence of ABPA of 2.5 per cent (estimated from secondary care cohorts). In India, the best estimate for the community prevalence of ABPA complicating asthma is about five per cent18. With this estimate, the case-load of ABPA in India was estimated to be about 1.4 million19. In a systematic review, the pooled prevalence of AS and ABPA complicating asthma in chest clinics was estimated to be about 28 [95% confidence interval (CI), 24-34%] and 13 per cent (95% CI, 8-19%), respectively14. The prevalence of ABPA is higher in patients with severe acute asthma compared to outpatient asthmatics. In a study of 57 patients with severe acute asthma admitted to an intensive care unit, the prevalence of ABPA was found to be 39 per cent (compared to 21% in the outpatient asthma group)20. The prevalence of ABPA complicating asthma reported in the last decade is summarized in Table I212223242526272829303132. The data suggest a high prevalence of AS and ABPA in special clinics (Table I) and a higher prevalence in India compared to other countries.
| Author (yr) | Type of study | Country | Prevalence of AS, n (%) | Prevalence of ABPA, n (%) |
|---|---|---|---|---|
| Prasad et al (2008)21 | Prospective | India | 74/244 (30.3) | 18/244 (7.4) |
| Agarwal et al (2010)22 | Prospective | India | 87/242 (35.9) | 54/242 (22.3) |
| Ghosh et al (2010)23 | Prospective | India | 54/215 (25.1) | 15/215 (6.9) |
| Sarkar et al (2010)24 | Prospective | India | 40/126 (31.7) | 10/126 (7.9)* |
| Ma et al (2011)25 | Prospective | China | 11/200 (5.5) | 5/200 (2.5) |
| Agin and Namavary (2012)26 | Prospective | Tehran | 42/201 (20.9) | - |
| Mathur and Mathur (2016)27 | Prospective | India | 27/300 (9) | 8/296 (2.7) |
| Kozlova et al (2017)28 | Prospective | Russia | 50/140 (36) | 5/140 (3.6) |
| Nath et al (2017)29 | Prospective | India | 135/350 (35.1) | 76/350 (21.7) |
| Kalaiyarasan et al (2018)30 | Prospective | India | 13/70 (18.6) | 9/70 (12.9) |
| Bhankhur et al (2019)31 | Prospective | India | - | 35/50 (70) |
| Savio et al (2019)32 | Prospective | India | 122/205 (59.6) | - |
*Includes fungi other than Aspergillus fumigatus
Pathogenesis of ABPA
Aspergillus is a ubiquitous mould, and its conidia (2-3.5 μm), being in the respirable range, can easily enter the airways. While exposure to large numbers of conidia of A. fumigatus may cause ABPA33, not all asthmatics develop the disorder despite dwelling in the same environment34. The pathogenesis of ABPA remains unclear. Two aspects are believed to be important in its development, namely the persistence of fungi in the airways (due to abnormal clearance) and skewed hyper-immune T-helper 2 (Th2) response (possibly due to the HLA-DR2/DR5 bearing dendritic cells). In ABPA, several defects in innate and adaptive immunity have been identified that lead to persistence of A. fumigatus and a distorted immune response Table II335363738394041. Vitamin D deficiency has been proposed as a pathogenetic factor in ABPA complicating CF4243; however, its role in ABPA complicating asthma is uncertain4445.
| Defects in innate immunity |
| Surfactant protein A2 gene polymorphisms |
| Mannose-binding lectin gene polymorphisms |
| Toll-like receptor 9 gene polymorphisms |
| Toll-like receptor 3 gene polymorphisms |
| CARD9 gene polymorphisms |
| EEA1 mutations |
| ZNF77 polymorphism |
| Adaptive immunity |
| HLA associations |
| Interleukin 4 receptor alpha polymorphisms |
| Interleukin 13 polymorphisms |
| Interleukin 10 promoter polymorphisms |
| Interleukin 15 polymorphisms |
| Tumour necrosis factor-α polymorphisms |
| Transforming growth factor-β polymorphisms |
| Others |
| CFTR gene mutation |
| CHIT1 gene mutations |
The conidia of A. fumigatus are immunologically inactive due to the presence of a surface hydrophobin RodA, which prevents immune recognition of the fungi by the host46. In asthmatic patients genetically predisposed to develop ABPA, defective clearance of the conidia of A. fumigatus allows them to germinate into hyphae. The germinating conidia shed their rodlet layer. This exposes the fungal proteins including β-d-glucan, galactomannan, and others. The phagocytes recognize these fungal proteins through their pattern recognition receptors and partially clear the fungi4748. The subsequent fungal growth causes exocytosis of several proteins, which is followed by the release of chemokines and cytokines and the activation of adaptive immune responses by Th cells49. The usual reaction of the human host is a Th1 CD4+ T-cell response, which eliminates the fungi secondary to macrophage and neutrophil-mediated phagocytosis50. On the contrary, the immune response in ABPA is a predominant Th2 CD4+ T-cell response (Fig. 1), with the release of interleukin (IL)-4, IL-5, IL-13, CCL17, IL-9 and others51. The Th2 response does not eradicate the fungi52 but generates an intense inflammatory reaction characterized by mast cell degranulation and influx of large numbers of eosinophils and neutrophils53. This causes the characteristic immunological (total and A. fumigatus-specific IgE and A. fumigatus-specific IgG synthesis)54 and pathological (mucus plugging, eosinophilic pneumonia and others) findings of ABPA. Persistent inflammation leads to bronchiectasis and if uncurbed pulmonary fibrosis and end-stage respiratory disease.
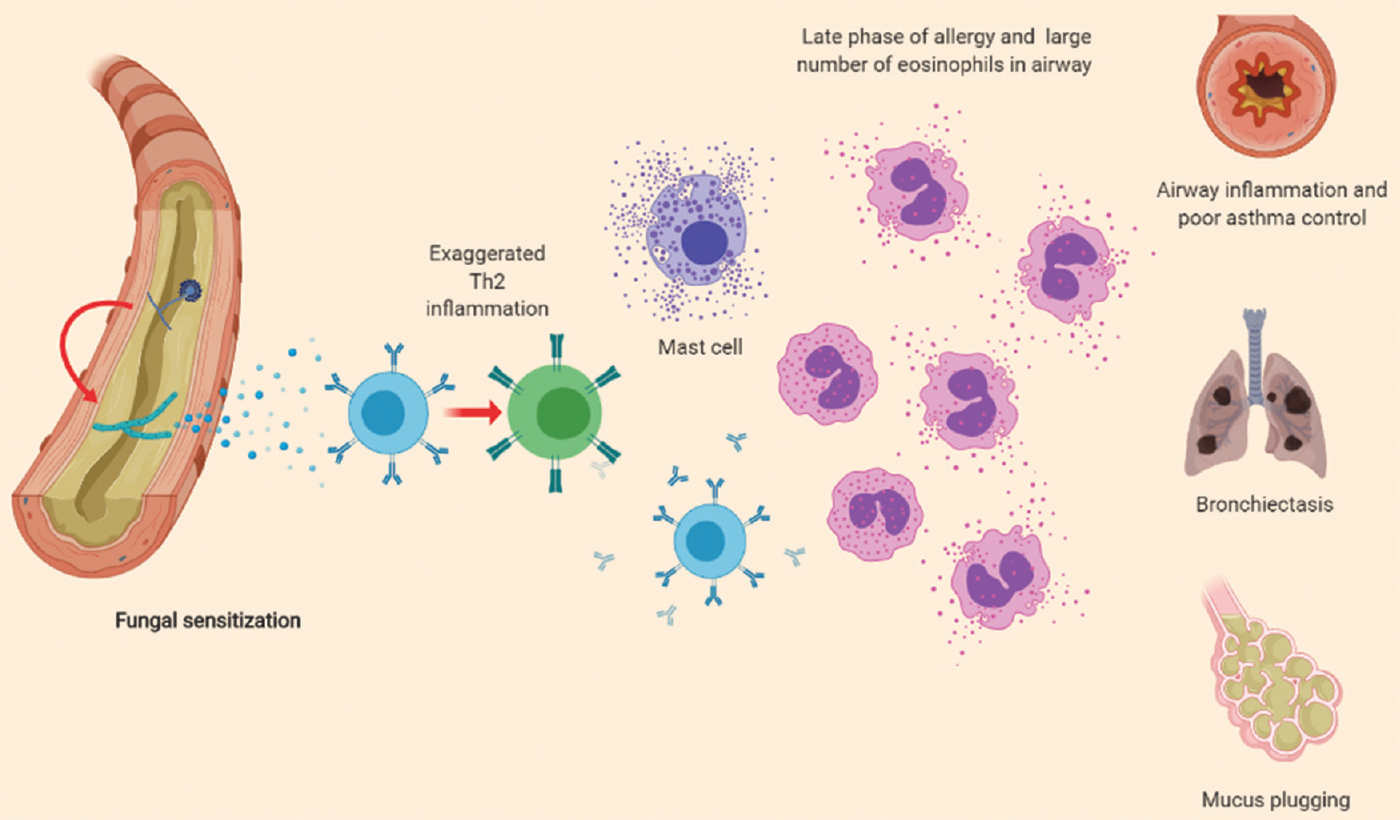
- Pathogenesis of allergic bronchopulmonary aspergillosis. Aspergillus conidia trapped in the airway mucus germinate into hyphae, in genetically predisposed individuals. The hyphae provide the antigenic stimulus for the allergic response, resulting in fungal sensitization. In susceptible individuals, an exaggerated T-helper 2 (Th2) immune response promotes further airway inflammation. This phase is characterized by recruitment of mast cells, increased production of immunoglobulin E (total as well as specific IgE to the fungus) and IgG antibodies to the fungi. The secreted chemokines and cytokines attract large number of eosinophils which attack the fungal hyphae, perpetuate further inflammation, finally culminating in end-organ damage and clinical manifestations. The red arrows indicate the steps where genetic predisposition plays a key role.
Pathology of ABPA
The diagnosis of ABPA is primarily immunological, and lung biopsy is not required in the majority55. Thus, limited information is available on the pathology of ABPA56. The usual pathological findings include bronchiectasis, with the dilated bronchi containing thick mucus plugs57 (Fig. 2, panel 1A). Histological examination reveals the presence of mucin (Fig. 2, panel 1A), Curschmann's spirals, Charcot-Leyden crystals and inflammatory cells (Fig. 2, panel 2). The fungi are sparsely demonstrated in the bronchiectatic cavities (Fig. 2, panels 3 and 4). The airways in ABPA are infiltrated by inflammatory cells, mainly the eosinophils but also the neutrophils57. The lung parenchyma contains a mixed chronic inflammatory response, often with prominent eosinophilia (Fig. 2, panel 5). In some patients with ABPA, fungi can also be demonstrated in the lung parenchyma58. The other findings include bronchiolitis obliterans with organizing pneumonia and bronchocentric granulomatosis5960 (Fig. 2, panel 6). Rarely, the course of ABPA may be complicated by invasive or chronic aspergillosis616263.
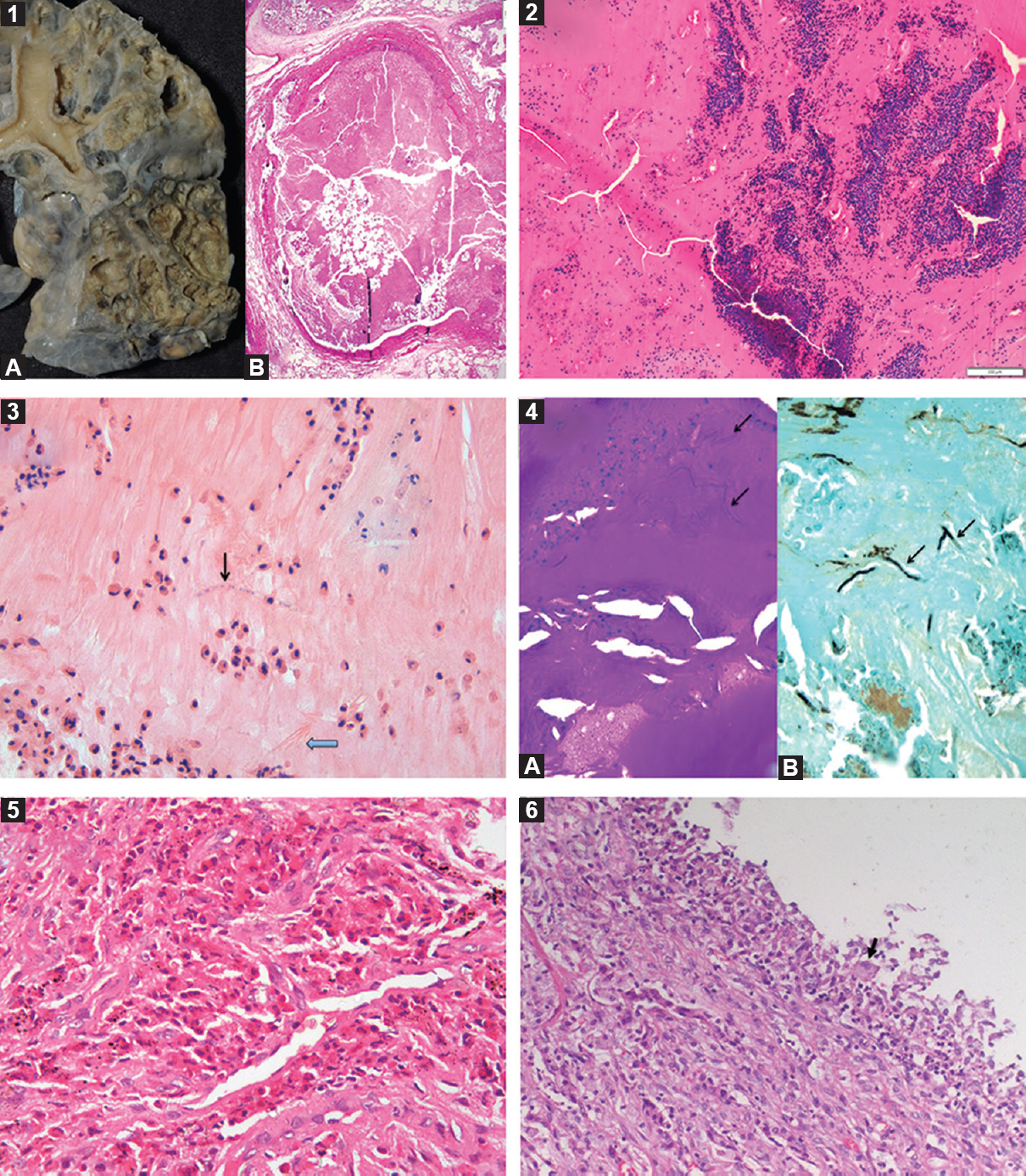
- A collage of histopathological findings in allergic bronchopulmonary aspergillosis. Gross photograph showing cystically dilated bronchi and bronchioles and lumen filled with brownish mucous plugs (panel 1A). Photomicrograph showing dilated bronchial lumen filled with allergic mucin (panel 1B; H and E, ×40). Low-power photomicrograph of allergic mucin having variegated appearance, containing mucin admixed with eosinophils, eosinophilic debris and other inflammatory cells arranged in a laminar pattern (panel 2; H and E, ×100). High-power photomicrograph of allergic mucin containing mucin admixed with eosinophils, Charcot laden crystals (thick arrow) and a few septate fungal hyphae (thin arrow) (panel 3; H and E, ×400). Photomicrographs of periodic acid-Schiff stain (panel 4A, ×400) and Grocott's stain (panel 4B, ×400) highlighting scattered fungal hyphae (arrows) within allergic mucin. Photomicrograph showing alveolar spaces filled with eosinophils indicative of eosinophilic pneumonia (panel 5; H and E, ×200). Photomicrograph of bronchocentric granulomatosis (panel 6) with occasional multinucleate giant cell (black arrow).
Clinical features
There is no age or gender preference for the development of ABPA64. Most patients present with poorly controlled asthma. Occasionally, patients may be asymptomatic, and ABPA is diagnosed on systematic screening of asthmatic patients. In one series of 155 patients with ABPA, almost 19 per cent had controlled asthma65. ABPA may also uncommonly occur in patients who do not have a history of bronchial asthma66. Other symptoms include haemoptysis, low-grade fever, weight loss and malaise. Expectoration of brownish mucus plugs is a characteristic symptom but seen in only 31-69 per cent of the patients656768. Clubbing is rare (16%), usually seen in those with long-standing bronchiectasis. On auscultation, polyphonic wheeze is the most common finding64, while coarse crackles are uncommon (15%)64. Physical examination may also reveal features of pulmonary hypertension and respiratory failure69. During exacerbations of ABPA, localized auscultatory findings can occur and need to be differentiated from other pulmonary illnesses.
Diagnosis of ABPA
Immunological investigations
Aspergillus fumigatus-specific immunoglobulin E (IgE): An elevated level of serum A. fumigatus-specific IgE (>0.35 kUA/l) is currently the most sensitive investigation in the diagnosis of ABPA and is also considered the preferred test for screening asthmatic patients for ABPA. In a large prospective study, the sensitivity and specificity of A. fumigatus IgE were found to be 100 and 70 per cent, respectively, in the diagnosis of ABPA70. Although serum A. fumigatus-specific IgE is useful in diagnosis, this is not helpful in the follow up of patients. In one study, the IgE increased (instead of decreasing) in 52 per cent of the patients after treatment, while it increased in only 39 per cent of the patients during an exacerbation71.
Aspergillus skin test: An immediate cutaneous reaction to A. fumigatus antigen (performed either using a skin prick or by intradermal injection) is analogous to the presence of raised IgE against A. fumigatus. The sensitivity of skin testing in the diagnosis of ABPA ranges from 88 to 94 per cent70 and thus can potentially miss 6-12 per cent of patients with ABPA. Moreover, skin testing is fraught with other problems including variable quality and lack of standardization of the antigen, the competence of the technical staff performing the test and the theoretical risk of anaphylaxis10. Hence, skin testing is currently not the favoured test for screening asthmatic patients for ABPA.
Serum total IgE levels: Measurement of the serum total IgE is a useful test in the diagnosis and follow up of patients with ABPA. A normal serum total IgE nearly excludes active ABPA as the cause of patient's symptoms. Although the sensitivity of serum total IgE (cut-off 500 IU/ml) in screening asthmatic patients for ABPA is good (96%), the specificity is poor (24%)70. Hence, it is not a good test for screening for ABPA. However, it is a good test in the follow up of patients because the serum total IgE levels start declining after treatment22. In most patients, the IgE level does not normalize following treatment. Hence, repeated measurements are required to determine the new baseline IgE value for every patient. An increase in serum total IgE along with clinical and radiological deterioration indicates an ABPA exacerbation3.
Aspergillus fumigatus-specific IgG: The precipitating IgG antibodies against A. fumigatus were the earliest immunological test used in the diagnosis of ABPA72. Unfortunately, A. fumigatus-specific IgG detected using double gel diffusion technique has a sensitivity of only 27 per cent in the diagnosis of ABPA6. The various in-house assays showed variable sensitivity and specificity while the commercial enzyme immunoassay methods for measuring A. fumigatus-specific IgG have a sensitivity exceeding 90 per cent673.
Peripheral blood eosinophil count: A total eosinophil count >1000 cells/μl is considered as an important criterion for the diagnosis of ABPA74. However, in one series, 60 per cent of the patients had an eosinophil count of <1000 cells/μl while 25 per cent had a count <500 cells/μl75. In our experience, an overreliance on eosinophil count for the diagnosis of ABPA is a significant cause for missed diagnosis of ABPA in India18. The accepted cut-off for peripheral blood eosinophil count is 500 cells/μl76.
Role of recombinant Aspergillus antigens: The currently used antigens for performing immunological assays in ABPA are crude extracts from A. fumigatus77. These antigens not only have variable diagnostic performance but can also cross-react with other fungal antigens. The World Health Organization (WHO) and International Union of Immunological Societies (IUIS) Allergen Nomenclature Sub-committee recognize 23 specific antigens of A. fumigatus78. Of these, five recombinant antigens (rAsp f1, f2, f3, f4 and f6) are commercially available. According to the concept of molecular allergy-based diagnostics, rAsp f1 and rAsp f2 are considered as the specific allergens of A. fumigatus as these have minimal cross-reactivity with other fungal antigens77. In a systematic review, the pooled sensitivity (97%) for diagnosing ABPA complicating asthma was best for IgE against a combination of rAsp f1 or f3 while the pooled specificity was the highest (99%) against a combination of f4 or f679. However, majority of the studies were from a few specialized centres of Europe and the United States. The major caveat was that none of the studies had specified any cut-off for the diagnosis of ABPA. In a recent study, the sensitivity and specificity of IgE against either rAsp f1 (cut-off, 4.465 kUA/l) or f2 (cut-off, 1.300 kUA/l) for diagnosing ABPA were 100 and 81 per cent, respectively80.
Basophil activation test (BAT): The BAT is an in vitro flow cytometry-based cellular assay that measures the activation of basophils upon allergen stimulation. The BAT identifies activated basophils with the aid of surface markers including CD63, CD193 and CD203c. Several studies suggest a promising role on the utility of BAT in the diagnosis of ABPA complicating CF818283. In our experience, there is a limited utility of BAT in ABPA complicating asthma both in the diagnosis of ABPA and differentiating ABPA from AS84. Moreover, this test has two important limitations, namely the requirement of flow cytometer and the fact that the test should be performed within a few hours of collection of the blood sample.
Radiological investigations
Chest radiograph: The chest radiographic findings in ABPA may be broadly classified as transient or fixed85. The characteristic fleeting opacities are found during ABPA exacerbations, whereas fixed abnormalities are encountered in the advanced stages. The most common finding described is consolidation. However, these descriptions are from the pre-computed tomography (CT) era86. In a later study, the areas of consolidation identified on the chest radiograph were found to represent mucus-filled bronchi on CT87. Other transient findings include tramline shadows, finger-in-glove opacities and toothpaste shadows86, all indicating mucus impaction of the bronchiectatic cavities. In the advanced stages, there can be fibrosis and collapse, affecting several lobes, which may indicate the development of chronic pulmonary aspergillosis6263. The most common finding is that of a normal chest radiograph87, suggesting that it is not the best investigation for delineating the radiological abnormalities of ABPA. However, a chest radiograph is useful in follow up as transient abnormalities disappear after the institution of therapy for ABPA.
Computed tomography of the chest: Thin-section (or high resolution) CT of the thorax is currently the imaging modality of choice for ABPA85. The most common finding on CT chest is bronchiectasis (Fig. 3). While central bronchiectasis is believed to be characteristic for the diagnosis of ABPA88, bronchiectases can reach the periphery in almost 40 per cent of the cases587. The pathognomonic radiological finding in ABPA is high-attenuation mucus (HAM), which is visually denser than the paraspinal skeletal muscle65899091 (Fig. 3). The presence of HAM indicates ABPA as the aetiology of the underlying bronchiectasis92. Other CT findings include the presence of non-hyper-attenuating mucoid impaction, centrilobular nodules, tree-in-bud opacities and mosaic attenuation. The uncommon radiologic manifestations include perihilar opacities simulating hilar lymphadenopathy93, miliary nodular opacities94, pleural effusions95, complete lung collapse96 and pulmonary masses97. It is pertinent to mention that ABPA can present without any radiological manifestations, which emphasizes the fact that the diagnosis of ABPA is primarily immunological.
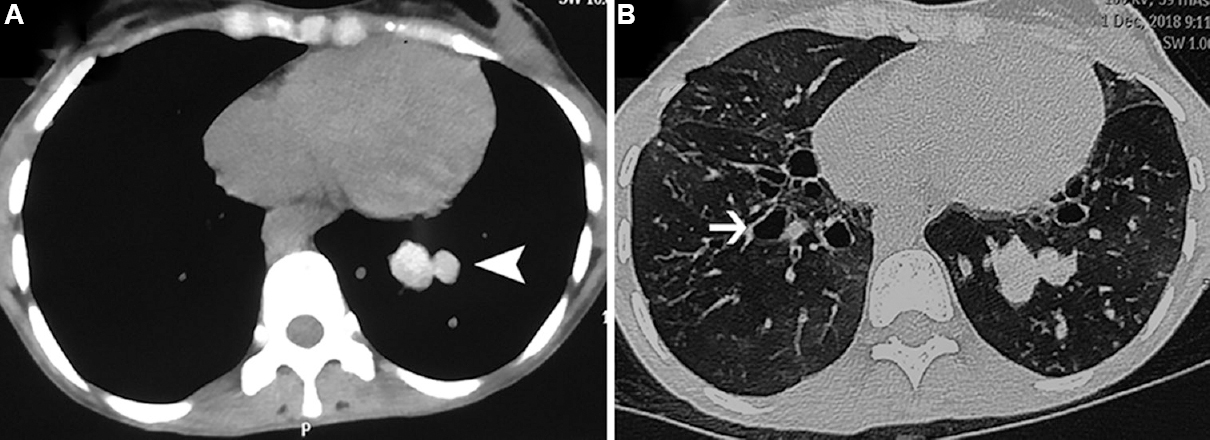
- Computed tomography chest images in a patient with allergic bronchopulmonary aspergillosis. The mediastinal window (panel A) shows the presence of high-attenuation mucus (arrow head), which is visually denser than para-spinal skeletal muscle. The lung window (panel B) shows bronchiectasis (thin arrow). Other findings discernable include centrilobular nodules, tree-in-bud opacities and mosaic attenuation.
Magnetic resonance imaging of the chest: Magnetic resonance imaging (MRI) has traditionally been avoided for the evaluation of lung due to the low proton density of lung and high propensity for an artefact. However, recent technological advancements have led to its use in respiratory pathologies including ABPA, both in asthma9899 and CF100. The counterpart of HAM on MRI seems to be inverted mucus impaction, which is characterized by a hyper-intense signal on T1-weighted images and hypo-intense signal on T2-weighted images100. The role of MRI in ABPA is still investigational, and it requires expertise. Hence, MRI is not advisable as a routine practice.
Other investigations
Sputum cultures: Patients with ABPA are colonized not only by the causative fungus but also by other fungi and bacteria. Growth of A. fumigatus in the sputum is supportive but not diagnostic of ABPA as the fungi are ubiquitous. Sputum cultures are however, essential as these can provide a clue to the presence of fungi other than A. fumigatus (ABPM). Sputum cultures are also valuable for performing drug-susceptibility testing and real-time molecular testing for resistance, of the isolates obtained, before treatment101.
Pulmonary function tests: These are essential in classifying the severity of the lung disease. Bronchoprovocation testing with Aspergillus antigens has been used in the past; however, it can precipitate acute bronchospasm102. The pulmonary function tests usually reveal obstructive defect with a reduction in diffusion capacity103.
Galactomannan detection: Galactomannan is a polysaccharide component of the Aspergillus cell wall. Serum or bronchoalveolar lavage fluid galactomannan is widely used in the diagnosis of invasive pulmonary aspergillosis. However, the sensitivity and specificity of serum galactomannan index (cut-off, 0.5) in ABPA are 25.7 and 82 per cent, respectively104. Bronchoalveolar lavage fluid galactomannan index was also found to have poor sensitivity105.
Thymus and activation-regulated chemokine (TARC): Due to the profound Th2 immune response, the overexpression of the thymus and activation-regulated chemokine (TARC, CCL17) has been evaluated in patients with ABPA. While some studies have found higher levels of TARC in patients with ABPA106107, others were not able to replicate these findings108. There is currently little utility of measuring TARC levels in patients with ABPA.
Diagnostic criteria and algorithm
The Rosenberg-Patterson criteria (8 major, 3 minor) were the most widely used yardstick for the diagnosis of ABPA in asthma74. Not only there was no agreement on the number of criteria that should be present to make a diagnosis, but also the cut-off value of various immunological tests was not specified55. The ISHAM-ABPA working group has proposed new criteria, which were published in 20133. After publication of these criteria, several new pieces of evidence have emerged. For instance, A. fumigatus-specific IgE and IgG are more sensitive than skin testing and serum A. fumigatus precipitins, respectively670. Moreover, inclusion of bronchiectasis may improve the sensitivity of the criteria (unpublished data). It is believed that minor modifications may improve the diagnostic performance of the criteria (Table III). The addition of recombinant Aspergillus antigens may further simplify the diagnostic algorithm of ABPA.
| Predisposing conditions |
| Asthma, cystic fibrosis |
| Obligatory criteria (both should be present) |
| Immediate cutaneous hyper-reactivity to Aspergillus antigens or Aspergillus fumigatus-IgE >0.35 kUA/l |
| Total IgE >1000 IU/ml |
| Other criteria (at least 2 out of 3) |
| Peripheral blood eosinophil count >500 cells/µl |
| Transient pulmonary infiltrates on chest radiograph |
| Presence of precipitins (IgG) against A. fumigatus |
| ISHAM-ABPA Working Group diagnostic criteria for ABPA (suggested modifications) |
| Predisposing conditions |
| Asthma, cystic fibrosis |
| Obligatory criteria (both should be present) |
| A. fumigatus-IgE >0.35 kUA/l |
| Total IgE >1000 IU/ml |
| Other criteria (at least 2 out of 3) |
| Peripheral blood eosinophil count >500 cells/µl |
| Bronchiectasis on computed tomography of the chest |
| A. fumigatus-IgG >27 mgA/l |
Source: Adapted with permission from Ref. 3
While investigating a patient with asthma, we first perform A. fumigatus-specific IgE (Fig. 4). If it is >0.35 kUA/l, the serum total IgE levels are assayed. If the serum total IgE is greater than 500 IU/ml, the other tests are performed, including a thin-section CT of the thorax, A. fumigatus-specific IgG and peripheral blood eosinophil count. Patients of ABPA without bronchiectasis on CT chest are labelled as serological ABPA (ABPA-S) while those with bronchiectasis and HAM are classified as ABPA-B and ABPA-HAM, respectively (Table IV). The asthmatic patients without AS with A. fumigatus-specific IgE are followed up every 2-3 yr. In those with raised A. fumigatus-specific IgE and serum total IgE <500 IU/ml, serum total IgE is repeated every year as 40 per cent of patients with AS can potentially develop ABPA14.
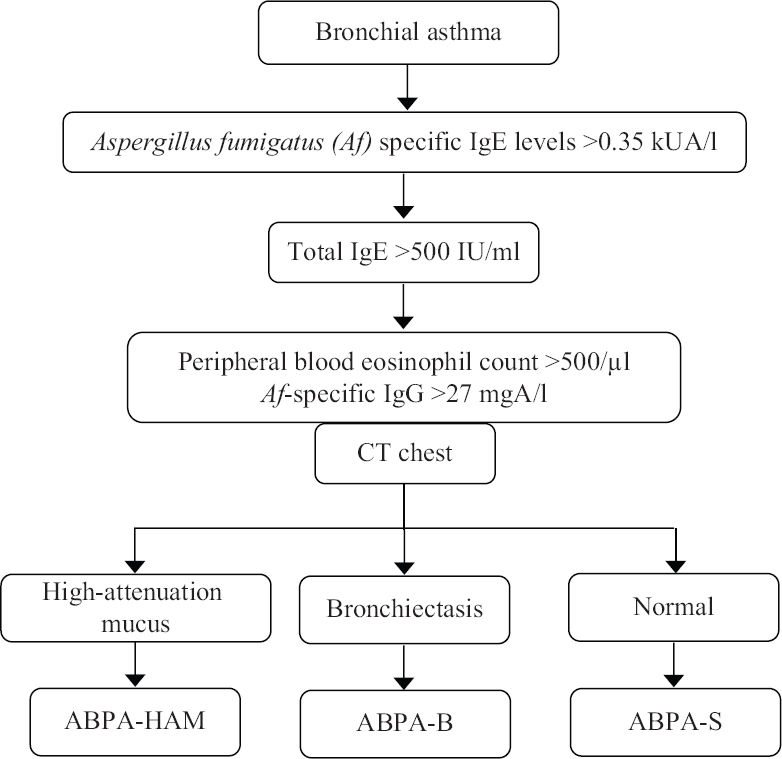
- Algorithm followed in the diagnostic work-up of allergic bronchopulmonary aspergillosis (ABPA). Reproduced with permission from Ref. 3.
| Classification | Features |
|---|---|
| ABPA-S | All the diagnostic features of ABPA (Table III) but no evidence of bronchiectasis on CT |
| ABPA-B | All findings of ABPA including bronchiectasis on CT of the chest |
| ABPA-HAM | All features of ABPA including HAM on CT of the chest |
| ABPA-CPF | ABPA with other radiologic features such as pulmonary fibrosis, bleb, bullae, pneumothorax, parenchymal scarring, emphysematous change, multiple cyst, fibrocavitary lesions, aspergilloma, pleural thickening |
CT, computed tomography; ABPA-S, serological ABPA; ABPA-B, ABPA with bronchiectasis; ABPA-HAM, ABPA with high attenuation mucus; ABPA-CPF, ABPA with chronic pleuropulmonary fibrosis Source: Reproduced with permission from Ref.3
Differential diagnosis
While the diagnosis of ABPA is usually straightforward if suspected, occasionally, the disorder needs to be differentiated from the following conditions: Aspergillus-sensitized bronchial asthma109; severe asthma with fungal sensitization, an entity described by the presence of fungal sensitization and severe asthma, where patients do not meet the criteria for ABPA110111; pulmonary tuberculosis (in high tuberculosis prevalence areas)112; eosinophilic pneumonia (acute and chronic) and Churg-Strauss syndrome113; and tropical pulmonary eosinophilia114.
Natural history and staging
The natural course of ABPA is characterized by recurrent exacerbations64. Furthermore, spontaneous improvement of symptoms and clearing of pulmonary opacities are common. If the disease is not recognized timely or treated appropriately, the inflammation can result in irreversible pulmonary damage.
Staging of ABPA
The ISHAM working group defines ABPA into seven stages (stages 0-6, Table V)3. Importantly, a patient does not necessarily advance from one stage to the other sequentially. Majority of the patients are diagnosed when they present for the first time with typical features of ABPA, including uncontrolled asthma (acute stage, stage 1). Presence or absence of mucoid impaction further defines this stage as 1a or 1b, respectively. Patients may have controlled asthma, and ABPA may be detected on routine screening (asymptomatic stage, stage 0). Stages 2-5 are designated in patients undergoing therapy. Response to treatment (stage 2) is characterized by a decline in the serum total IgE by at least 25 per cent, along with clinical and radiological improvement. With treatment, serum total IgE falls progressively, and the nadir is now defined as a new baseline. Exacerbation is defined by the presence of clinical and radiologic worsening and an increase in the serum total IgE by at least 50 per cent from the new baseline (stage 3). Remission (stage 4) is established by the presence of clinical, immunological (<50% increase from new baseline) and radiologic stability for at least six months, without any ABPA-specific therapy. Patients who require glucocorticoids for controlling ABPA or asthma are classified as stage 5a (treatment-dependent ABPA) or 5b (glucocorticoid-dependent asthma), respectively. Finally, patients with ABPA may develop advanced disease with extensive bronchiectasis and evidence of either pulmonary hypertension or respiratory failure and are labelled as advanced ABPA (stage 6)3.
| Stage | Definition | Features |
|---|---|---|
| 0 | Asymptomatic | No previous diagnosis of ABPA |
| Controlled asthma (according to Indian guidelines) | ||
| Fulfilling the diagnostic criteria of ABPA (Table IV) | ||
| 1 | Acute | No previous diagnosis of ABPA |
| Symptoms consistent with ABPA | ||
| Satisfying the diagnostic criteria of ABPA | ||
| 1a | With mucoid impaction | Mucoid impaction observed on thoracic imaging |
| 1b | Without mucoid impaction | Absence of mucoid impaction on thoracic imaging |
| 2 | Response | Clinical and/or radiological improvement and decline in serum total IgE by ≥25% of baseline at 8 wk |
| 3 | Exacerbation | Clinical and/or radiological worsening and increase in serum total IgE by at least 50% from the new baseline established during response/remission |
| 4 | Remission | Sustained clinical and radiological improvement and serum total IgE levels persisting at or below baseline (or increase by <50%) for ≥6 months off treatment |
| 5a | Treatment-dependent ABPA | Two or more exacerbations within six months of stopping therapy or clinical and/or radiological worsening, along with increase in serum total IgE levels, on tapering oral steroids/azoles |
| 5b | Glucocorticoid- dependent asthma | Systemic glucocorticoids required for control of asthma while the ABPA activity is controlled (as indicated by serum total IgE and thoracic imaging) |
| 6 | Advanced ABPA | Extensive bronchiectasis due to ABPA on chest imaging along with either cor pulmonale and/or chronic Type II respiratory failure |
Source: Reproduced with permission from Ref. 3
Treatment of ABPA
The treatment of ABPA comprises two tenets: (i) the use of glucocorticoids as anti-inflammatory agents to suppress the immune hyper-reactivity, and (ii) the use of anti-fungal agents to reduce the fungal burden in the airways115116. The goals of treatment include the following: (i) reduction of pulmonary inflammation, (ii) control of asthma, (iii) treatment of acute symptoms of ABPA, (iv) prevention of ABPA exacerbations, and (v) halt the onset or progression to bronchiectasis. It is important that any environmental source responsible for continuous exposure to A. fumigatus is eliminated.
Glucocorticoids
Oral glucocorticoids: Oral glucocorticoids are currently the preferred treatment in the management of ABPA (Table VI)25117. However, the dose and duration of treatment were unclear. Our group has reported the efficacy of two different glucocorticoid dosing protocols in ABPA118. Ninety two patients with asthmatic ABPA were randomized to receive either high-dose (0.75 mg/kg/day for 6 wk, 0.5 mg/kg/day for 6 wk, taper by 5 mg every 6 wk; total duration: 8-10 months) or low-dose (0.5 mg/kg/day for 2 wk, 0.5 mg/kg/day on alternate days for 8 wk, taper by 5 mg every 2 wk; total duration: 3-5 months) prednisolone. The numbers of patients with exacerbation after one year (40.9 vs. 50%, P=0.59) and glucocorticoid-dependent ABPA after two years of treatment (11.4 vs. 14.6%, P=0.88) were similar in the two groups. The occurrence of adverse reactions was significantly higher in the high-dose arm. Thus, low-dose glucocorticoids are as effective as high-dose and are associated with lesser side effects. However, in this study, the number of patients with a response after six weeks was higher in the high-dose group118. To overcome this limitation, a slightly higher glucocorticoid dose (prednisolone: 0.5 mg/kg for 4 wk, 0.25 mg/kg for 4 wk, 0.125 mg/kg for 4 wk, taper by 5 mg every week; total duration: 4 months) was tested. With this regimen, in two subsequent randomized trials, there was no response failure after six weeks of treatment119120.
| Oral glucocorticoids |
| Prednisolone: 0.5 mg/kg for 4 wk, 0.25 mg/kg for 4 wk, 0.125 mg/kg for 4 wk, then tapered by 5 mg every wk to continue for a total duration of at least 4 months |
| Indication: First-line treatment of ABPA, both in acute-stage and during exacerbation |
| Oral azoles |
| Itraconazole: 200 mg twice a day for 24 wk Indication: Second exacerbation of ABPA; glucocorticoid-dependent ABPA; alternative to glucocorticoids as first-line treatment of ABPA, especially in those with increased propensity for glucocorticoid-related side effects |
| Follow up and monitoring |
| Patients are followed up with history and physical examination, chest radiograph, spirometry and measurement of total IgE levels every 8 wk (to determine the new baseline IgE) |
| Important points |
| A 25% decline in serum total IgE along with clinical and/or radiological improvement, indicates a satisfactory response to therapy |
| A clinical or radiological worsening along with a ≥50% increase in the new baseline IgE points to an ABPA exacerbation |
| Worsening of symptoms in the absence of radiological or immunological worsening (serum total IgE) suggests an asthma exacerbation |
| Monitor for adverse effects e.g., hypertension, hyperglycaemia, in case of glucocorticoids; nausea, vomiting, diarrhoea, elevated liver enzymes, in case of azoles |
| Monitor for drug-drug interactions |
| Prophylaxis for osteoporosis (with glucocorticoid therapy): oral calcium and bisphosphonates |
Source: Adapted with permission from Ref.3
Inhaled glucocorticoids (ICSs): Inhaled glucocorticoids (ICSs) achieve suitable concentrations in the airways, are associated with significantly fewer side effects and are the first-line therapy of bronchial asthma. These have also been used as anti-inflammatory agents in ABPA. In a randomized trial involving 32 patients of ABPA, there was no benefit of 400 μg/day of inhaled beclomethasone over placebo121. In another study, 21 patients with ABPA-S were managed with ICS and long-acting beta-2 agonists (formoterol/budesonide, 24/1600 μg/day). Although there was a clinical improvement in patients treated with ICS, none could completely achieve asthma control. In addition, the IgE levels increased significantly. Subsequent treatment with oral glucocorticoids led to an improvement in the control of asthma and a decline in IgE levels122. Thus, ICS alone is useful only for the control of asthma in patients of ABPA.
Intravenous pulse doses of glucocorticoids: Pulse doses of glucocorticoids (intravenous infusion of 15 mg/kg of methylprednisolone for 3 consecutive days) have been used in children with ABPA as an alternative to daily glucocorticoid therapy123. Pulse glucocorticoid therapy may also be useful in refractory ABPA exacerbation124. This is especially seen in those with long-term glucocorticoid use, which can lead to downregulation of glucocorticoid receptors, thereby leading to a steroid-resistance state. The use of pulse doses of glucocorticoid may overcome the steroid-resistance by its non-genomic effects, independent of the glucocorticoid receptor.
Antifungal agents
Antifungal agents act by reducing the fungal load, thereby minimizing the impetus for the ongoing inflammatory activity125. Antifungal agents were not considered as a reasonable therapy in ABPA, till the advent of itraconazole. The currently available triazoles have fewer side effects and seem to be an attractive option in the management of ABPA. Several randomized trials have reported the use of itraconazole in ABPA119126127. In one study, 55 patients with glucocorticoid-dependent ABPA (stage 5) were randomized to receive either oral itraconazole (400 mg/day) or placebo for 16 wk. Itraconazole was superior to placebo in the composite response (diminution in glucocorticoid dose by at least 50% and decline in serum total IgE by at least 25%; and either increase in exercise capacity by ≥25% or improvement in pulmonary function by ≥25% or resolution of pulmonary opacities); however, there was no difference when each outcome was evaluated independently126. The other trial randomized 29 stable ABPA patients (stages 2, 4 and 5) to receive itraconazole (400 mg/day orally) or placebo. There was a decline in sputum inflammatory markers, serum total IgE and the frequency of exacerbations requiring glucocorticoids127.
Another approach is to use itraconazole alone in patients with the acute stage (stage 1) of ABPA, thereby altogether avoiding the exposure of glucocorticoids. In a recent study, 131 patients [prednisolone (n=63), itraconazole (n=68)] of stage 1 ABPA were randomized to receive either oral itraconazole (400 mg/day) or prednisolone for four months119. The proportion of subjects showing response at six weeks were significantly higher in those receiving prednisolone versus itraconazole (100 vs. 88%, P=0.007). However, the reduction in serum total IgE after six weeks and three months, and the number of patients s with exacerbations after one and two years of treatment was similar in the two groups. Adverse reactions were significantly more in the glucocorticoid arm. Though prednisolone was more effective than itraconazole in inducing a response in acute-stage ABPA, itraconazole with fewer side effects was an acceptable alternative agent in the treatment of acute-stage ABPA.
Newer azoles: Voriconazole is an alternative antifungal agent to itraconazole. In a trial, 50 patients were randomized to receive either prednisolone or voriconazole120. The response to treatment after six weeks and three months was similar in the two groups. The numbers of patients with exacerbations after one and two years were also similar in the two groups. The decline in serum total IgE, the change in lung function and quality of life score and the time to first exacerbation were similar in the two groups. In our experience, many patients are unable to tolerate voriconazole due to photo-toxicity. Newer azoles (posaconazole, isavuconazole) have also been evaluated for their efficacy in ABPA128129. Because of the higher cost of therapy, the newer azoles are generally reserved in those with itraconazole failures.
Nebulized amphotericin B: Inhaled amphotericin achieves high concentrations in the airways well above the minimal inhibitory concentration of A. fumigatus (0.5 mg/l), with negligible serum concentrations130. Thus, there is clinical efficacy with minimal side effects130131132. Chishimba et al133 evaluated the use of nebulized amphotericin B in 10 ABPA patients (failing azole therapy or experiencing side effects with the azoles). Nebulized amphotericin B was found to be effective in only two patients indicating limited efficacy of nebulized amphotericin B in the management of ABPA exacerbations. Another approach is to first induce response/remission with either glucocorticoids or oral azoles and then maintain the response with nebulized amphotericin. This strategy was evaluated in 21 patients with recurrent exacerbations (≥2) of ABPA after inducing response with prednisolone134. Patients were randomized to receive either nebulized amphotericin B (deoxycholate preparation; 10 mg twice daily, thrice a week) plus nebulized budesonide or nebulized budesonide alone. The primary outcome (time to first exacerbation) was similar; however, the number of patients experiencing ABPA exacerbations was lower in the amphotericin arm (8.3 vs. 66.7%, P=0.016). The conventional preparation of amphotericin B has detergent properties due to the deoxycholate component. The deoxycholate depletes the pulmonary surfactant and can potentially cause bronchospasm135. The lipid formulations have longer pulmonary retention and no effects on lung surfactant. Hence, future trials should preferably use lipid preparations.
Other therapies
Anti-IgE therapy: While omalizumab, a monoclonal antibody is indicated in patients with severe allergic asthma136, it has also been evaluated in ABPA137. The aim of therapy with omalizumab is to decrease the serum total IgE to <21 IU/ml, and the dose required to achieve this is 0.016 mg/kg/IU (IgE/ml)138. An upper limit of IgE of 1500 IU/ml and a maximum dose of omalizumab 1200 mg monthly have been specified by the manufacturer. In ABPA, the serum total IgE is highly elevated and the dose required is very high. In one study, a 600 mg daily dose of subcutaneous omalizumab was used. The study was prematurely terminated due to a significant dropout rate (clinical trials.gov; NCT00787917). In a crossover trial, Voskamp et al139 randomized 13 patients of ABPA to four-month treatment with omalizumab (375 mg subcutaneous every 2 wk) or placebo, followed by a three-month washout period. Omalizumab not only reduced the number of exacerbations during the treatment phase but also decreased the exhaled nitric oxide levels and reduced the basophil sensitivity to A. fumigatus and the basophil FcεRI and surface-bound IgE levels. Currently, omalizumab remains a treatment option in patients with ABPA who do not tolerate first-line treatments or are refractory to other therapies140.
Anti-Th2 therapies: ABPA is characterized by an intense Th2 inflammation associated with excessive production of Th2 cytokines IL-4 and IL-5, which suggests a role for anti-IL-5 therapies116. There are reports on the efficacy of mepolizumab, a monoclonal antibody against IL-5 (100 mg subcutaneous every 4 wk) and benralizumab, a monoclonal antibody against IL-5Rα (30 mg subcutaneous every 4 wk for the first three doses, then every 8 wk) in patients with ABPA141142. More studies are required to define the place of anti-Th2 therapies in ABPA.
Supportive therapies: Supportive therapies include nebulized hypertonic saline (7%, 3-5 ml) to reduce the sputum viscosity143. The use of hypertonic saline may occasionally cause bronchospasm. Thus, its administration should be preceded by salbutamol inhalation. Nebulized hypertonic saline is not recommended in patients with FEV1 (forced expiratory volume in one second) <1.l, and the first dose should be given under supervision. Although one study used bronchoscopy to clear mucus in all patients with ABPA-B or ABPA-HAM144, we use therapeutic bronchoscopy only in patients with airway collapse (segmental or larger) that persists despite 3-4 wk of oral glucocorticoids96.
Anti-inflammatory therapy with statins or azithromycin has been evaluated in patients with bronchiectasis, not specifically ABPA145. The European Respiratory Society (ERS) guidelines advocate long-term antibiotic treatment for adults with bronchiectasis who have three or more exacerbations per year145. The guidelines also suggest the following in patients with bronchiectasis and three or more infections per year: (i) long-term therapy with an inhaled antibiotic for adults with chronic P. aeruginosa infection, (ii) long-term treatment with macrolides for chronic P. aeruginosa infection where an inhaled antibiotic is not viable due to any reason, (iii) additional long-term treatment with macrolides for chronic P. aeruginosa infection in patients with a high exacerbation frequency despite inhaled antibiotics, (iv) long-term treatment with macrolides in patients not infected with P. aeruginosa, (v) long-term oral antibiotic (based on antibiotic susceptibility) therapy in patients not infected with P. aeruginosa where treatment with macrolides is not practical due to any reason, and (vi) long-term treatment with an inhaled antibiotic in patients not infected with P. aeruginosa where oral antibiotic prophylaxis is not accomplishable due to any reason. The improvement in exacerbations is balanced against the risk of drug-related side effects and of acquiring antibiotic resistance.
Pneumococcal and influenza vaccination is recommended in all patients of ABPA-B and ABPA with poorly controlled asthma. A poor response to 23-valent polysaccharide pneumococcal vaccine in ABPA patients was noted in a study146. Thus, either a combination of polysaccharide and 13-valent conjugate vaccine or a delay of vaccination until the patient is off glucocorticoids may result in a superior response. Long-term oxygen therapy is required in patients with advanced ABPA147. Lung transplantation remains the only option for patients with advanced lung disease. ABPA can recur in the donor lungs148, and has been successfully treated in the post-transplant setting with nebulized amphotericin B149.
Practical approach to treatment
A suggested protocol for the management of ABPA is shown in Figure 5. As most patients require prolonged therapy, the treatment goals should be met with minimal adverse reactions. One should not start any specific medicine for ABPA in patients without bronchiectasis (ABPA-S), provided the asthma is well controlled150. However, most patients with bronchiectasis or fleeting pulmonary opacities, even if asymptomatic, should be treated, because the presence of bronchiectasis suggests end-organ damage. Patients in stage 1 (acute stage) should be managed with glucocorticoids (Table VI). High doses of inhaled steroids should be as avoided as a single agent in the management of ABPA. An alternative option is to use oral itraconazole, especially in those with an increased propensity for glucocorticoid-related adverse effects. Newer azoles (voriconazole, posaconazole or isavuconazole) can be used in those who either do not respond or are intolerant or experience adverse effects with itraconazole. There is a risk of development of triazole resistance with long-term therapy101, and resistance to one azole can lead to cross-resistance to other azoles151. Whether a combination of oral itraconazole and glucocorticoids is superior to glucocorticoids alone in acute stage ABPA (stage 1) remains unknown.
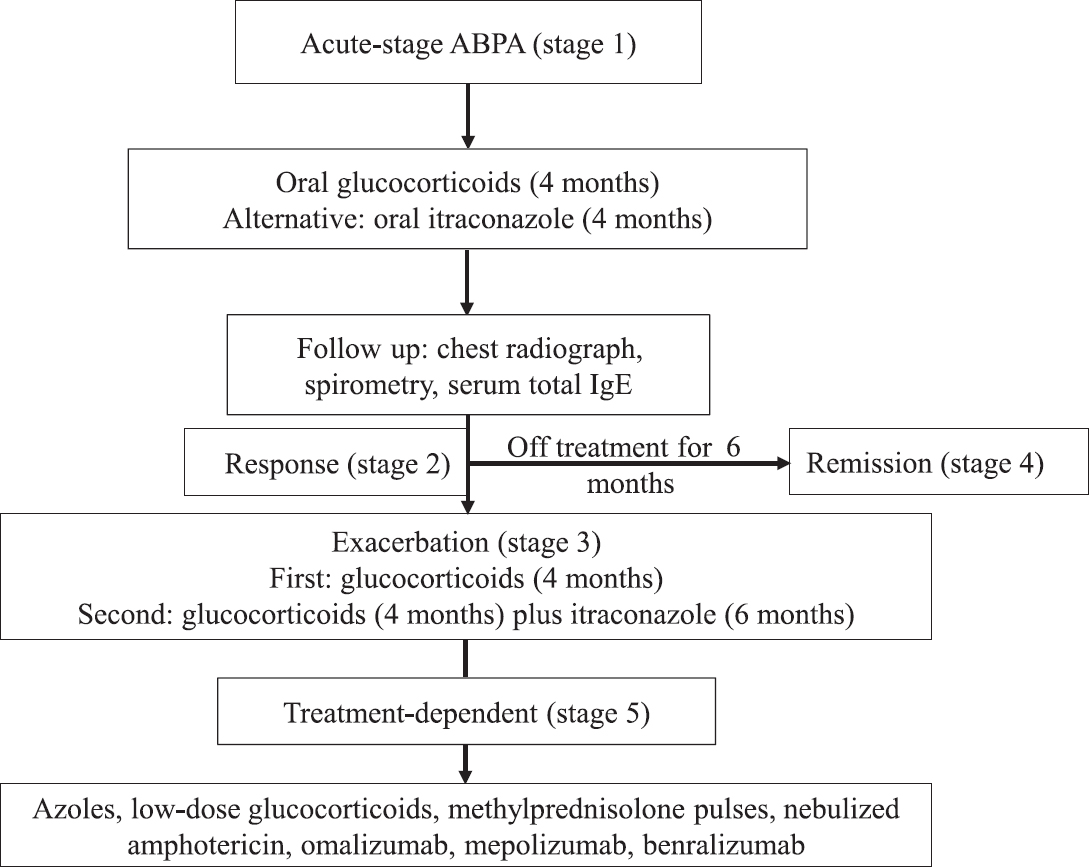
- Suggested treatment approach for allergic bronchopulmonary aspergillosis (ABPA).
Patients should be monitored at least every eight weeks with serum total IgE, chest radiograph, and spirometry (Table VI). A good response to therapy is indicated by a clinical, spirometric and radiological improvement and a reduction in serum total IgE by about 25 per cent (stage 2)22152. It may be relevant to reaffirm that there is no role of A. fumigatus-specific IgE and IgG in monitoring treatment responses in patients with ABPA671. The aim of therapy is not normalization of IgE levels. About 40-50 per cent of patients experience an exacerbation (stage 3)52265118119120, which is characterized by clinical and radiological deterioration along with 50 per cent increase in the new baseline serum total IgE value. The first ABPA exacerbation is treated with glucocorticoids. In patients with subsequent exacerbation, a combination of glucocorticoids and itraconazole is recommended. The dose and duration of treatment are similar to that used in acute stages.
Patients with recurrent exacerbations or treatment-dependent ABPA (stage 5) may be managed with oral itraconazole, low-dose glucocorticoids, monthly pulses of methylprednisolone, nebulized amphotericin B, omalizumab or anti-Th2 therapies. Patients on long-term therapy should be advised about the side effects of treatments.
ABPA in special situations and related conditions
In children
The diagnosis and treatment protocol in children are similar to adults153. However, glucocorticoids should be administered at the lowest possible dose and the shortest possible duration, due to concerns regarding growth retardation. A lower threshold should be maintained for the use of antifungal azoles, nebulized amphotericin, omalizumab or mepolizumab. Where ABPA is glucocorticoid-dependent, monthly doses of methylprednisolone should be considered.
During pregnancy
The treatment of choice in pregnancy is glucocorticoids. While itraconazole has not been associated with increased risk of major congenital anomalies154, the rates of miscarriage are higher in the itraconazole-exposed group154155. Newer azoles are contraindicated in pregnancy except in a life-threatening situation without any alternative. Nebulized amphotericin B can be safely used during pregnancy155. The use of omalizumab has been found to be without any congenital abnormalities, prematurity or low birth weight156. The safety of mepolizumab in pregnancy is not known.
ABPA complicating cystic fibrosis
ABPA is a dreaded complication of CF and is associated with rapid loss of lung function, higher rates of microbial colonization and poorer nutritional status157158159. The critical element in pathogenesis is the exposure of bronchial mucosa to high levels of Aspergillus, secondary to abnormal mucus and cellular abnormalities resulting from the CF transmembrane conductance regulator mutations4. In a systematic review, the prevalence of AS and ABPA in CF was found to be 39 per cent (95% CI, 33-45%) and nine per cent (95% CI, 7-11%), respectively160. The recognition of ABPA in CF is arduous as lung disease secondary to CF per se shares several similarities with ABPA. For instance, wheezing can occur secondary to intercurrent infections in CF; transient pulmonary opacities, bronchiectasis and mucus plugging are manifestations of CF-related lung disease, even without ABPA. There are also challenges in diagnosis. Several patients demonstrate fluctuating immunological responses to A. fumigatus; others experience a spontaneous decline in many immunologic parameters, including serum total IgE and A. fumigatus-specific IgE values161. In this regard, BAT in response to stimulation by Aspergillus allergens is useful in differentiating CF-ABPA from AS and Aspergillus colonization in CF818283108. In addition, sputum real-time Aspergillus polymerase chain reaction and sputum galactomannan along with traditional immunological tests (serum total IgE, A. fumigatus-specific IgE and IgG) have been used for enhanced recognition of ABPA in CF162. The treatment of ABPA in CF is akin to ABPA in asthma. However, the treatment issues are complicated by the coexisting malabsorption, as oral medications, especially itraconazole capsules, are poorly absorbed. Furthermore, the use of glucocorticoids can either induce or worsen CF-related diabetes.
ABPA sans bronchial asthma
Though ABPA most commonly occurs in those with bronchial asthma, it can occasionally develop without underlying asthma94. However, the majority (97%) of patients with ABPA without asthma have underlying bronchiectasis. Because of the absence of asthma, patients with ABPA without asthma are initially misdiagnosed as bronchogenic carcinoma163, pulmonary tuberculosis94 and others. Recently, we have shown that ABPA sans asthma is a distinct subset of ABPA, associated with a better lung function and fewer occurrence of ABPA exacerbations66.
ABPA complicating other conditions
ABPA has been reported in other pulmonary disorders including bronchiectasis164, post-tubercular lung disease165166, Kartagener's syndrome167, Macleod's syndrome168, chronic obstructive pulmonary disease169170171, chronic granulomatous disease and hyper-IgE syndrome172. Larger observations are required to establish a definite association and clinical significance.
Allergic bronchopulmonary mycosis
Several thermo-tolerant fungi other than A. fumigatus have been shown to cause an ABPA-like syndrome173174. The frequency of ABPM is far less when compared to ABPA. The diagnosis of ABPM is similar to ABPA except that sensitization has to be documented against that particular fungus175.
Sinobronchial allergic mycosis
Patients with ABPA can have coexistent allergic fungal rhinosinusitis (AFRS); this combination is referred to as sinobronchial allergic mycosis. AFRS is an entity characterized by mucoid impaction of the para-nasal sinuses similar to ABPA176. The pathogenesis of AFRS, analogous to ABPA, represents a hyper-immune response to the presence of Aspergillus in the sinuses177. The patient usually presents with nasal stuffiness, rhinorrhoea, headache and epistaxis. The sinusitis can often be radiologically demonstrated. However, the reference standard for diagnosis is functional endoscopic sinus surgery (FESS), which is both diagnostic and therapeutic178.
The cornerstone of therapy is surgical debridement using FESS. The inflammatory material is removed from the sinuses, thereby providing aeration. Patients also receive preoperative glucocorticoids (prednisolone: 0.5 mg/kg/day) for two weeks to lessen the inflammation. After surgery, glucocorticoids (prednisolone: 0.5 mg/kg/day for 4 wk, then tapered) are continued the next 2-5 months179. Intranasal steroids and nasal irrigation with saline are required indefinitely. Short courses of oral glucocorticoids are required in patients with worsening of symptoms despite medical management. Although antifungal azoles are widely used in AFRS, their role remains unproven due to the absence of high-quality studies180.
Conclusions
A high-index of suspicion for ABPA should be maintained in all patients with bronchial asthma in the specialized clinics, irrespective of the level of control. Host susceptibility is central to the pathogenesis while host immunologic responses are the primary determinants of the clinical, biological, pathological and radiological features of this disorder. ABPA is often misdiagnosed for a variety of pulmonary diseases. However, the disorder needs to be diagnosed early and treated appropriately to prevent the development or progression of bronchiectasis. The diagnostic and classification criteria proposed by the ISHAM would go a long way in the early identification of this disorder. The best investigation in the diagnosis of ABPA is A. fumigatus-specific IgE and the most specific finding is the presence of HAM on CT chest. The use of A. fumigatus-specific IgG instead of the traditional precipitins will further improve the sensitivity of the diagnostic criteria. Glucocorticoids are the treatment of choice; antifungal triazoles are the alternative therapy. Finally, early diagnosis and appropriate treatment are associated with appropriate outcomes.
Financial support & sponsorship: The first author (RA) received grant support from Cipla, India, on research in ABPA and also received consultancy fees from Pulmatrix Inc., USA.
Conflicts of Interest: None.
References
- Allergic bronchopulmonary aspergillosis: Review of literature and proposal of new diagnostic and classification criteria. Clin Exp Allergy. 2013;43:850-73.
- [Google Scholar]
- Allergic bronchopulmonary aspergillosis in cystic fibrosis-state of the art: Cystic Fibrosis Foundation Consensus Conference. Clin Infect Dis. 2003;37(Suppl 3):S225-64.
- [Google Scholar]
- Allergic bronchopulmonary aspergillosis: Lessons from 126 patients attending a chest clinic in North India. Chest. 2006;130:442-8.
- [Google Scholar]
- Role of Aspergillus fumigatus-specific IgG in diagnosis and monitoring treatment response in allergic bronchopulmonary aspergillosis. Mycoses. 2017;60:33-9.
- [Google Scholar]
- Diagnosis of bronchopulmonary aspergillosis is often made too late. Med Klin (Munich). 1993;88:353-6.
- [Google Scholar]
- Developments in the diagnosis and treatment of allergic bronchopulmonary aspergillosis. Expert Rev Respir Med. 2016;10:1317-34.
- [Google Scholar]
- Role of recombinant Aspergillus fumigatus antigens in diagnosing Aspergillus sensitization among asthmatics. Mycoses 2020 doi: 101111/myc13124
- [Google Scholar]
- IgE sensitization to Aspergillus fumigatus is associated with reduced lung function in asthma. Am J Respir Crit Care Med. 2010;182:1362-8.
- [Google Scholar]
- Clinical significance of Aspergillus sensitisation in bronchial asthma. Mycoses. 2011;54:e531-8.
- [Google Scholar]
- Aspergillus hypersensitivity and allergic bronchopulmonary aspergillosis in patients with bronchial asthma: Systematic review and meta-analysis. Int J Tuberc Lung Dis. 2009;13:936-44.
- [Google Scholar]
- Epidemiology of allergic bronchopulmonary aspergillosis. In: Pasqualotto AC, ed. Aspergillosis: From diagnosis to prevention. New York: Springer; 2010. p. :671-88.
- [Google Scholar]
- Total IgE levels and asthma prevalence in the US population: Results from the national health and nutrition examination survey 2005-2006. J Allergy Clin Immunol. 2009;124:447-53.
- [Google Scholar]
- Global burden of allergic bronchopulmonary aspergillosis with asthma and its complication chronic pulmonary aspergillosis in adults. Med Mycol. 2013;51:361-70.
- [Google Scholar]
- Burden and distinctive character of allergic bronchopulmonary aspergillosis in India. Mycopathologia. 2014;178:447-56.
- [Google Scholar]
- Estimation of the burden of chronic and allergic pulmonary aspergillosis in India. PLoS One. 2014;9:e114745.
- [Google Scholar]
- Aspergillus hypersensitivity and allergic bronchopulmonary aspergillosis in patients with acute severe asthma in a respiratory intensive care unit in North India. Mycoses. 2010;53:138-43.
- [Google Scholar]
- A study on prevalence of allergic bronchopulmonary aspergillosis in patients of bronchial asthma. Internet J Pulm Med. 2008;9:1-6.
- [Google Scholar]
- Clinical significance of decline in serum IgE levels in allergic bronchopulmonary aspergillosis. Respir Med. 2010;104:204-10.
- [Google Scholar]
- Aspergillus hypersensitivity and allergic bronchopulmonary aspergillosis among asthma patients in Eastern India. J Indian Med Assoc. 2010;108:863-5.
- [Google Scholar]
- Occurrence of allergic bronchopulmonary mycosis in patients with asthma: An Eastern India experience. Lung India. 2010;27:212-6.
- [Google Scholar]
- Prevalence of allergic bronchopulmonary aspergillosis in Chinese patients with bronchial asthma. Zhonghua Jie He He Hu Xi Za Zhi. 2011;34:909-13.
- [Google Scholar]
- Frequency of specific immunoglobulin G antibodies and immediate skin test reactivity to Aspergillus fumigatus antigen among adults with allergic asthma: Tehran. Int J Med Toxicol Forensic Med. 2012;2:97-102.
- [Google Scholar]
- Prevalence of allergic broncho pulmonary aspergillosis in patients with asthma attending allergy clinic in a North West Indian tertiary care institute. Int J Biomed Adv Res. 2016;7:230-4.
- [Google Scholar]
- Allergic bronchopulmonary aspergillosis in patients with asthma: Results of a prospective study. Ter Arkh. 2017;89:13-6.
- [Google Scholar]
- Prevalence of Aspergillus hypersensitivity and allergic bronchopulmonary aspergillosis in patients with bronchial asthma at a tertiary care center in North India. Lung India. 2017;34:150-4.
- [Google Scholar]
- Prevalence of allergic bronchopulmonary as pergillosis in asthmatic patients: A prospective institutional study. Indian J Tuberc. 2018;65:285-9.
- [Google Scholar]
- Prevalence of allergic bronchopulmonary aspergillosis among patients with severe bronchial asthma in a tertiary care hospital in Northern India. Indian J Pathol Microbiol. 2019;62:111-3.
- [Google Scholar]
- A cross-sectional study of skin prick test to Aspergillus fumigatus antigen in asthmatic patients seen at a tertiary healthcare center. Indian J Allergy Asthma Immunol. 2019;33:19-24.
- [Google Scholar]
- Two year follow-up of a garbage collector with allergic bronchopulmonary aspergillosis (ABPA) Am J Ind Med. 2000;37:438-42.
- [Google Scholar]
- A questionnaire-based study on the role of environmental factors in allergic bronchopulmonary aspergillosis. Lung India. 2014;31:232-6.
- [Google Scholar]
- Allergic bronchopulmonary aspergillosis: Lessons learnt from genetics. Indian J Chest Dis Allied Sci. 2011;53:137-40.
- [Google Scholar]
- Genetic susceptibility to Aspergillus fumigatus infections. Int J Med Microbiol. 2011;301:445-52.
- [Google Scholar]
- Link between CFTR mutations and ABPA: A systematic review and meta-analysis. Mycoses. 2012;55:357-65.
- [Google Scholar]
- Genetic susceptibility to allergic bronchopulmonary aspergillosis in asthma: A genetic association study. Allergy Asthma Clin Immunol. 2016;12:47.
- [Google Scholar]
- Mutations in EEA1 are associated with allergic bronchopulmonary aspergillosis and affect phagocytosis of Aspergillus fumigatus by human macrophages. PLoS One. 2018;13:e0185706.
- [Google Scholar]
- Lung colonization by Aspergillus fumigatus is controlled by ZNF77. Nat Commun. 2018;9:3835.
- [Google Scholar]
- CARD9S12N facilitates the production of IL-5 by alveolar macrophages for the induction of type 2 immune responses. Nat Immunol. 2018;19:547-60.
- [Google Scholar]
- Vitamin D3 attenuates Th2 responses to Aspergillus fumigatus mounted by CD4+ T cells from cystic fibrosis patients with allergic bronchopulmonary aspergillosis. J Clin Invest. 2010;120:3242-54.
- [Google Scholar]
- Vitamin D supplementation decreases Aspergillus fumigatus specific Th2 responses in CF patients with Aspergillus sensitization: A phase one open-label study. Asthma Res Pract. 2015;1 pii: 3
- [Google Scholar]
- Vitamin D levels in asthmatic patients with and without allergic bronchopulmonary aspergillosis. Mycoses. 2018;61:344-9.
- [Google Scholar]
- A randomised trial of Vitamin D in acute-stage allergic bronchopulmonary aspergillosis complicating asthma. Mycoses. 2019;62:320-7.
- [Google Scholar]
- Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature. 2009;460:1117-21.
- [Google Scholar]
- Pattern recognition pathways leading to a Th2 cytokine bias in allergic bronchopulmonary aspergillosis patients. Clin Exp Allergy. 2015;45:423-37.
- [Google Scholar]
- Cytokines induce effector T-helper cells during invasive aspergillosis; what we have learned about T-helper cells? Front Microbiol. 2015;6:429.
- [Google Scholar]
- Anti-Aspergillus human host defence relies on type 1 T helper (Th1), rather than type 17 T helper (Th17), cellular immunity. Immunology. 2010;130:46-54.
- [Google Scholar]
- The Th1/Th2 paradigm in allergic bronchopulmonary aspergillosis. J Lab Clin Med. 1998;131:194-6.
- [Google Scholar]
- Inflammatory cells and airway defense against Aspergillus fumigatus. Immunol Allergy Clin North Am. 1998;18:619-40.
- [Google Scholar]
- Controversies in allergic bronchopulmonary aspergillosis. Int J Respir Care. 2010;6:53.
- [Google Scholar]
- A pathologic study of allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol. 1988;81:718-25.
- [Google Scholar]
- Allergic bronchopulmonary aspergillosis. Clinical and pathologic study of three cases. Chest. 1971;59:33-9.
- [Google Scholar]
- Allergic bronchopulmonary aspergillosis: Evidence of limited tissue invasion. Am Rev Respir Dis. 1975;111:232-6.
- [Google Scholar]
- Bronchocentric granulomatosis: A complication of allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol. 1977;59:83-90.
- [Google Scholar]
- The pathology of pulmonary disorders due to Aspergillus spp. Arch Pathol Lab Med. 2008;132:606-14.
- [Google Scholar]
- Acute invasive pulmonary aspergillosis complicating allergic bronchopulmonary aspergillosis: Case report and systematic review. Mycopathologia. 2015;180:209-15.
- [Google Scholar]
- Development of chronic pulmonary aspergillosis in adult asthmatics with ABPA. Respir Med. 2015;109:1509-15.
- [Google Scholar]
- Is there an overlap in immune response between allergic bronchopulmonary and chronic pulmonary aspergillosis? J Allergy Clin Immunol Pract. 2019;7:969-74.
- [Google Scholar]
- Clinical manifestations and natural history of allergic bronchopulmonary aspergillosis. Aspergillosis: From diagnosis to prevention 2010:707-24.
- [Google Scholar]
- Clinical significance of hyperattenuating mucoid impaction in allergic bronchopulmonary aspergillosis: An analysis of 155 patients. Chest. 2007;132:1183-90.
- [Google Scholar]
- Allergic bronchopulmonary aspergillosis (ABPA) sans asthma: A distinct subset of ABPA with a lesser risk of exacerbation. Med Mycol. 2020;58:260-3.
- [Google Scholar]
- Prevalence of allergic bronchopulmonary aspergillosis in patients with bronchial asthma. Asian Pac J Allergy Immunol. 2000;18:181-5.
- [Google Scholar]
- Eight-year study of allergic bronchopulmonary aspergillosis in an Indian teaching hospital. Mycoses. 2002;45:295-9.
- [Google Scholar]
- Pulmonary hypertension as a presenting manifestation of allergic bronchopulmonary aspergillosis. Indian J Chest Dis Allied Sci. 2009;51:37-40.
- [Google Scholar]
- Diagnostic performance of various tests and criteria employed in allergic bronchopulmonary aspergillosis: A latent class analysis. PLoS One. 2013;8:e61105.
- [Google Scholar]
- Utility of IgE (total and Aspergillus fumigatus specific) in monitoring for response and exacerbations in allergic bronchopulmonary aspergillosis. Mycoses. 2016;59:1-6.
- [Google Scholar]
- Pulmonary aspergillosis: Diagnostic and immunological significance of antigens and C-substance in Aspergillus fumigatus. J Pathol Bacteriol. 1964;88:141-51.
- [Google Scholar]
- Comparison of six Aspergillus-specific IgG assays for the diagnosis of chronic pulmonary aspergillosis (CPA) J Infect. 2016;72:240-9.
- [Google Scholar]
- Clinical and immunologic criteria for the diagnosis of allergic bronchopulmonary aspergillosis. Ann Intern Med. 1977;86:405-14.
- [Google Scholar]
- Clinical relevance of peripheral blood eosinophil count in allergic bronchopulmonary aspergillosis. J Infect Public Health. 2011;4:235-43.
- [Google Scholar]
- Cut-off values of serum IgE (total and A. fumigatus -specific) and eosinophil count in differentiating allergic bronchopulmonary aspergillosis from asthma. Mycoses. 2014;57:659-63.
- [Google Scholar]
- Serological diagnosis of allergic bronchopulmonary mycosis: Progress and challenges. Allergol Int. 2016;65:30-6.
- [Google Scholar]
- Allergen Nomenclature. Available from: http://wwwallergenorg/searchphpSpecies=Aspergillus%20fumigatus
- Utility of recombinant Aspergillus fumigatus antigens in the diagnosis of allergic bronchopulmonary aspergillosis: A systematic review and diagnostic test accuracy meta-analysis. Clin Exp Allergy. 2018;48:1107-36.
- [Google Scholar]
- Diagnostic cutoffs and clinical utility of recombinant Aspergillus fumigatus Antigens in the diagnosis of allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol Pract. 2020;8:579-87.
- [Google Scholar]
- Blood basophil activation is a reliable biomarker of allergic bronchopulmonary aspergillosis in cystic fibrosis. Eur Respir J. 2016;47:177-85.
- [Google Scholar]
- The role of basophil activation test in allergic bronchopulmonary aspergillosis and Aspergillus fumigatus sensitization in cystic fibrosis patients. J Cyst Fibros. 2016;15:587-96.
- [Google Scholar]
- The basophil surface marker CD203c identifies Aspergillus species sensitization in patients with cystic fibrosis. J Allergy Clin Immunol. 2016;137:436-43e9.
- [Google Scholar]
- The utility of the basophil activation test in differentiating asthmatic subjects with and without allergic bronchopulmonary aspergillosis. Mycoses. 2020;63:588-95.
- [Google Scholar]
- Pictorial essay: Allergic bronchopulmonary aspergillosis. Indian J Radiol Imaging. 2011;21:242-52.
- [Google Scholar]
- The spectrum of radiologic findings in allergic bronchopulmonary aspergillosis. Radiology. 1978;127:301-7.
- [Google Scholar]
- Chest radiographic and computed tomographic manifestations in allergic bronchopulmonary aspergillosis. World J Radiol. 2012;4:141-50.
- [Google Scholar]
- High-attenuation mucus in allergic bronchopulmonary aspergillosis: Another cause of diffuse high-attenuation pulmonary abnormality. Am J Roentgenol. 2006;186:904.
- [Google Scholar]
- An alternate method of classifying allergic bronchopulmonary aspergillosis based on high-attenuation mucus. PLoS One. 2010;5:e15346.
- [Google Scholar]
- Radiologic criteria for the diagnosis of high-attenuation mucus in allergic bronchopulmonary aspergillosis. Chest. 2016;149:1109-10.
- [Google Scholar]
- High attenuation mucoid impaction in allergic bronchopulmonary aspergillosis. World J Radiol. 2010;2:41-3.
- [Google Scholar]
- Case report: A rare cause of miliary nodules - Allergic bronchopulmonary aspergillosis. Br J Radiol. 2009;82:e151-4.
- [Google Scholar]
- Pleural effusion in a patient with allergic bronchopulmonary aspergillosis. Respir Care. 2012;57:1509-13.
- [Google Scholar]
- A rare cause of acute respiratory failure - allergic bronchopulmonary aspergillosis. Mycoses. 2011;54:e223-7.
- [Google Scholar]
- Pulmonary masses in allergic bronchopulmonary aspergillosis: Mechanistic explanations. Respir Care. 2008;53:1744-8.
- [Google Scholar]
- MRI: A new paradigm in imaging evaluation of allergic bronchopulmonary aspergillosis? Chest. 2015;147:e58-9.
- [Google Scholar]
- Evaluation of 3T lung magnetic resonance imaging in children with allergic bronchopulmonary aspergillosis: Pilot study. Eur J Radiol. 2019;111:88-92.
- [Google Scholar]
- Allergic bronchopulmonary aspergillosis in cystic fibrosis: MR imaging of airway mucus contrasts as a tool for diagnosis. Radiology. 2017;285:261-9.
- [Google Scholar]
- High-frequency triazole resistance found in nonculturable Aspergillus fumigatus from lungs of patients with chronic fungal disease. Clin Infect Dis. 2011;52:1123-9.
- [Google Scholar]
- Allergic broncho-pulmonary aspergillosis Clinical immunology 2 Skin, nasal and bronchial tests. Clin Allergy. 1971;1:415-32.
- [Google Scholar]
- Acute and chronic pulmonary function changes in allergic bronchopulmonary aspergillosis. Am J Med. 1979;67:631-7.
- [Google Scholar]
- Performance of serum galactomannan in patients with allergic bronchopulmonary aspergillosis. Mycoses. 2015;58:408-12.
- [Google Scholar]
- The utility of galactomannan antigen in the bronchial washing and serum for diagnosing pulmonary aspergillosis. Respir Med. 2013;107:1094-100.
- [Google Scholar]
- Chemokines indicate allergic bronchopulmonary aspergillosis in patients with cystic fibrosis. Am J Respir Crit Care Med. 2006;173:1370-6.
- [Google Scholar]
- Comparison of serum markers for allergic bronchopulmonary aspergillosis in cystic fibrosis. Eur Respir J. 2008;31:36-42.
- [Google Scholar]
- Blood basophils from cystic fibrosis patients with allergic bronchopulmonary aspergillosis are primed and hyper-responsive to stimulation by Aspergillus allergens. J Cyst Fibros. 2012;11:502-10.
- [Google Scholar]
- The link between fungi and severe asthma: A summary of the evidence. Eur Respir J. 2006;27:615-26.
- [Google Scholar]
- Randomized controlled trial of oral antifungal treatment for severe asthma with fungal sensitization: The fungal asthma sensitization trial (FAST) study. Am J Respir Crit Care Med. 2009;179:11-8.
- [Google Scholar]
- Allergic bronchopulmonary aspergillosis or pulmonary tuberculosis: A case of mistaken identity? Lung India. 2015;32:529-30.
- [Google Scholar]
- Allergic bronchopulmonary mycosis due to schizophyllum commune treated effectively with voriconazole. Intern Med. 2018;57:2553-7.
- [Google Scholar]
- Concurrent sensitization to Aspergillus fumigatus in tropical pulmonary eosinophilia. Monaldi Arch Chest Dis. 2016;81:736.
- [Google Scholar]
- Diagnosis and treatment of pulmonary aspergillosis syndromes. Chest. 2014;146:1358-68.
- [Google Scholar]
- Treatment options in severe fungal asthma and allergic bronchopulmonary aspergillosis. Eur Respir J. 2014;43:1487-500.
- [Google Scholar]
- A randomised trial of glucocorticoids in acute-stage allergic bronchopulmonary aspergillosis complicating asthma. Eur Respir J. 2016;47:490-8.
- [Google Scholar]
- A randomized trial of itraconazole vs prednisolone in acute-stage allergic bronchopulmonary aspergillosis complicating asthma. Chest. 2018;153:656-64.
- [Google Scholar]
- A randomised trial of voriconazole and prednisolone monotherapy in acute-stage allergic bronchopulmonary aspergillosis complicating asthma. Eur Respir J. 2018;52 pii: 1801159
- [Google Scholar]
- Report to the research committee of the british thoracic association. Br J Dis Chest. 1979;73:349-56.
- [Google Scholar]
- Role of inhaled corticosteroids in the management of serological allergic bronchopulmonary aspergillosis (ABPA) Intern Med. 2011;50:855-60.
- [Google Scholar]
- Intravenous monthly pulse methylprednisolone treatment for ABPA in patients with cystic fibrosis. J Cyst Fibros. 2009;8:253-7.
- [Google Scholar]
- Pulse methylprednisolone in allergic bronchopulmonary aspergillosis exacerbations. Eur Respir Rev. 2014;23:149-52.
- [Google Scholar]
- What is the current place of azoles in allergic bronchopulmonary aspergillosis and severe asthma with fungal sensitization. Expert Rev Respir Med. 2012;6:363-71.
- [Google Scholar]
- A randomized trial of itraconazole in allergic bronchopulmonary aspergillosis. N Engl J Med. 2000;342:756-62.
- [Google Scholar]
- Anti-inflammatory effect of itraconazole in stable allergic bronchopulmonary aspergillosis: A randomized controlled trial. J Allergy Clin Immunol. 2003;111:952-7.
- [Google Scholar]
- Voriconazole and posaconazole improve asthma severity in allergic bronchopulmonary aspergillosis and severe asthma with fungal sensitization. J Asthma. 2012;49:423-33.
- [Google Scholar]
- Successful treatment of allergic bronchopulmonary aspergillosis with isavuconazole: Case report and review of the literature. Open Forum Infect Dis. 2017;4:ofx040.
- [Google Scholar]
- Role of inhaled amphotericin in allergic bronchopulmonary aspergillosis. J Postgrad Med. 2014;60:41-5.
- [Google Scholar]
- Nebulised liposomal amphotericin B for Aspergillus lung diseases: Case series and literature review. Mycoses. 2015;58:173-80.
- [Google Scholar]
- Efficacy of nebulised liposomal amphotericin B in the attack and maintenance treatment of ABPA. Eur Respir J. 2012;39:1261-3.
- [Google Scholar]
- Efficacy and safety of nebulised amphotericin B (NAB) in severe asthma with fungal sensitisation (SAFS) and allergic bronchopulmonary aspergillosis (ABPA) J Asthma. 2015;52:289-95.
- [Google Scholar]
- A pilot randomized trial of nebulized amphotericin in patients with allergic bronchopulmonary aspergillosis. J Asthma. 2016;53:517-24.
- [Google Scholar]
- A review on the clinical use of inhaled amphotericin B. J Aerosol Med Pulm Drug Deliv. 2009;22:213-27.
- [Google Scholar]
- Guidelines for diagnosis and management of bronchial asthma: Joint ICS/NCCP (I) recommendations. Lung India. 2015;32:S3-42.
- [Google Scholar]
- Beneficial effects of omalizumab therapy in allergic bronchopulmonary aspergillosis: A synthesis review of published literature. Respir Med. 2017;122:33-42.
- [Google Scholar]
- Pharmacodynamics of omalizumab: Implications for optimised dosing strategies and clinical efficacy in the treatment of allergic asthma. Curr Med Res Opin. 2003;19:491-8.
- [Google Scholar]
- Clinical efficacy and immunologic effects of omalizumab in allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol Pract. 2015;3:192-9.
- [Google Scholar]
- Combination omalizumab and mepolizumab therapy for refractory allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol Pract. 2017;5:1137-9.
- [Google Scholar]
- Allergic bronchopulmonary aspergillosis successfully treated with benralizumab. J Allergy Clin Immunol Pract. 2019;7:1633-5.
- [Google Scholar]
- Allergic bronchopulmonary aspergillosis and related allergic syndromes. Semin Respir Crit Care Med. 2011;32:682-92.
- [Google Scholar]
- Therapeutic bronchoalveolar lavage with conventional treatment in allergic bronchopulmonary aspergillosis. J Coll Physicians Surg Pak. 2015;25:359-62.
- [Google Scholar]
- European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J. 2017;50 pii: 1700629
- [Google Scholar]
- Response to pneumococcal polysaccharide vaccination in patients with chronic and allergic aspergillosis. Vaccine. 2015;33:7271-5.
- [Google Scholar]
- Recurrence of allergic bronchopulmonary aspergillosis in the posttransplant lungs of a cystic fibrosis patient. Chest. 1997;112:281-2.
- [Google Scholar]
- Allergic bronchopulmonary aspergillosis in a lung transplant patient successfully treated with nebulized amphotericin. J Heart Lung Transplant. 2002;21:1237-41.
- [Google Scholar]
- Is treatment of serological ABPA similar to that of ABPA with bronchiectasis? J Allergy Clin Immunol Pract. 2017;5:1474.
- [Google Scholar]
- Epidemiological cutoffs and cross-resistance to azole drugs in Aspergillus fumigatus. Antimicrob Agents Chemother. 2008;52:2468-72.
- [Google Scholar]
- Serum IgE as an important aid in management of allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol. 1984;74:68-71.
- [Google Scholar]
- First-trimester itraconazole exposure and pregnancy outcome: A prospective cohort study of women contacting teratology information services in Italy. Drug Saf. 2009;32:239-44.
- [Google Scholar]
- Antifungal drugs during pregnancy: An updated review. J Antimicrob Chemother. 2015;70:14-22.
- [Google Scholar]
- The xolair pregnancy registry (EXPECT): The safety of omalizumab use during pregnancy. J Allergy Clin Immunol. 2015;135:407-12.
- [Google Scholar]
- Allergic bronchopulmonary aspergillosis in cystic fibrosis: Role of atopy and response to itraconazole. Chest. 1999;115:364-70.
- [Google Scholar]
- Effect of allergic bronchopulmonary aspergillosis on lung function in children with cystic fibrosis. Am J Respir Crit Care Med. 2006;174:1211-20.
- [Google Scholar]
- Allergic bronchopulmonary aspergillosis in cystic fibrosis A European epidemiological study Epidemiologic registry of cystic fibrosis. Eur Respir J. 2000;16:464-71.
- [Google Scholar]
- Prevalence of Aspergillus sensitization and allergic bronchopulmonary aspergillosis in cystic fibrosis: Systematic review and meta-analysis. Clin Exp Allergy. 2015;45:1765-78.
- [Google Scholar]
- Variability in parameters of allergic bronchopulmonary aspergillosis in patients with cystic fibrosis. J Allergy Clin Immunol. 1991;88:390-4.
- [Google Scholar]
- Novel immunologic classification of aspergillosis in adult cystic fibrosis. J Allergy Clin Immunol. 2013;132:560-6e10.
- [Google Scholar]
- A pulmonary cavitated mass complicating long-standing allergic bronchopulmonary aspergillosis. Respir Med Extra. 2005;1:39-42.
- [Google Scholar]
- Allergic bronchopulmonary aspergillosis and sensitization to Aspergillus fumigatus in chronic bronchiectasis in adults. Clin Allergy. 1985;15:571-9.
- [Google Scholar]
- An unusual association between Mycobacterium tuberculosis and Aspergillus fumigatus. Monaldi Arch Chest Dis. 2008;69:32-4.
- [Google Scholar]
- Prevalence of Aspergillus sensitisation in pulmonary tuberculosis-related fibrocavitary disease. Int J Tuberc Lung Dis. 2014;18:850-5.
- [Google Scholar]
- Allergic bronchopulmonary aspergillosis in an adult with Kartagener syndrome. BMJ Case Rep. 2015;2015 pii: bcr2015211493
- [Google Scholar]
- Allergic bronchopulmonary aspergillosis complicating Swyer-James-Macleod's syndrome: Case report and review of literature. Eur Ann Allergy Clin Immunol. 2016;48:99-102.
- [Google Scholar]
- Allergic bronchopulmonary aspergillosis complicating chronic obstructive pulmonary disease. Mycoses. 2008;51:83-5.
- [Google Scholar]
- Aspergillus hypersensitivity in patients with chronic obstructive pulmonary disease: COPD as a risk factor for ABPA? Med Mycol. 2010;48:988-94.
- [Google Scholar]
- Aspergillus sensitisation in bidi smokers with and without chronic obstructive lung disease. Mycoses. 2017;60:381-6.
- [Google Scholar]
- Sensitization to Aspergillus species in the congenital neutrophil disorders chronic granulomatous disease and hyper-IgE syndrome. J Allergy Clin Immunol. 1999;104:1265-72.
- [Google Scholar]
- Allergic fungal airway disease: Pathophysiologic and diagnostic considerations. Curr Opin Pulm Med. 2015;21:39-47.
- [Google Scholar]
- Prevalence of sensitization to Aspergillus flavus in patients with allergic bronchopulmonary aspergillosis. Med Mycol. 2019;57:270-6.
- [Google Scholar]
- Allergic bronchopulmonary mycosis due to fungi other than Aspergillus: A global overview. Crit Rev Microbiol. 2014;40:30-48.
- [Google Scholar]
- Concomitant allergic bronchopulmonary aspergillosis and allergic Aspergillus sinusitis: A review of an uncommon association*. Clin Exp Allergy. 2001;31:1896-905.
- [Google Scholar]
- Allergic fungal rhinosinusitis: An attempt to resolve the diagnostic dilemma. Arch Otolaryngol Head Neck Surg. 2006;132:173-8.
- [Google Scholar]
- Are allergic fungal rhinosinusitis and allergic bronchopulmonary aspergillosis lifelong conditions? Med Mycol. 2017;55:87-95.
- [Google Scholar]
- Medical management of allergic fungal rhinosinusitis following endoscopic sinus surgery: An evidence-based review and recommendations. Int Forum Allergy Rhinol. 2014;4:702-15.
- [Google Scholar]






