Translate this page into:
AGT, CYP11B2 & ADRB2 gene polymorphism & essential hypertension (HT): A meta-analysis
For correspondence: Dr Hoh Boon-Peng, Division of Applied Biomedical Sciences & Biotechnology, School of Health Sciences, IMU University, Kuala Lumpur 57000, Malaysia e-mail: hoh.boonpeng@gmail.com
-
Received: ,
Abstract
Background & objectives
The results of the genetic association studies between the selected candidate genes and hypertension (HT) contradicted across different populations. Majority of the meta-analyses carried out did not consider population genetic ancestry as a confounding factor. Therefore, this meta-analysis attempted to consolidate and re-evaluate the findings of the association between the selected candidate variants (AGT-rs699, CYP11B2-rs1799998, ADRB2-rs1042713 and rs1042714) and HT, by categorizing the genotyping data based on known genetic ancestry, and/or major geographical populations.
Methods
Publications were retrieved from PubMed, Cochrane and World of Science. The included articles were further divided into different populations based on their known genetic and/or geographical ancestry.
Results
AGTrs699-G was significantly associated with HT among Indians for (i) allele [P=0.03, Odds ratio (OR): 1.37, 95% Confidence Interval (CI): 1.03–1.82], and (ii) dominant mode of inheritance (P=0.009, OR:1.45, 95% CI: 1.09–1.91). CYP11B2rs1799998-G was significantly associated with HT in Europeans for (i) allele (P=6.9 × 10–5, OR: 0.82, 95% CI: 0.74–0.9), (ii) recessive (P=6.38 × 10-5, OR: 0.7, 95% CI: 0.59–0.83) and (iii) dominant mode of inheritance (P=0.008, OR: 0.81, 95% CI: 0.7–0.94). ADRB2-rs1042713-G was significantly associated with HT in east Asians for (i) allele (P=0.01, OR: 1.26, 95% CI: 1.05–1.51), and (ii) recessive mode of inheritance (P=0.04, OR: 1.36, 95% CI: 1.01–1.83).
Interpretation & conclusions
Different genotype and allele frequencies in diverse populations result in different genetic associations with HT across populations. This meta-analysis finding provides an update and summary of the genetic association between the selected simple nucleotide polymorphism (SNPs) and HT across different populations and essential insights into selecting appropriate pharmacogenetic marker(s) for effective HT management in populations of different ancestries.
Keywords
ADRB2-AGT
CYP11B2
essential hypertension
meta-analysis
populations
Hypertension (HT) is an escalating problem in modern society, affecting nearly a third of the adult population globally. It is a major risk factor for cardiovascular complications, including stroke and left ventricular hypertrophy, among others1. HT is caused by the interplays between individual susceptible genetic variants and environmental factors2. Studies have shown that genetics accounts for 30–60 per cent of the blood pressure (BP) variability3,4,5, yet the molecular pathophysiology of HT is inconclusive, as evidenced by the discovery of over 1000 genetic loci associated with BP regulation6.
Essential HT (ESH), which accounts for ⁓95 per cent of all HT cases7, is primarily considered a salt-sensitive (SS) phenomenon8. Salt-sensitive HT (SS-HT) is classified into two sub-intermediate phenotypes based on the plasma renin activity, normal and low renin, in response to upright posture and dietary salt restriction8. Notably, angiotensinogen (AGT) has been associated with normal renin SS-HT, while beta-2 adrenergic receptor (ADRB2) and aldosterone synthase (CYP11B2) are associated with low renin SS-HT8.
AGT and CYP11B2 are two important components in the renin-angiotensin-aldosterone system that are responsible for water and sodium homeostasis, hence BP regulation. AGT-rs699G allele is attributed to elevated plasma angiotensinogen9,10,11. CYP11B2 is involved in the terminal steps of the aldosterone biosynthesis12.The variant rs1799998 has been reported to alter its transcription binding site of putative steroidogenic factor-1, leading to downregulation of CYP11B2 promoter activity thence altered expression of CYP11B213,14,15. ADRB2 mediates effects on vascular tone and cardiac contractility via the sympathetic nervous system16,17. It attenuates vasodilation that leads to the elevation of vascular resistance17, hence salt excretion and HT18. ADRB2-46AA/79CC (rs1042713- AA/rs1042714-CC) was associated with a disproportionate elevation of aldosterone levels during liberal salt intake19.
Despite promising evidence on the functionality of these candidate genetic variants, their associations with HT have been contradicting20-24. Besides potentially unclear intermediate phenotyping, we postulated that the variability of minor allele frequencies across different populations could potentially be a reason for this equivocal conclusion.
Although meta-analysis offers an alternative to solve a problem associated with genetic association studies, most of the meta-analyses on the candidate gene have not considered the genetic ancestry of the studied populations as a potential confounding factor to the statistical analysis. Different genetic ancestries often result in variability of selected allele frequencies owing to evolutionary processes, for example, migration, admixture and natural selection25. Therefore, this meta-analysis attempted to consolidate and re-evaluate the findings of the association between the selected candidate variants (AGT-rs699, CYP11B2-rs1799998, and ADRB2-rs1042713 and rs1042714) and HT, by categorizing the genotyping data retrieved from the publications included based on known genetic ancestry, and/or major geographical populations.
Methods
Search Strategy
A comprehensive systematic literature search was conducted in several databases: PubMed, Cochrane and Web of Science (WOS). Publications related to a case-control genetic association study for AGT (rs699), CYP11B2 (rs1799998), ADRB2 (rs1042713 and rs1042714) and HT were retrieved. The terms and keywords used when performing the search were tabulated in Supplementary Table I.
The search was conducted independently by one author (NHM) and the full text was retrieved for possible relevant studies. The search was conducted until January 2023.
The following criteria were used during articles search: (i) article written in English; (ii) full-text article; (iii) case-control study; (iv) aged 18 yr and above; (v) ESH; (vi) available candidate single nucleotide polymorphisms (SNPs) genotype profile for HT and normotensive (NT) for AGT-rs699, ADRB2-rs1042713 and rs1042714 and CYP11B2-rs1799998; (vii) HT defined as BP ≥140 in systolic blood pressure or ≥90 diastolic blood pressure mmHg and both or on anti-hypertensive medication; (viii) NT defined as BP <140/90mmHg.
Articles were excluded based on following criteria: (i) samples with secondary HT, or with any known systemic diseases; (ii) publication from letters, review articles, meta-analysis, abstracts, and meeting reports; (iii) duplicating first and corresponding authors; (iv) suspected genotyping error (reported frequencies significantly deviated from expected or potential in genotyping error potentially due to flipping strand issue).
Study selection
Publications that fulfilled the inclusion and exclusion criteria were further divided into different populations based on geographical continents/known to share the same genetic ancestry: (i) European, (ii) Southern Chinese, (iii) East Asian: Northern Chinese and Japanese, (iv) Southern Indian, (v) Northern Indian, (vi) Latin American, (vii) African and (viii) the Middle East.
Data extraction
The data from eligible publications was extracted independently by three reviewers (NHM, IJR and KAM). Any discrete publication results were referred to the fourth reviewers (HBP). A standardized data collection form was piloted, containing information on the name of the first author, year of publications, ethnicities, number of cases and control. The genotype of AGT (rs699), CYP11B2 (rs1799998) and ADRB2 (rs1042713 and rs1042814) genotypes were extracted and categorized based on populations. The eligible articles included in this study are listed in Supplementary Table II.
Quality assessment of included studies
Two reviewers (IJR and KAM) independently assessed the quality of the included studies. An adapted version of the modified Newcastle-Ottawa Quality Assessment Scale Form was used to perform a quality assessment of case-control studies for this systematic review (Supplementary Table III).
Statistical analysis
Meta-analysis was performed using MetaGenyo, an online tool for meta-analysis of genetic association study (https://metagenyo.genyo.es/). Association between the candidate variants and HT was assessed using odds ratio (OR) with 95% confidence interval (Cl). In this study, either fixed or random-effect models were acquired based on the significance of inter-study heterogeneity (P< 0.05).
To assess possible publication bias, a funnel plot was generated (Supplementary Fig. 1) and evaluated using Egger’s test. A sensitivity test was also performed to examine the robustness of the meta-analysis. MetaGenyo requires a minimum of three publications for a meta-analysis to be carried out. Therefore, populations with less than three publications were excluded from the analysis.
Results
AGT-rs699:
Characteristics of the included studies
Overall, 947 publications were retrieved: PubMed (n=772 publications), Cochrane (n=22 publications) and WOS (n=153 publications). After applying pre-determined stringent filtering criteria, 48 publications remained. Overall, 12,336 HT and 10,784 NT individuals were involved in this analysis: (i) East Asian (Northern Han Chinese and Japanese), involving 4313 HT and 3565 NT: (a) Southern Han Chinese consisted of 1063 HT and 959 NT; (b) Southern Indian consisted of 1196 HT and 1131 NT; (c) North Indian consisted of 1244 HT and 1009NT; and (d) West Asia consisted of 359 HT and 281 NT. (ii) Europe consisted of 3601 HT and 3269 NT; (iii) Latin America consisted of 401 HT and 371 NT. Figure summarizes the flow search process for AGT-rs699. A similar flow search applies to other variants.
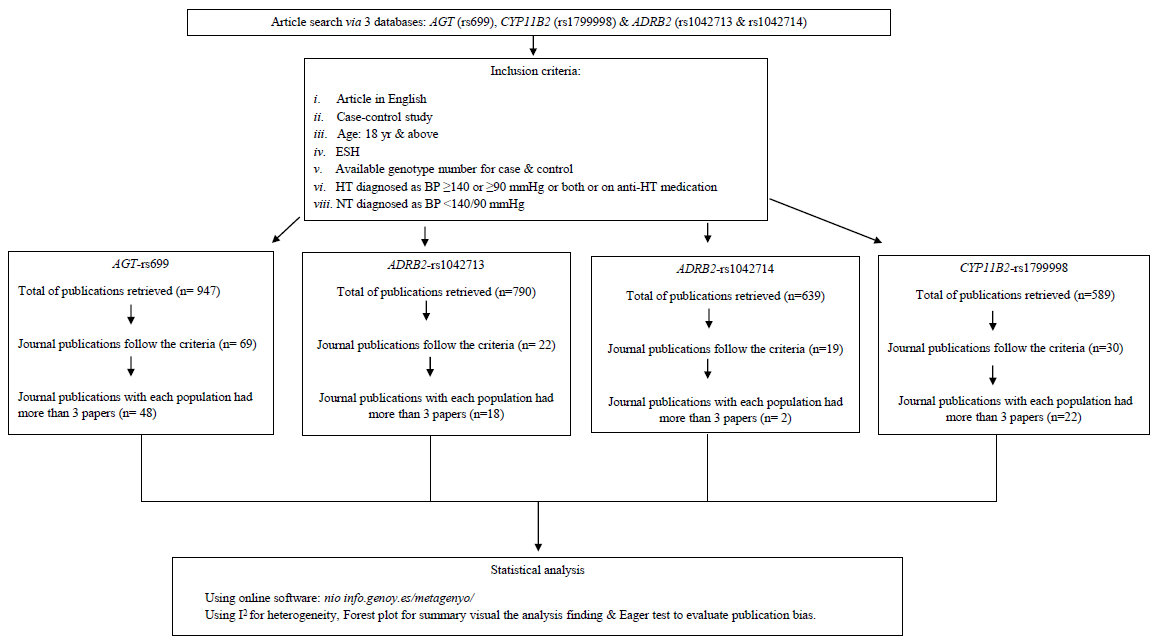
- Summary of the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow search process for AGT-rs699, CYP11B2 (rs1799998) and ADRB2 (rs1042713 and rs1042714). ESH, established hypertension; HT, hypertension; BP, blood pressure; NT, normotensive.
Meta-analysis result
Eight subgroup analyses revealed significant association between rs699-G and HT among the South Asian population (Southern India + Northern India) in allele [P=0.03, OR: 1.37, 95% CI: 1.03–1.82, Pheterogeneity= 0.0001]and dominant mode of inheritance (GG + GA vs. AA) (P=0.009, OR: 1.45, 95% CI: 1.09–1.91, Pheterogeneity= 0.0086) under random model effect.
Publication bias
With an exception in the allelic model of the West Asian (P=0.0008), Egger’s test revealed no publication bias in other populations. The funnel plot result is shown in Supplementary Figure 2.
Sensitivity test
The sensitivity test revealed no significant change observed between the overall OR value and in each omitted publication OR value, as the percentage between the two ORs is less than 20 per cent, indicating the meta-analysis results were reliable. A similar finding was observed for other included variants in this study. The summary of the AGT-rs699 finding is shown in Supplementary Table IV and Supplementary Figure 3.
CYP11B2-rs1799998:
Characteristics of the included studies
A total of 589 publications were retrieved for CYP11B2-rs1799998: PubMed (n=180), Cochrane (n=49) and WOS (n=360). Twenty-two publications remained in subsequent analysis: Japanese (4), European (5), Indian (5) and Northern Chinese (8). Overall, 11,745 HT and 11,014 NT individuals were included: (i) European (3037 HT and 2018 NT), (ii) Northern Han Chinese (4603 HT and 4113 NT), (iii) Japanese (2666 HT and 3502 NT), (iv) Indian (1439 HT and 1381 NT).
Meta-analysis results
Meta-analysis revealed significant association between rs1799998-G and HT among the European population (P=0.00012, OR: 1.21, 95% CI: 1.1–1.33, Pheterogeneity = 0.55), in both recessive (GG + GA vs. AA) (P=0.008, OR: 1.22, 95% CI: 1.05–1.42, Pheterogeneity = 0.97), and dominant mode of inheritance (GG vs. GA + AA) (P=0.00019, OR: 1.38, 95% CI: 1.16–1.62, Pheterogeneity = 0.18), under the fixed-effect model. The association effect was stronger when the article by Bengra et al26 was excluded. This is possibly due to the difference in G and A allele frequencies reported in the publication as compared to other included articles. Analysis showed a significant association between HT and the allele G (P=6.9×10-5, OR: 1.22, 95% CI: 1.1–1.34, Pheterogeneity = 0.69), in recessive (GG + GA vs. AA) (P=0.008, OR: 1.22, 95% CI: 1.05–1.42, Pheterogeneity = 0.97) and dominant mode of inheritance (GG vs. GA + AA) (P=6.8×10-5, OR: 1.42, 95% CI: 1.19–1.68, Pheterogeneity = 0.3) under the fixed effect model.
Publication bias
Egger’s test suggested no publication bias was detected in the included articles (Supplementary Fig. 3). The summary of the CYP11B2-rs1799998 finding is shown in Supplementary Table IV and Supplementary Figure 2.
ADRB2-rs1042713:
Characteristics of the included studies
A total of 790 publications were retrieved: PubMed (n=607), Cochrane (n=2) and WOS (n=159), of which 18 publications remained in meta-analysis representing three populations: European (11), African American (3) and East Asian (4; covering 2 for Northern Han Chinese and 2 for Japanese). Overall, 5739 HT and 3681 NT subjects were included: (i) European: 2922 HT and 1844 NT; (ii) African American: 329 HT and 336 NT; and (iii) East Asian: 2128 HT and 1501 NT.
Meta-analysis results
Meta-analysis results revealed a significant association between HT among the East Asian (Northern Han and Japan) in rs1042713-G allele (P=0.01, OR: 1.26, 95% CI: 1.05–1.51, Pheterogeneity = 0.05), and recessive mode of inheritance (GG + GA vs. AA) (P=0.04, OR: 1.36, 95% CI: 1.01–1.83, Pheterogeneity = 0.07), under the random model effect. A similar finding was also observed in the African-American population in the dominant mode of inheritance (GG vs. GA + AA) (P=0.03, OR: 0.68, 95% CI: 0.48–0.97, Pheterogeneity = 0.84) under the fix model effect.
Publication bias
Egger’s test suggested publication bias in studies involving African-American population (P=0.02) but not in others. The summary of the ADRB2-rs1042713 finding is listed in Supplementary Table IV and Supplementary Figure 2 and 3.
ADRB2-rs1042714:
Characteristics of the included studies
Overall, 639 publications were retrieved: PubMed (n=508), Cochrane (n=22) and WOS (n=158), of which 12 publications were included: European (7); and East Asian (5; covering two from Southern Chinese and Japanese), comprising 4953 HT and 3359 NT individuals: (i) European: 2250 HT and 1435 NT, (ii) Northern Han Chinese (1298 HT and 936 NT), (iii) East Asian consisting of 2706 HT and 1924 NT: (a) Northern Han Chinese (1298 HT and 936 NT); (b) Southern Han Chinese (288 HT and 149 NT) and (c) Japanese (1120 HT and 839 NT).The summary of the flow search process for ADRB2-rs1042714 is shown in Figure.
Meta-analysis results
No appreciative association between ADRB2-rs1042714 and HT was observed as shown in Supplementary Table IV.
Publication bias
The Egger’s test suggested no publication bias was detected.
Discussion
In this review, we categorized the data based on geographical populations. Significant genetic association was observed between HT and (i) AGT-rs699 G risk allele among the South Asian (Southern + Northern Indian); (ii) CYP11B2-rs1799998 G risk allele among the European; and (iii) ADRB2-rs1042713 G risk allele observed among the east Asian (Northern Han Chinese and Japanese).
AGT-rs699 G allele was associated with elevated AGT plasma levels, leading to elevated BP27. However, its association with HT across different populations was not universal, as evident by the inconsistency of the meta-analysis performed in this study. This is not surprising as studies have revealed that the AGT-rs699 diversity is correlated to the geographical latitude of the studied populations28,29 whilst the derived allele frequency increases moving away from the equator, nearly all populations close to the equator were homozygous or heterozygous for the ancestral allele, for what other studies have identified as being the AGT risk allele. However, our results contradict the study performed on the African population30. The AGT-rs699 surprisingly was not associated with HT in the European population, even though Europeans contributed more HT samples than the South Asians in this study. The recruitment of a small sample size in the European population partly could be a reason leading to sampling error31, hence a non-significant finding. When the sample size is large, the sampling variance of the effect size can be assumed to be approximately known and normal32.At least 500 HT individuals should be acquired in each publication to increase the power of meta-analyses20, as a small number could result in insufficient statistical power to estimate the effect of the variant on the disease33. In addition, the allele frequencies may appear heterogenous solely due to the nature of sampling variability when the trial sizes are small21. However, because the sample size per publication was not restricted in this study, the effect of heterogeneity may have been compromised.
ADRB2-rs1042713A allele carriers exhibited significantly lower basal blood flow and attenuated elevation in forearm blood flow as opposed to the G allele34, perhaps due to the variability in vascular responsiveness to isoproterenol in the vascular bed associated with ADRB2-rs104271335. In contrast, it remains unclear whether the ADRB2-rs1042713 influences the β2-mediated vasodilation that affects the blood flow36, BP control and new onset of HT37. Our finding is similar to Yan et al17 in 2020. Although previous meta-analyses reported a significant association between the ADRB2-rs1042713-G allele and HT in Africa20, we did not observe similar results. We speculated the contradiction may be due to unclassified intermediate phenotypes, resulting in the dilution of detection power.
The CYP11B2-rs1799998T allele leads to downregulation of CYP11B2 promoter13 hence influencing the level of aldosterone synthesis38. In contrast to this review, a previous publication reported a significant association between CYP11B2-rs1799998 and HT in the Northern and Southern Chinese13.
On a separate note, whether the rs1799998 G or T allele is more prevalent in HT is conflicting–some argued that the G allele carrier had a lower risk of HT39,40; while others claimed the opposite41. We speculate that, first, heterogeneity of the population studied. Most of the studies were based on the population genetic ancestry not controlled. Second, the phenotype of the genes involved in sodium/volume homeostasis, in this case, CYP11B2, low renin and BP levels are likely dependent on the environmental factors15. Third, different minor allele between different populations may have complicated the association signal42. Finally, genetic association analysis between CYP11B2-rs1799998 and BP by gender was observed43, thus conceivably confounded sampling neutrality, as aldosterone level can be influenced by gender bias44.
In this meta-analysis, we focused on ESH without co-existing other diseases, such as diabetes and heart disease, thus reducing potential analysis bias. Further, this study categorized the publication genotyping data according to known major geographical populations and hence mitigates the influence introduced by the variability of allele frequencies among different populations. Findings of this review summarised that different genotypes and allele frequencies in diverse populations with different ancestries result in different genetic associations with HT across the population and, therefore, the treatment may be different across populations.
The study has some limitations. First, the phenotypic characterization of HT for some of the publications was not documented as most of the studies defined HT by elevated BP measurements, but whether they were SS was not diagnosed. Knowing that different intermediate phenotypes of HT are associated with selected genetic variations8, unclassified intermediate phenotypes could have diluted the statistical power to detect a true genetic association. Second, the sample size included in meta-analyses for some publications in this study was small (n=30). This may have led to insufficient statistical power to detect fluctuated risk estimate45. Owing to the small effect size (OR: 1–1.61) of the variants of interest, the statistical power of this study may have been too weak to detect statistical association46. Third, our findings only included the articles published in the English language, which may not be generalizable to all countries and settings.
Overall, this review provides an update and summary of the genetic association between the selected SNPs and HT across populations with different genetic ancestries. Owing to different genetic susceptibility across different populations, levels of genetic variation in populations of different genetic ancestries should be considered if the pharmacogenetic approach is applied to the management of HT. Further meta-analyses are required to warrant the findings of this study.
Acknowledgment
Permission to publish this review was obtained from the Director-General of Health, Malaysia. This paper is dedicated to the memory of Academician Emeritus Professor Dato’ Dr Khalid Yusoff.
Financial support & sponsorship
This study was funded by the Ministry of Higher Education Malaysia under Long Term Research Grant Scheme (Grant code: 600-RMI/LRGS 5/3 (2/2011)-2).
Conflicts of Interest
None.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing of the manuscript and no images were manipulated using AI.
References
- Hypertension and cardiovascular risk: General aspects. Pharmacol Res. 2018;129:95-9.
- [CrossRef] [PubMed] [Google Scholar]
- The genetics of essential hypertension. Br J Clin Pharmacol. 2001;51:5-11.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The genetics of blood pressure and hypertension: the role of rare variation. Cardiovasc Ther. 2011;29:37-45.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Programming of essential hypertension: What pediatric cardiologists need to know. Pediatr Cardiol. 2015;36:1327-37.
- [CrossRef] [PubMed] [Google Scholar]
- Association of established blood pressure loci ith 10‐year change in blood pressure and their ability to predict incident hypertension. J Am Heart Assoc. 2020;9:e014513.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Essential hypertension: part I: definition and etiology. Circulation. 2000;101:329-35.
- [CrossRef] [PubMed] [Google Scholar]
- Genetics of human primary hypertension: Focus on hormonal mechanisms. Endocr Rev. 2019;40:825-56.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Molecular basis of human hypertension: role of angiotensinogen. Cell. 1992;71:169-80.
- [CrossRef] [PubMed] [Google Scholar]
- Genes and environment in blood pressure control--salt intake again shows its importance. Am J Clin Nutr. 2008;88:255-6.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular genetics of human hypertension: role of angiotensinogen. Endocr Rev. 1997;18:662-77.
- [CrossRef] [PubMed] [Google Scholar]
- Correlation between CYP11B2 polymorphism and the risk of preeclampsia. Medicine (Baltimore). 2020;99
- [CrossRef] [Google Scholar]
- The–344C/T polymorphism in the CYP11B2 gene is associated with essential hypertension in the Chinese. J Renin Angiotensin Aldosterone Syst. 2014;15:150-5.
- [PubMed] [Google Scholar]
- Angiotensin II and potassium regulate human CYP11B2 transcription through common cis elements. Mol Endocrinol. 1997;11:638-49.
- [CrossRef] [PubMed] [Google Scholar]
- Aldosterone synthase gene (CYP11B2) polymorphisms and enhanced cardiovascular risk. In: Çalışkan M, Erol O, Öz GC, eds. The recent topics in genetic polymorphisms. Intech Open; 2019.
- [Google Scholar]
- Genetics of salt-sensitive hypertension. Curr Hypertens Rep. 2011;13:55-66.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Association between the A46G polymorphism (rs1042713) in the β2-adrenergic receptor gene and essential hypertension susceptibility in the Chinese population: A PRISMA-compliant meta-analysis. Medicine (Baltimore). 2020;99:e23164.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- β2-Adrenergic receptor genotype affects the renin-angiotensin-aldosterone system response to the Dietary Approaches to Stop Hypertension (DASH) dietary pattern. Am J Clin Nutr. 2010;92:444-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- β-2 adrenergic receptor diplotype defines a subset of salt-sensitive hypertension. Hypertension. 2006;48:892-900.
- [CrossRef] [PubMed] [Google Scholar]
- A46G and C79G polymorphisms in the β2-adrenergic receptor gene (ADRB2) and essential hypertension risk: a meta-analysis. Hypertens Res. 2010;33:1114-23.
- [CrossRef] [PubMed] [Google Scholar]
- Angiotensinogen gene and essential hypertension in the Japanese: extensive association study and meta-analysis on six reported studies. J Hypertens. 1999;17:757-63.
- [CrossRef] [PubMed] [Google Scholar]
- M235T polymorphism of the angiotensinogen gene and hypertension in Chinese. J Hypertens. 1998;16:1137-40.
- [CrossRef] [PubMed] [Google Scholar]
- T235 variant of the angiotensinogen gene and blood pressure in the Chilean population. J Hypertens. 1998;16:829-33.
- [CrossRef] [PubMed] [Google Scholar]
- Contribution of four polymorphisms in renin-angiotensin-aldosterone-related genes to hypertension in a Thai population. Int J Hypertens. 2019;2019:4861081.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Allele frequency dynamics in a pedigreed natural population. Proc Natl Acad Sci USA. 2019;116:2158-64.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Genotyping of essential hypertension single-nucleotide polymorphisms by a homogeneous PCR method with universal energy transfer primers. Clin Chem. 2002;48:2131-40.
- [CrossRef] [PubMed] [Google Scholar]
- Association of renin-angiotensin and endothelial nitric oxide synthase gene polymorphisms with blood pressure progression and incident hypertension: prospective cohort study. J Hypertens. 2008;26:1780.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Natural selection and population history in the human angiotensinogen gene (AGT): 736 complete AGT sequences in chromosomes from around the world. Am J Hum Genet. 2004;74:898-916.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The hypertension pandemic: An evolutionary perspective. Physiology (Bethesda). 2017;32:112-25.
- [CrossRef] [PubMed] [Google Scholar]
- Genetic factors contributing to hypertension in African‐based populations: A systematic review and meta‐analysis. J Clin Hypertens (Greenwich). 2018;20:485-95.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Bias caused by sampling error in meta-analysis with small sample sizes. PLoS One. 2018;13:e0204056.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A guide to conducting a meta-analysis with non-independent effect sizes. Neuropsychol Rev. 2019;29:387-96.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Genetic associations with hypertension: meta-analyses of six candidate genetic variants. Genet Test Mol Biomarkers. 2013;17:736-42.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinical Concerns with Inhaled-Agonists: adult Asthma. Clin Rev Allergy Immunol. 2006;31:197-208.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of common polymorphisms of the β2-adrenergic receptor on agonist-mediated vascular desensitization. N Engl J Med. 2001;345:1030-5.
- [CrossRef] [PubMed] [Google Scholar]
- Exercise capacity after coarctation repair relates to the c. 46A> G genomic polymorphism of the ß2-adrenoreceptor and the c. 704T> C angiotensinogen polymorphism. Eur J Prev Cardiol. 2012;19:199-204.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors and myocardial infarction in patients with obstructive sleep apnea: impact of β2-adrenergic receptor polymorphisms. BMC Med. 2007;5:1-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- T allele of− 344C/T polymorphism in aldosterone synthase gene is not associated with resistant hypertension. Hypertens Res. 2009;32:159-62.
- [CrossRef] [PubMed] [Google Scholar]
- Influence of CYP11B2 gene polymorphism on the prevalence of hypertension and the blood pressure in Japanese men: interaction with dietary salt intake. J Nutrigenet Nutrigenomics. 2008;1:252-8.
- [CrossRef] [PubMed] [Google Scholar]
- Association between single-nucleotide polymorphisms in six hypertensive candidate genes and hypertension among northern Han Chinese individuals. Hypertens Res. 2014;37:1068-74.
- [CrossRef] [PubMed] [Google Scholar]
- Aldosterone synthase gene (CYP11B2) C-344T polymorphism, plasma aldosterone, renin activity and blood pressure in a multi-ethnic population. J Hypertens. 2004;22:1895-901.
- [CrossRef] [PubMed] [Google Scholar]
- Genetic variants associated with complex human diseases show wide variation across multiple populations. Public Health Genomics. 2010;13:72-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Age-and sex-dependent association of the–344C/T polymorphism of CYP11B2 with blood pressure in European populations. J Hum Hypertens. 2007;21:333-6.
- [CrossRef] [PubMed] [Google Scholar]
- Sex differences in renin-angiotensin-aldosterone system affect extracellular volume in healthy subjects. Am J Physiol Renal Physiol. 2018;314:F873-8.
- [CrossRef] [PubMed] [Google Scholar]
- Genetic associations with hypertension: meta-analyses of six candidate genetic variants. Genet Test Mol Biomarkers. 2013;17:736-42.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]






