Translate this page into:
A systematic review on hydroxyurea therapy for sickle cell disease in India
For correspondence: Dr Harpreet Kaur, Division of Epidemiological & Communicable Diseases, Indian Council of Medical Research, New Delhi 110 029, India e-mail: kaurh.hq@icmr.gov.in
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Sickle cell disease (SCD) constitutes frequently inherited haemoglobin disorders and poses a significant health burden in India. Hydroxyurea (HU), the most commonly used drug, has shown promising results in the clinical management of SCD. The present systematic review was undertaken to assess the efficacy and toxicity of HU in Indian sickle cell patients.
Methods:
A systematic review of studies on HU therapy was conducted to identify the application of HU and its outcome(s) across India. PubMed, Scopus and Cochrane Library was used as data sources for various studies on the efficacy and toxicity of HU therapy for treatment for SCD in India published between January 2001 and October 2021. Two authors independently extracted the data on study design, patient characteristics and therapeutic outcomes of HU in order to determine the study quality of the present review.
Results:
Overall, 14 studies were included for a systematic analysis. Of these 11 were prospective, two cross-sectional and one double-blind randomized controlled trial. Low-dose HU (10 mg/kg/day) was found to reduce the rates of vaso-occlusive crisis and hospitalization as well as decreased the requirement of blood transfusion in SCD patients. The foetal haemoglobin (HbF) level was recorded in 13 (80%) studies all of whom reported an elevation in the HbF levels, with a mean increase in per cent HbF from 15.8 to 21.4 per cent across studies. The common adverse events were reversible, mild-to-moderate cytopenia and anaemia.
Interpretation & conclusions:
The findings of the present review suggest that there is still insufficient information presently to determine the long-term or major adverse effects on organ damage, fertility as well as pregnancy on the use of HU therapy for SCD. Long-term multi-centric studies are thus required to address these problems.
Keywords
Foetal haemoglobin
haemoglobinopathies
hospitalization
hydroxyurea
sickle cell disease
vaso-occlusive crisis
The abnormalities associated with the globin chain of haemoglobin give rise to a group of disorders called haemoglobinopathies, the most common of which include sickle cell disease (SCD) and β-thalassaemia. Sickle haemoglobin (HbS) polymerizes on deoxygenation, leading to distortion and sickle shape of the red blood cells1. India accounts for approximately 40,000 sickle homozygous births every year2. SCD is a notable contributor of childhood mortality and premature death in the adult population2-4, thus inflicting a substantial economic burden on the affected families and the health sector. The sickle cell gene in India represents an occurrence of the HbS mutation known as the Asian haplotype5 and is widely distributed in central, western and eastern States of the sub-continent along with some parts of southern India6,7. The carrier frequency of the sickle cell gene is even higher in some parts of the central, western and southern States in India7. SCD in Indians is believed to have a relatively milder clinical presentation, compared to that among Africans8 due to various reasons such as the high prevalence of the putative potential genetic modifiers such as Arab Indian (AI) β-globin haplotype, high foetal haemoglobin (HbF) levels, the coinheritance of α-thalassaemia and high polymorphic frequency of Xmn1 gene site9-15. Evidence suggests that low socio-economic conditions and poverty could lead to adverse outcomes of the disease10,16.
The National Health Mission (NHM), Government of India (GoI) initiated the SCD programme in high-burden districts, directed at the mass screening of tribal groups along with the provision of care utilizing public health facilities17. Despite screening, the availability and accessibility of established therapies such as preventive and therapeutic hydroxyurea (HU) and pneumococcal immunization in public health institutions are sub-optimal18. The NHM suggests 10-15 mg/kg/day of HU in a single daily dose to be initiated after two years of age. The dose can be escalated every 6-8 wk, if no major toxicity is observed until the desired endpoints (viz. decrease in pain, increase in HbF by 15-20 per cent, increase in Hb level if severely anaemic, improved wellbeing and acceptable myelotoxicity). The maximum tolerated dose (MTD) is 35 mg/kg/day17.
Preliminary review of literature suggested that the management of SCD in India appears to vary and the studies are mostly conducted in a certain population/region only. Therefore, in the context of the treatment of SCD patients with HU, the present review was conducted to assess the efficacy and toxicity of HU in Indian sickle cell patients.
Material & Methods
Data sources: We searched biomedical literature databases of PubMed, Scopus and Cochrane Library for various studies published during the last 20 years (between January 2001 and October 2021), using the keywords, namely sickle cell anaemia (SCA), SCD, hydroxyurea (HU), hydroxycarbamide and India in different combinations.
Data extraction: The abstracts of all the selected studies, identified through web search, were reviewed independently by two authors (A.P. and S.B.) and the final decision of selecting the articles was reached by consensus. While extracting the data, relevant parameters such as author’s name, journal name, year of publication, study design, objectives, methodology, results and outcomes and other factors that can affect outcomes were carefully noted in an excel sheet. This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA, http://www.prisma-statement.org) as per the IJMR guidelines for systematic reviews & meta-analysis. With the search strategy, a total of 93 articles were identified, of which 14 studies fulfilled the review criteria and were thus selected. A detailed evidence table was prepared from these 14 studies. The efficacy and effectiveness of HU therapy were taken together as the studies were not easily distinguishable.
Inclusion/exclusion criteria: All prospective, retrospective, observational cohort, descriptive, prospective and randomized placebo-controlled trials reporting the efficacy and toxicity of HU therapy for SCD in India were included in the study. Studies on SCD that did not include the efficacy and toxicity of HU therapy in India were excluded from the present review. Data from unpublished studies, non-peer reviewed journals and from publications which reported data from previously included studies, were also excluded from the current review.
Quality assessment & risk of bias: The quality of the randomized and non-randomized studies was assessed by using a modified methodological quality checklist developed by Downs and Black19. The checklist consisted of 27 questions on reporting, external validity, internal validity bias confounding and power. Twenty six questions were scored as yes (=1) or no/unable to determine (=0) and one question of power was scored on a 3-point scale. The overall study quality was categorized as excellent (23-28), good (18-22), fair (13-17) and poor (<13). The discussion between the authors was sought to resolve the disagreements, when the difference in scoring was more than one.
Statistical analysis: The percentage agreement between the independent reviewers and Cohen’s kappa coefficient were calculated using IBM SPSS® Statistics for Windows, version 19 (IBM Corp., Armonk, NY, USA).
Results
Data synthesis: Of the 93 articles identified using the search strategy, two studies were identified as duplicate and removed. Of the remaining 91 studies, 71 records were further removed after screening as these were either conducted in animals or were irrelevant (Figure). From the remaining 20 records, two retrospective studies, one review and one descriptive study, which did not assess the efficacy of HU therapy along with one case study of two patients, were further excluded. Fourteen20-33 articles met the inclusion criteria and were chosen for further qualitative analysis (Tables I and II). Study design of the 14 studies included prospective cohort (n=11), cross-sectional (n=2) and double-blind randomized controlled (n=1) trial. The percentage agreement and the inter-rater variability (Cohen’s kappa coefficient) ranged between 70-89 and 0.4-0.8 per cent, respectively. The source of participants, inclusion criteria and patient characteristics were well defined in all the studies. Adherence to interventions was good and properly reported in nine (64.2%) studies20,21,23,25,26,28,30,32,33.
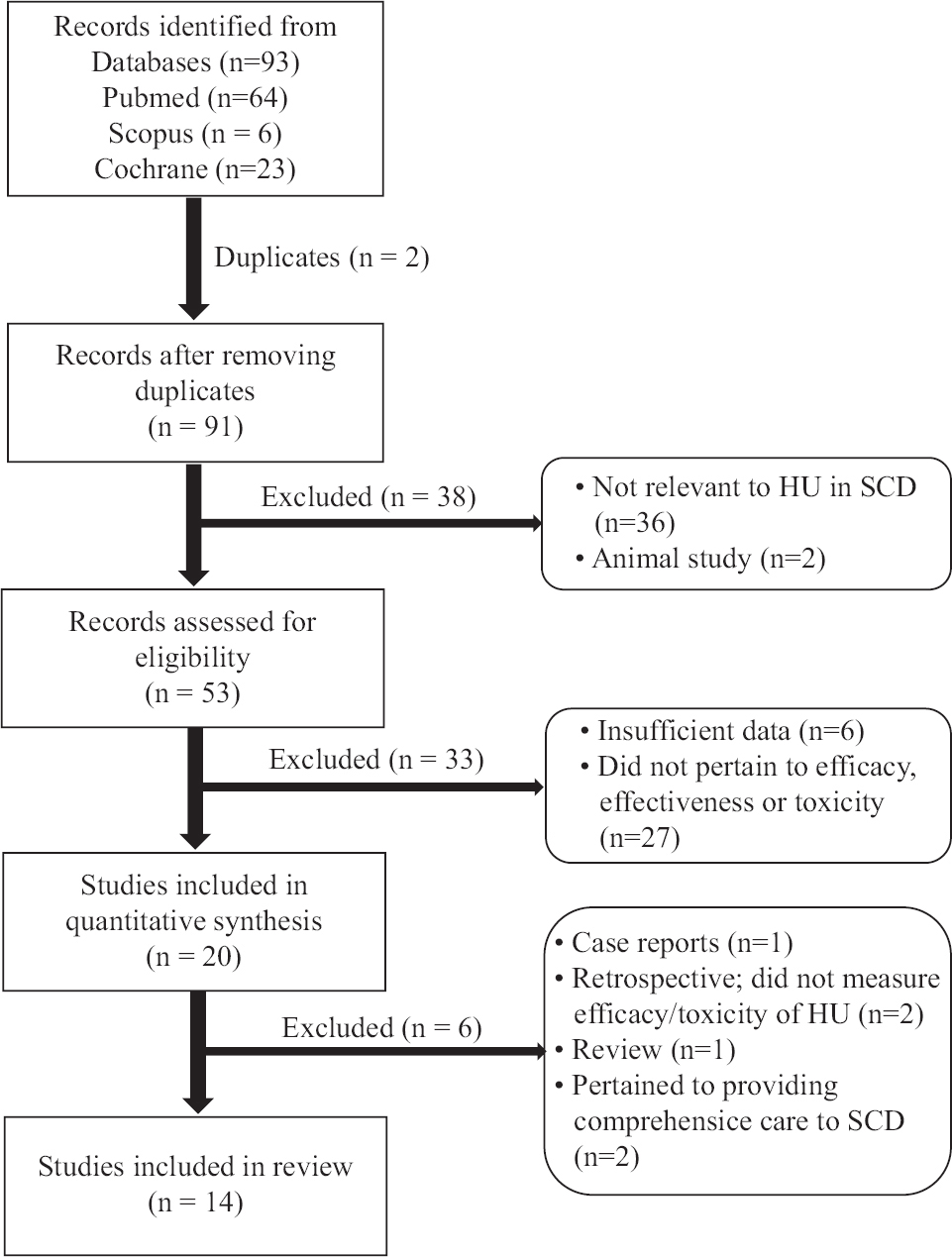
- Summary of literature search and review process as per the PRISMA guidelines. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses
| Study | Study design and aims | Inclusion criteria | Exclusion criteria | Study group | HU dose (mg/kg/day) | Follow up (months) | Genotype, n | Haplotype (%) |
|---|---|---|---|---|---|---|---|---|
| Patel et al6, 2012 | Prospective open-label observational study to assess the effectiveness of very low and fixed dose of HU (10 mg/kg/day) in sickle cell anaemia patients | SCA, ≥3 painful crises in the previous 12 months, > 2 BT in the last 12 months | Hb S/β-thalassaemia, Hb S/Hb E, Hb S/Hb C, Hb S/Hb D-Punjab, children < three years, adults >60 yr; Patients on special programme/trial that may affected their clinical/haematological status; who could not be followed up; refused to come for regular checkups; had taken HU for <80.0% of days; refuse to give consent | HU group Control group | 10 | 24 | NR | Asian haplotype (89.0) |
| Sethy et al3, 2018 | Prospective cohort study on beneficial effect of low fixed dose of HU in SCD (HbSS) patients | Age ≥18 yr, 2 attacks of VOC/year and/or rate of transfusion 1-2 units/month | Pregnant women, HIV, patients on medications that could potentially enhance HU toxicity, serum creatinine > ULN for age and ALT > twice the ULN for age | HU | 10 | 12 | NR | Arab-Indian haplotype |
| Barma et al29, 2020 | Prospective cohort study to evaluate clinical and haematological response to HU in SCA patients | SCD, children, age group 5-14 yr | Children with active liver and kidney disease, who refuse to take HU therapy, already on HU therapy | HU+ group HU− group | 20 | 24 | NR | NR |
| Singh et al30, 2010 | Prospective cohort study to evaluate the efficacy and impact of HU in SCD | Homozygous SCD, history of severe, recurrent VOC, pain requiring ≥3 hospitalization/year, stroke or ACS and severe or symptomatic anaemia (Hb <7 g/dl) | Pregnant women, sexually active and unwilling to use contraception, patients with active liver disease, previously treated with HU or other anti-sickling agents and history of significant non-compliance with recommended medical care | HU | 20 | 12 | NR | NR |
| Jain et al23, 2013 | Prospective longitudinal study evaluate the efficacy of fixed low-dose HU in children with SCA | SCA patients with ≥3 episodes of VOC or BTs, ≥1 episode of ACS or cerebrovascular stroke or sequestration crisis | Pregnancy, HIV infection or medications that could potentially enhance HU toxicity, serum creatinine > ULN for age and ALT > twice the ULN for age | HU | 10 | 60 | NR | NR |
| Deshpande et al27, 2016 | Prospective cohort study to validate the beneficial effects of HU in SCD patients | SCD patients treated at 1 hospital | NR | HU | 15 and built up to MTD till 30 | 12 | NR | NR |
| Italia et al21, 2009 | Prospective cohort study to investigate the efficacy and safety of HU in severe manifestations where the βs gene is linked to the Arab-Indian haplotype and is associated with higher HbF levels | Frequent VOC ≥ five per year, CNS affected at least once during their lifetime, ACS >2 times during their lifetime, AVN of the femur head | NR | HU group Control group | 10-15 | 24 | Normal α genotype - 72.7%; single α gene deletion (−α3.7/αα or −α4.2/αα) - 22.0%; 2 α gene deletions (−α3.7/−α3.7) −5.1% | Arab-Indian haplotype (−98.7) |
| Mohanty et al24, 2017 | Hospital-based cross-sectional study to evaluate iron status of SCA and its correlation with other parameters which may influence it | Adult, age >18 yr, no history of other comorbidities such as genetic/metabolic diseases that may alter the iron load, chronic infection, inflammatory/autoimmune/liver and renal diseases | Patients with double heterozygous states or any other haemoglobinopathies | HU group Control group | 20 | 60 | NR | Arab-Indian haplotype (−90.4) |
| Dehury et al28, 2015 | Prospective open label observational study to assess low and fixed dose of HU is effective and safe in HbSb + thalassaemia patients with IVS1-5 (G-C) mutation | VOC/dactylitis≥3 episodes previous 12 months, ≥2 BT in the last 12 months | b-thalassaemia mutation other than IVS1-5 (G-C), patients part of special programme/trial that may affect their clinical/haematological status, patients not followed up regularly, who refused to consent | HU | 10 | 24 | HbSb + thalassaemia | NR |
| Jain et al20, 2012 | Double-blind RCT to evaluate the efficacy and toxicity of fixed low-dose HU therapy in SCA patients | SCA, frequent VOC requiring hospitalization (> three per year), frequent BT requirement (> three per year) | HIV, any chronic illness that could potentially enhance HU toxicity | HU Placebo | 10 | 18 | NR | NR |
| Patel et al25, 2014 | Prospective cohort study to investigate the effect of HU on compound heterozygotes for sickle cell-haemoglobin D-Punjab | VOC/dactylitis ≥3 episodes previous 12 months, ≥2 BT in the last 12 months | NR | HU | 10 | 24 | NR | NR |
| Somkuwar et al22, 2020 | Prospective longitudinal study to evaluate the toxicities/adverse drug events and beneficial effects by clinical, haematological and biochemical parameters in SCD children | ≥2 acute painful events per year requiring hospitalization, frequent BT ( > three per year), ACS, cerebrovascular event, AVN of femur | SCD children < five years age, other systemic illness, regularly on drugs such as theophylline, oestrogen or calcium channel blockers, deranged haematological/renal/hepatic laboratory parameters and parents not willing for participation | HU | 10 | 24 | NR | NR |
| Sahoo et al32, 2017 | Prospective cohort study to evaluate the potential impact of HU on seminal fluid parameters and fertility of men with SCD | Painful crises ≥3 episodes in previous one year, ≥2 BT in last one year | Hb S/β-thalassaemia, Hb S/Hb E, Hb S/Hb C, Hb S/Hb D Punjab and others; male patients <18 yr and >45 yr; patients who refused to give consent; and patients who had taken HU for <80% of days | HU | 10 | 26 | NR | NR |
| Oberoi et al31, 2014 | Retrospective cohort study to assess the response to HU in HbSD-Punjab patients | HbSD-Punjab was confirmed by HPLC, Hb electrophoresis at alkaline (8.6) and acidic pH (6.0), sickling test and by family screening | NR | HU | 20 | 120 | NR | NR |
Hb, haemoglobin; HbF, foetal Hb; HU, hydroxyurea; HbS, sickle Hb; SCA, sickle cell anaemia; SCD, sickle cell disease; VOC, vaso-occlusive crisis; MCH, mean corpuscular haemoglobin, MCV, Mean corpuscular volume; BT, blood transfusion; WBC, white blood cell; CBC, complete blood count; ACS, acute chest syndrome; CNS, central nervous system, AVN, avascular necrosis; RCT, randomized controlled trial; MTD, maximum tolerated dose; NR, not reported; ALT, alanine transaminase; HbSD, heterozygous haemoglobin Sand haemoglobin D; HbSS, homozygous haemoglobin S; HbSb, sickle beta plus thalassaemia; HPLC, high-performance liquid chromatography; ULN, upper limit of normal; HbD, haemoglobin D-Punjab; HbE, haemoglobin E; HbC, haemoglobin C; MTD, maximum tolerated dose
| Study | Time of study/observation (months) | Group | n | Age (mean or range) | Deaths | HbF (%) | Hb | MCV |
|---|---|---|---|---|---|---|---|---|
| Patel et al26, 2012 | 24 | Control | 45 | Age matched | NA | 19.7±5.7 (P=0.10) | 9.8±1.6 (P=0.08) | 77.8±9.5 (P=0.78) |
| Group I | 27 | 9.3±4.1 | NA | 22.5±7.3*** | 9.1±1.2*** | 85.4±8.8*** | ||
| Group II | 91 | 27.3±8.7 | NA | 22.5±6.3*** | 10.1±1.6 (0.007) | 86.1±7.9*** | ||
| Sethy et al33, 2018 | 12 | 128 | <18 yr | NA | 21.66±6.7 (P>0.05) | 9.78±1.13*** | 90.78±6.59*** | |
| Barma et al29, 2020 | 18 | HU | 52 | 5-14 yr | NA | 11.67±1.11** | 7.36±0.5*** | 86.26±1.6** |
| Non-HU | 48 | 5-14 yr | NA | 6.39±0.91** | 4.84±0.3*** | 79.37±0.91** | ||
| Singh et al30, 2010 | 12 | 24 | 19.85 | NA | 19.17 | 9.98 (P=0.253) | 89.87 (0.0045) | |
| Jain et al23, 2013 | 25 | 144 | <18 yr | NA | 21.98±5.22** | 9.66±1.58*** | 88.3±11.10** | |
| Deshpande et al27, 2016 | 12 | 11 | 0-10 yr | NA | 23.22 (P-0.008) | 9.63 (P=0.006) | NA | |
| 22 | 11-20 yr | 24.7 | 10.9 (P=0.01) | NA | ||||
| 26 | 21-30 yr | 23.24 | 10.69*** | NA | ||||
| 11 | >30 yr | 27.86 | 10.98 (P=0.04) | NA | ||||
| Italia et al21, 2009 | 24 | Group I, pre-HU | 29 | 18-35 yr | 23.1±5.2** | 10.7±1.5** | 95.4±11.8** | |
| Post-HU | NA | |||||||
| Group II, pre-HU | 25 | 5-17 yr | 24.4±6.3** | 9.4±1.9*** | 94.5±10.6** | |||
| Post-HU | NA | |||||||
| Group III, pre-HU | 23 | 18-35 yr | 26.9±10** | 9.8±1.7*** | 77.2±12** | |||
| Post-HU | NA | |||||||
| Mohanty et al24, 2017 | 60 | Patients | 208 | 18-48 | NA | NA | NA | |
| Control | 52 | 20-45 | NA | NA | NA | |||
| Dehury et al28, 2015 | 24 | Group I | 37 | <18 yr | NA | 20.0±7.0*** | 9.2 (1.7) median (IQR)* | 76.0 (6.95) Median (IQR) |
| Group II | 67 | ≥18 yr | 19.6±7.0*** | 9.6 (2.8) median (IQR)* | 77.2 (10.5) Median (IQR) | |||
| Jain et al20, 2012 | 18 | HU | 30 | 12.73±4.4 | NA | 24.00±5.90** | 9.29±0.55*** | NA |
| Placebo | 30 | 11.73±4.08 | NA | 18.92±5.77 | 7.90±0.58 | NA | ||
| Patel et al25, 2014 | 24 | HU | 37 | <18 yr | 21.2±7.2*** | 9.8±1.8*** | 96.7±12.4 (P=0.0014) | |
| Non-HU | 67 | ≥18 yr | 20.8±6.9 (P=0.1434) | 9.3±2.5 (P=0.07) | 86.2±11.04 (0.5449) | |||
| Somkuwar et al22, 2020 | 24 | 36 | NA | NA | 18.13±5.79*** | 9.27±1.26 (P=0.0012) | NA | |
| Sahoo et al32, 2017 | 3 | HU | 50 | 25.8 (19-45 yr) | NA | 23.138±5.4 | NA | NA |
| 3 | Non-HU | 50 | 26.02 (18-45 yr) | 17.958±6.8 | NA | NA | ||
| Oberoi et al31, 2014 | NA | HU | 5 | 8.2 yr (3.3-14.5) | NA | NA | 8.24 | NA |
| Non-HU | 5 | 11.79 | 6.76 | 89.95 | ||||
| Study | Reticulocyte count | Pain/VOC events | BT (mean±SD, events or % patients) | Hospitalization | Toxicity in HU group (n) | |||
| Patel et al26, 2012 | NA | 4.8/year | NA | NA | Skin and nail pigmentation (2), Thrombocytopenia (10) | |||
| NA | 0.5/year | 19/20 (95.0% reduction) | NA | |||||
| NA | NA | |||||||
| Sethy et al33, 2018 | NA | 0.23±0.92 | 0.52±1.27 events/year** | NA | Thrombocytopenia (2), neutropenia (2), hepatic dysfunction (4), renal dysfunction (3) | |||
| Barma et al29, 2020 | NA | 6.33/year pre-HU, 3.19/year post-HU | 2.40 events/year | None | Thrombocytopenia (2), neutropenia (2), myelotoxicity (2), decrease in bilirubin and ALT levels | |||
| NA | 5.83/year | 5.21 events/year | None | None | ||||
| Singh et al30, 2010 | 1.67/year | NA | Post-HU 2.25, P=0.03 | Mild reversible bone marrow suppression | ||||
| Jain et al23, 2013 | 168.43±123.5 | 0.15±0.47 | 0.15±0.58** | 0.29±0.73** | Neutropenia (5), Thrombocytopenia (4), hepatic dysfunction (8), renal dysfunction (3), AIDS (1) | |||
| Deshpande et al27, 2016 | 3.56 | 88% reduction | 0.09 events/year/patient, P=0.1669 | NA | None | |||
| 2.15 | 84% reduction | 0.09 events/year, P=0.008 | ||||||
| 2.85 | 84% reduction | 0.12 events/year, P=0.06 | ||||||
| 2.67 | 91% reduction | 0.55 events/year, P=0.8 | ||||||
| Italia et al21, 2009 | 9.9±4.8 | 71% | 46% | 44 | NR | |||
| 5.6±2.5 | ||||||||
| 11.1±6.9 | 67 | 47% | 63 | |||||
| 8.9±7.4 | ||||||||
| 10.2±5.3 | 71 | 47% | 51 | |||||
| 7.2±4.3 | ||||||||
| Mohanty et al24, 2017 | NA | 90 individuals (≥three per year) | NA | NA | NR | |||
| NA | 0 | NA | NA | |||||
| Dehury et al28, 2015 | NA | 0.5/year | 0 events/year*** | 0 events/year*** | Reduced ANC (1), Thrombocytopenia (4), short term myelotoxicity (5), altered sperm parameters (2) | |||
| NA | 0/year | 0 events/year*** | 0 events/year*** | |||||
| Jain et al20, 2012 | 1.15±0.10 | 8/18 | 0.13±0.43** | 0.70±1.28** | Reduced TLC, reduced reticulocytes, nausea (2), skin rash (3) | |||
| 1.81±0.67 | 32/300 | 1.98±0.82 | 9.59±2.94 | |||||
| Patel et al.25, 2014 | NA | 0.4±0.60/year | 0.02±0.08/year | NA | None | |||
| NA | 0.74±0.66/year | 0.09±0.2/year | NA | |||||
| Somkuwar et al.22, 2020 | 144.42±64.02 | 0.25±0.5/year | 0.30±0.57 | NA | Anaemia (5), neutropenia (3), Thrombocytopenia (4), renal toxicity (4), hepatic toxicity (3) | |||
| Sahoo et al.32, 2017 | NA | NA | NA | Oligospermia (10), azoospermia (5) | ||||
| NA | NA | NA | ||||||
| Oberoi et al.31, 2014 | NA | No or decrease in painful VOC | NA | NA | Transient neutropenia (1) | |||
| 14.32 | NA | NA | ||||||
P *< 0.05; **<0.001; ***<0.0001. Hb, haemoglobin; HbF, foetal Hb; HU, hydroxyurea; VOC, vaso-occlusive crisis; MCV, mean corpuscular volume; BT, blood transfusion; WBC, white blood cell; CBC, complete blood count; ACS, acute chest syndrome; CNS, central nervous system; AVN, avascular necrosis; NA, not available; IQR, interquartile range; ALT, alanine transaminase; SD, standard deviation; ANC, absolute neutrophil count; TLC, total leucocyte count; NR, not reported
While selected 10 studies20,22-24,26,27,29,30,32,33 assessed the efficacy/toxicity of HU therapy in SCD, two articles each were on beta-thalassaemia21,28 and HbSD-Punjab25,31. Of the total 14 studies reviewed, five (35.7%) studies20,22,27,29,31 were conducted in paediatric patients, six (42.8%)21,24,25,30,32,33 in adults patients and three (21.4%) in others26-28 in paediatric as well as adult patients with SCD. The duration of both observation and the follow up of patients, however, varied in the studies.
Efficacy & effectiveness of hydroxyurea (HU): It was noted that the majority of the studies indicated the efficacy of low-dose HU therapy in reducing the frequency of pain crises, blood transfusions and hospitalization. Of the 14 studies, 12 used the fixed-dose method20,22-26,28-33, and two used the standard dose escalation method21,27. In the two studies that used the dose escalation method, while one study started with a low dose of 10 mg/kg/day and escalated to 15 mg/kg/day, the other one started from 15 mg/kg/day and escalated to 30 mg/kg/day21,27. On the other hand, of the 12 studies that used the fixed dose method, eight (57.14%) studies showed a beneficial effect of low fixed dose (10 mg/kg/day) of HU in reducing vaso-occlusive crisis (VOC) and transfusion requirements in the treated patients20,22,23,25,26,28,32,33 and four (28.6%) used a standard dose of 20 mg/kg/day24,29-31. The frequency of pain crises decreased significantly in 12 (75%) studies20-31. In five prospective studies22,23,29,30,33, the VOC declined from 4.92 to 1.098 events/yr after HU therapy. The requirement of blood transfusion reportedly decreased in 10 (62.5%) studies20-23,25-29,33. The frequency of hospitalization also decreased as reported in six studies20,21,23,28-30. The randomized controlled trial (RCT)20 conducted with the fixed low-dose HU (10 mg/kg/day) was found to be effective in ameliorating SCA-related events with 95, 94.6 and 93.1 per cent reductions in the rates of VOC, blood transfusions and hospitalizations, respectively, when compared with the placebo group20. The duration of hospitalization was less in the HU group (3.1±1.2) compared to the counterparts in the placebo group (7.1±2.1 days)20.
A cohort study by Deshpande et al27 observed a significant reduction in the number of pain crises which required hospitalization across age groups. A decline in the requirement for blood transfusion in all patients was also reported, however, it was significant only in the age group of 11-20 yr27. Another study measured the efficacy of low-dose HU in paediatric and adult SCA and reported a significant reduction in the painful crises in both the groups. The control and HU therapy groups had different painful crisis rates at the end of two years, with the control and HU groups having 4.8 and 0.5 crises per year, respectively, showing a difference of 89.6 per cent. Ninety five per cent of patients who received HU treatment became transfusion independent26. A study conducted by Italia et al21, in patients with severe manifestations reported that after two years of HU therapy, 78 per cent of patients showed clinical improvement with a reduction of VOC and transfusion requirements.
Predictors of response: The HbF level was recorded in 12 (75%) studies20-23,25-32, all of which reported an elevation in the HbF levels. The mean per cent HbF across these studies increased from 15.8 to 21.4 per cent. Jain et al23, in a study on children with SCA, showed a higher increase in HbF levels in patients with lower baseline HbF levels, suggesting that HbF response to HU is variable. Italia et al21 showed a significant correlation between clinical improvement and the HbF and F-cell response. There was a positive correlation between the rise in HbF level and the reduction in episodes and severity of sickle cell crises.
Toxicity of hydroxyurea (HU): Compliance, adverse effects and inadequate follow up for monitoring play as significant barriers to HU therapy34. Compliance to treatment was reported in nine (64.2%) studies4,21,23,25,26,28,30,32,33. Drug toxicities and adverse events were indicated in 10 (71.4%) studies. Follow up compliance was reportedly good in seven (50%) studies20,21,23,25-27,32 and loss to follow up was reported in five (35.7%) studies22,28-30,33. Four studies21,24,25,27, on the other hand, did not report any toxicities.
Thrombocytopenia and neutropenia were the most common toxicities observed in the nine studies20,22,23,26-28,30,31,33. A few studies22,23,29,33 reported renal and hepatic dysfunction, but whether this was due to HU or as a consequence of SCD requires further evaluation. There are, however, inadequate data regarding the long-term risk of HU and its impact on fertility and reproduction. Dehury et al28 observed minimal gonadal toxicity. Sahoo et al32 found a significant alteration in seminal fluid parameters exacerbated by low-dose HU therapy in male SCD patients in the age group of 15-45 yr. Pigmentation of skin and nail and other adverse events like nausea were also reported in these studies.
Discussion
This systematic review evaluates the efficacy of HU therapy and its toxicity in SCD patients in India. This review included one ‘excellent’20 and six ‘good’23,24,26,29-31 quality studies, as assessed by the cumulative scores obtained by two independent authors using Downs and Black Checklist19. Although HU was found to be beneficial in reducing the VOC, blood transfusion and requirement of hospitalization, much ground still remains to be covered in the better documentation of HU for SCD in Indian patients. A majority (57.14%) of the published evidence from India have reportedly initiated and continued with the low and fixed dose of HU (10 mg/kg/day) in severely affected children and also showed a significant reduction in the recurrent painful episodes, need for frequent blood transfusion and of the frequency of hospitalization along with other SCA related crises20,22,23,25,26,28,32,33. Furthermore, transient cytopenia was the most common side effect observed, which required temporary discontinuation of HU in 6 studies22,23,26,28,30,33.
In context of dose escalation of HU as prescribed by NHM17, the authors believe that while this may confer additional clinical benefits, due to limited facilities to monitor toxicity, administering a higher dose may become a barrier to the treatment. Therefore, dose escalation may be advisable only if there is no reduction in the pain crisis and the requirement of blood transfusion. From this systematic review it also emerged that there was a better compliance with the low dose, which can be further reinforced with the coordinated efforts between a physician and his patients. Thus, on the basis of an RCT20 and other observational studies20,22,23,25,26,28,32,33 highlighting the beneficial effects of low- and fixed-dose HU therapy in SCD patients, it can be concluded that MTD may not be necessary to achieve a therapeutic effect.
A case study35 of two female sickle beta-thalassaemia patients on HU therapy who managed through their pregnancies after discontinuing HU therapy was identified, however, this study was not able to contribute to drawing inference related to teratogenic effect of HU due to its limited extrapolation.
Through this systematic review, several key gaps and challenges associated with HU therapy in a resource-limited setting like India were identified. Although considerable work has been done in India on SCD, these studies remain limited to a handful of centres and hence have limited impact at the national level. The National Heart, Lung and Blood Institute guidelines36 offered that HU should be utilized more broadly, including HU initiation in all infants with sickle cell, at nine months of age, regardless of clinical severity, with regular counselling, whereas in India, the healthcare providers initiate HU therapy to only symptomatic SCD children due to fear of toxicity as well as lack of availability of paediatric dose. Due to the unavailability of low-dose HU tablets, initiating a low/standard-dose treatment became a tedious task for the service providers as the available HU tablets (500 mg) need to be opened up, weighed accordingly and provided in new packages23. In India, even with high HbF levels, severe manifestations among some SCD patients have been reported, where the role of genetic modifiers needs to be studied. A variation in the dosage of HU therapy due to a lack of a definite regimen was also reflected in these studies.
In order to address these gaps and challenges, multi-centric RCTs that can compare the low dose with standard dose and doses up to MTD are required while assessing the pharmacokinetics while also exploring the role of genetic modifiers. There is a paucity of evidence on the long-term benefits of HU in preventing chronic complications of SCD and its effects on fertility and reproduction as a long-term follow up of the patients on HU therapy is lacking. Thus, it becomes imperative to evaluate the long-term safety profile of HU in both children and adults.
There are several limitations in our study. The studies reviewed included patients enrolled from various age groups and with severe clinical manifestations. Furthermore, one double-blind RCT20 could be retrieved from the search.
This review suggests that there is need to conduct research focusing on varied ethnicity in different areas with a clear distinction of the tribal/non-tribal population, as patients’ response across the population could vary. This will not only help in identifying the SCD burden but will also help in identifying the various genetic modifiers across different populations responsible for phenotypic variability. Therefore, more studies are required on individual pharmacokinetic, pharmacodynamic and pharmacogenomic differences contributing to the phenotypic variability in both the dosing of and response to HU therapy. There is also a need in India to develop guidelines based on consensus, especially regarding the dose and duration of HU therapy. These guidelines can be further refined, as and when new data become available. Early detection, treatment and general awareness about SCA in the community are also needed, which can be achieved by community education and partnership as well as better access to healthcare facilities for all economic classes.
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- Understanding α-globin gene regulation and implications for the treatment of β-thalassemia. Ann N Y Acad Sci. 2016;1368:16-24.
- [Google Scholar]
- Global burden of sickle cell anaemia in children under five, 2010-2050:Modelling based on demographics, excess mortality, and interventions. PLoS Med. 2013;10:e1001484.
- [Google Scholar]
- Neonatal screening and the clinical outcome in children with sickle cell disease in central India. PLoS One. 2016;11:e0147081.
- [Google Scholar]
- Sickle cell anemia from central India:A retrospective analysis. Indian Pediatr. 2012;49:911-3.
- [Google Scholar]
- Sickle cell disease:Thoughts for India from the Jamaican cohort study. Front Med. 2021;8:2054.
- [Google Scholar]
- Sickle cell disease in tribal populations in India. Indian J Med Res. 2015;141:509-15.
- [Google Scholar]
- Clinical, hematologic and molecular variability of sickle cell-βthalassemia in western India. Indian J Hum Genet. 2010;16:154-8.
- [Google Scholar]
- Geographical survey of βS-globin gene haplotypes:Evidence for an independent Asian origin of the sickle-cell mutation. Am J Hum Genet. 1986;39:239-44.
- [Google Scholar]
- Effect of alpha-thalassemia on sickle-cell anemia linked to the Arab-Indian haplotype in India. Am J Hematol. 1997;55:104-9.
- [Google Scholar]
- Phenotypic effect of α-globin gene numbers on Indian sickle β-thalassemia patients. J Clin Lab Anal. 2014;28:110-3.
- [Google Scholar]
- Sickle cell disease among tribes of Andhra Pradesh and Orissa, India. Anthropol Anz. 2002;60:169-74.
- [Google Scholar]
- Community expansion and gene geography of sickle cell trait and G6PD deficiency, and natural selection against malaria:Experience from tribal land of India. Cardiovasc Hematol Agents Med Chem. 2012;10:3-13.
- [Google Scholar]
- Burden &pattern of illnesses among the tribal communities in central India:A report from a community health programme. Indian J Med Res. 2015;141:663-72.
- [Google Scholar]
- Socioeconomic and demographic characteristics of sickle cell disease patients from a low-income region of northeastern Brazil. Rev Bras Hematol Hemoter. 2015;37:172-7.
- [Google Scholar]
- Prevention and control of hemoglobinopathies in India - Thalassemias, sickle cell disease and other variant hemoglobins. Available from: http://nhm.gov.in/images/pdf/programmes/RBSK/Resource_Documents/Guidelines_on_Hemoglobinopathies_in India.pdf
- Initial outcomes of a comprehensive care model for sickle cell disease among a tribal population in rural western India. Int J Community Med Public Health. 2016;3:1282-7.
- [Google Scholar]
- The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377-84.
- [Google Scholar]
- Low fixed-dose hydroxyurea in severely affected Indian children with sickle cell disease. Hemoglobin. 2012;36:323-32.
- [Google Scholar]
- Hydroxyurea in sickle cell disease –A study of clinico-pharmacological efficacy in the Indian haplotype. Blood Cells Mol Dis. 2009;42:25-31.
- [Google Scholar]
- Short-term safety and beneficial effects of hydroxyurea therapy in children with sickle cell disease. Indian J Child Health. 2020;7:29-32.
- [Google Scholar]
- Efficacy of fixed low dose hydroxyurea in Indian children with sickle cell anemia:A single centre experience. Indian Pediatr. 2013;50:929-33.
- [Google Scholar]
- Variability of iron load in patients of sickle cell anaemia (HbSS):A study from Eastern India. J Clin Diagn Res. 2017;11:EC19-22.
- [Google Scholar]
- The effect of hydroxyurea on compound heterozygotes for sickle cell-hemoglobin D-Punjab –A single centre experience in eastern India. Pediatr Blood Cancer. 2014;61:1341-6.
- [Google Scholar]
- Low dose hydroxyurea is effective in reducing the incidence of painful crisis and frequency of blood transfusion in sickle cell anemia patients from eastern India. Hemoglobin. 2012;36:409-20.
- [Google Scholar]
- Hydroxyurea in sickle cell disease:Our experience in Western India. Indian J Hematol Blood Transfus. 2016;32:215-20.
- [Google Scholar]
- Low and fixed dose of hydroxyurea is effective and safe in patients with HbSβ(+) thalassemia with IVS1-5(G?C) mutation. Pediatr Blood Cancer. 2015;62:1017-23.
- [Google Scholar]
- Effect of hydroxyurea on clinical and haematological profile of children with sickle cell anaemia. Int J Res Rev. 2020;7:494-9.
- [Google Scholar]
- Effective control of sickle cell disease with hydroxyurea therapy. Indian J Pharmacol. 2010;42:32-5.
- [Google Scholar]
- HbSD-Punjab:Clinical and hematological profile of a rare hemoglobinopathy. J Pediatr Hematol Oncol. 2014;36:e140-4.
- [Google Scholar]
- Study of seminal fluid parameters and fertility of male sickle cell disease patients and potential impact of hydroxyurea treatment. J Assoc Physicians India. 2017;65:22-5.
- [Google Scholar]
- Beneficial effect of low fixed dose of hydroxyurea in vaso-occlusive crisis and transfusion requirements in adult HbSS patients:A prospective study in a tertiary care center. Indian J Hematol Blood Transfus. 2018;34:294-8.
- [Google Scholar]
- Clinical manifestations of sickle cell disease in India:Misconceptions and reality. Curr Opin Hematol. 2018;25:171-6.
- [Google Scholar]
- Exposure to hydroxyurea during pregnancy in sickle-βThalassemia:A report of 2 cases. J Clin Pharmacol. 2010;50:231-4.
- [Google Scholar]
- Management of sickle cell disease:Summary of the 2014 evidence-based report by expert panel members. JAMA. 2014;312:1033-48.
- [Google Scholar]






