Translate this page into:
A retrospective analysis of serological & molecular testing data on dengue fever in Kolkata & adjacent districts during 2016-2019
For correspondence: Dr Shanta Dutta, ICMR-National Institute of Cholera and Enteric Diseases, P-33, C.I.T. Road, Scheme-XM, Beliaghata, Kolkata 700 010, West Bengal, India e-mail: shanta1232001@gmail.com
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Regional Virus Research and Diagnostic Laboratory established at ICMR-National Institute of Cholera and Enteric Diseases (NICED) regularly receives samples for dengue screening and serotyping from patients of acute febrile illness (AFI) from Kolkata and adjacent districts. In this study, data over a three year period (August 2016-July 2019) was retrospectively analyzed to provide insight into the epidemiological trends of dengue fever in this region.
Methods:
Serological screening of dengue was performed by detection of NS1 antigen and/or immunoglobulin M (IgM) antibody. Dengue serotyping was done by conventional or real-time reverse transcriptase–PCR. The data were analyzed to describe the distribution of dengue with respect to age of patient, duration of fever on the day of blood collection and month of the year. Zip codes were used for spatial plotting.
Results:
Out of the 24,474 samples received from Kolkata and its adjacent districts (Hooghly, Howrah, North and South 24 Parganas), 38.3 per cent (95% confidence interval: 37.7-38.9%) samples were screened positive for dengue. The correlation between age and dengue positivity was found to be weak. A combination of dengue NS1 antigen and dengue IgM antibody detection may be a better option for detecting dengue positivity compared to a single test. Most AFI cases were tested from August to November during the study period, with maximum dengue positivity noted during September (45.9%). The predominant serotype of 2016, dengue virus serotype 1 (DENV-1), was almost entirely replaced by DENV-2 in 2017 and 2018.
Interpretation & conclusions:
Dengue continues to be an important cause of AFI in the region and round-the-year preventive measures are required for its control. Serotype switching is alarming and should be monitored routinely.
Keywords
Dengue fever
dengue serotyping
Kolkata
mapping
Dengue fever is the most common arboviral infection worldwide, with an estimated 40 per cent of the world’s population living in dengue-endemic areas1. This is caused by dengue virus (DENV) which belongs to the genus Flavivirus under the family Flaviviridae2. It is transmitted to humans mainly through Aedes mosquitoes, the most important species being Aedes aegypti3. DENV is serologically distinguishable mainly as DENV-1, DENV-2, DENV-3 and DENV-42. Clinically, dengue fever presents a wide spectrum of symptoms ranging from self-limiting mild fever to plasma leakage with or without haemorrhagic manifestations4. Several factors such as age at infection, viral load and DENV serotype with its specific genotype/lineage have been suggested to contribute to variations in the severity of illness5.
According to WHO, the incidence of dengue has grown dramatically in recent decades with half of the global population at risk6. India contributed 34 per cent of the 96 million apparent dengue infections estimated to have occurred globally in 20107. The first major confirmed dengue epidemic took place in 1963-1964 in Kolkata2,5. Subsequently after its absence of more than three decades, another major outbreak was reported in Delhi in 19968. Over the years, dengue fever has established itself as endemic over most parts of India2,5,9. Studies have also indicated a shift in age group involvement from children to young adults2. Co-circulation of all well-known DENV 1-4 serotypes with frequent change in the predominant serotype has been observed2,5,9-15. The definite increase in the frequency of outbreaks alongside co-circulation of the four major serotypes hints at hyperendemicity of dengue fever in India2.
The Regional Virus Research and Diagnostic Laboratory (VRDL) at Indian Council of Medical Research (ICMR) – National Institute of Cholera and Enteric Diseases (NICED), Kolkata, India, is involved with timely identification, reporting and research on viral diseases and other agents of public health importance and epidemic potential16. The laboratory receives samples for serological screening and serotyping of dengue from the eastern region of India, mainly from the city of Kolkata and its adjacent districts such as Hooghly, Howrah and North and south 24 Parganas. The region is endemic for dengue and has experienced multiple dengue outbreaks over the last three years. In the present study, the three-year data have been analyzed to describe the epidemiology of laboratory-confirmed dengue cases among the clinically suspected patients with respect to age, seasonality, geographic distribution and the changing trend of prevalent serotype in and around Kolkata, West Bengal, India.
Material & Methods
This retrospective cross-sectional study was carried out on stored samples. Prior approval was procured from the Institutional Ethics Committee.
Study participants: Acute-phase blood samples, collected from patients with acute febrile illness (AFI) referred from hospitals attached to VRDL-NICED or from cases of suspected dengue outbreaks referred by State health authorities, were screened for dengue from August 2016 to July 2019. Cases from the districts of Kolkata, Hooghly, Howrah and North and South 24 Parganas were selected for the study. Dengue serotyping was performed on the NS1 antigen-positive samples.
Serological testing for dengue detection: Enzyme-linked immunosorbent assays (ELISAs) were performed on serum samples. Dengue NS1 antigen detection was performed using Panbio Dengue Early ELISA kit (Standard Diagnostics Inc., 01PE40, Republic of Korea), while dengue immunoglobulin M (IgM) antibody detection was performed using Panbio Dengue IgM Capture ELISA kit (Standard Diagnostics Inc., 01PE20, Republic of Korea) or NIV Dengue IgM Antibody Capture ELISA kit (ICMR-National Institute of Virology, Pune, India) as per the availability. Tests were performed and interpreted according to the manufacturers’ instructions.
Dengue serotyping: RNA extraction was performed using a commercial RNA extraction kit (QIAamp viral RNA Mini Kit, QIAGEN, Hilden, Germany). Dengue serotyping during 2016-2017 was performed using a nested PCR method modified from that originally developed by Lanciotti et al17. Dengue serotyping during 2018 was performed using in-house developed real-time reverse transcriptase-PCR (RT-PCR) method (ICMR-National Institute of Virology, Pune), shared as a standard operating protocol of VRDL network of laboratories18. The reaction setup involved detection of DENV-1, DENV-2 and DENV-3 in one reaction and DENV-4, Chikungunya virus (CHIKV) and beta-actin (internal control) in the second reaction. The sequences of primers and probes were reported earlier19,20.
Data analysis: Information on patient demography, duration of fever and test results was collected from laboratory record systems. The entire retrieved information was anonymized by compiling the data into spreadsheet, and rows pertaining to each patient information were swapped randomly. Data cleaning was performed on anonymized patient data. For the purpose of the study, equivocal results were considered negative. The complete data were entered and analyzed in R version 4.0.1. Dengue positivity data were analyzed with respect to age of patient, duration of fever on the day of blood collection and month of receipt. Chi-square tests were performed for comparison of categorical variables and P<0.05 was considered as significant. If the null hypothesis was rejected as per P value, the effect size (Cramer’s V) was calculated to determine the strength of correlation between the variables.
Spatial plotting of data on dengue positivity rate: For spatial plotting of the dengue positivity rate, postal zip code data were used. Postal zip code was found to be the most consistent data available in the records that could provide geolocation yet preserving patient privacy and anonymity. For records where zip codes were not available, the residential addresses were used to identify the zip codes. The positivity rates of dengue in Kolkata and adjoining areas were determined for each zip code area, from where at least 10 samples were received. These zip codes were geocoded and plotted as a heat map which is a 2D density plot using an R programming language (free and open-source software environment for statistical computing and graphics). The spatial plotting was achieved using ggplot2 (open-source data visualization package for R). For the underlying map, the free and open-source R package ggmap was used which is a collection of functions to visualize spatial data and models on top of static maps from various online sources, e.g. Google Maps21.
Results
Serum samples from a total of 24,474 individuals with AFI were received at VRDL-NICED from August 2016 to July 2019 which belonged to patients from Kolkata (49.5%) and its adjacent four districts – Hooghly (13.8%), Howrah (0.4%), North 24 Parganas (25.1%) and South 24 Parganas (11.2%). A total of 9,379 [38.3%; 95% confidence interval (CI) 37.7-38.9%] individuals were screened positive for dengue by at least one of the dengue serological tests – NS1 antigen or dengue IgM. Most of the samples collected were from individuals of 20 to 29 yr of age group (28.8%), while those from patients aged 60 yr or more were the least (3.2%). Dengue positivity was noted maximum in 20-29 yr of age group (43.3%) and the least in patients aged nine years or below (21.9%). The age of the dengue-positive patients ranged from six months to 97 yr, with a median of 27 yr (IQR: 19-37) (Table I). From the total number of observations, 23,604 (870 observations for which age was not available were excluded from statistical analysis), Chi-square statistic was 456.897; P=1.609e-95, i.e. <0.05. Since the null hypothesis is rejected as per the P value, we calculated the effect size (Cramer’s V)=0.139. It should be noted that a small P value with a small effect size signifies a weak correlation between age and positivity.
| Age groups (yr) | 2016-2017 | 2017-2018 | 2018-2019 | Total tested | Total positive (%) | |||
|---|---|---|---|---|---|---|---|---|
| Tested | Positive (%) | Tested | Positive (%) | Tested | Positive (%) | |||
| ≤9 | 877 | 242 (27.6) | 256 | 54 (21.1) | 325 | 24 (7.4) | 1458 | 320 (21.9) |
| 10-19 | 2881 | 1203 (41.8) | 1757 | 740 (42.1) | 261 | 53 (20.3) | 4899 | 1996 (40.7) |
| 20-29 | 3343 | 1451 (43.4) | 2462 | 1117 (45.4) | 427 | 131 (30.7) | 6232 | 2699 (43.3) |
| 30-39 | 2444 | 1021 (41.8) | 1935 | 838 (43.3) | 363 | 108 (29.8) | 4742 | 1967 (41.5) |
| 40-49 | 1574 | 589 (37.4) | 1329 | 495 (37.2) | 237 | 54 (22.8) | 3140 | 1138 (36.2) |
| 50-59 | 887 | 264 (29.8) | 787 | 242 (30.7) | 180 | 27 (15.0) | 1854 | 533 (28.7) |
| ≥60 | 590 | 132 (22.4) | 526 | 154 (29.3) | 163 | 17 (10.4) | 1279 | 303 (23.7) |
| Data not available | 870 | 423 (48.6) | 0 | 0 | 0 | 0 | 870 | 423 (48.6) |
A total of 15,180 patients were screened for NS1 antigen only, of these 5782 (38.1%; 95% CI: 37.3-38.9%) patients were positive; 5457 patients were screened for dengue IgM antibody only, of these 2041 (37.4%; 95% CI: 36.1-38.7%) patients were positive. Out of 3837 tested for both NS1 antigen and dengue IgM antibody, dengue was positive in 1554 (40.5%; 95% CI: 38.9-42.1%) patients, based on detection of NS1 antigen (n=570, 14.9%), IgM antibodies (n=484, 12.6%) or both (n=500, 13.0%).
Table IIA shows the agreement of Dengue NS1 and IgM test results with the duration of fever grouped into three days or less, four to seven days and eight days or more. Cohen’s kappa statistic that measures interrater reliability clearly indicated that there was poor agreement between NS1 and IgM results in all three intervals chosen22. Table IIB shows the association of dengue NS1 and IgM test results in dengue seropositive patients with duration of fever in similar groups. NS1 positivity was compared between intervals ≤3 days and 4-7 days (P>0.05), 4-7 days and ≥8 days (P<0.05, effect size: 0.11; weak correlation) and ≤3 days and ≥8 days (P<0.05, effect size: 0.20; weak correlation). IgM positivity was compared between intervals ≤3 days and 4-7 days (P<0.05, effect size: 0.13; weak correlation), 4-7 days and ≥8 days (P<0.05, effect size: 0.09; weak correlation) and ≤3 days and ≥8 days (P<0.05, effect size 0.32; some correlation).
| Days of fever | NS1+ IgM− (%) | NS1− IgM+ (%) | NS1+ IgM+ (%) | NS1− IgM− (%) | Total | Per cent agreement | Cohen’s kappa statistics | Level of agreement |
|---|---|---|---|---|---|---|---|---|
| ≤3 | 102 (19.6) | 53 (10.2) | 35 (6.7) | 331 (63.5) | 521 | 70.23 | 0.13 | None |
| 4-7 | 438 (15.8) | 365 (13.1) | 423 (15.2) | 1551 (55.9) | 2777 | 71.08 | 0.31 | Minimal |
| ≥8 | 30 (5.6) | 66 (12.2) | 42 (7.8) | 401 (74.4) | 539 | 82.19 | 0.36 | Minimal |
IgM, immunoglobulin M
| Days of fever | NS1+ IgM− (%) | NS1− IgM+ (%) | NS1+ IgM+ (%) | Total |
|---|---|---|---|---|
| ≤3 | 102 (19.6) | 53 (10.2) | 35 (6.7) | 190 |
| 4-7 | 438 (15.8) | 365 (13.1) | 423 (15.2) | 1226 |
| ≥8 | 30 (5.6) | 66 (12.2) | 42 (7.8) | 138 |
Fig. 1 shows the month-wise distribution of AFI cases and dengue positivity over three years (August 2016-July 2017, August 2017-July 2018 and August 2018-July 2019). The maximum clustering of cases was seen during monsoon and post-monsoon months of August to November. Average dengue positivity ranged from lowest in April (5.1%) to highest in September (45.9%).
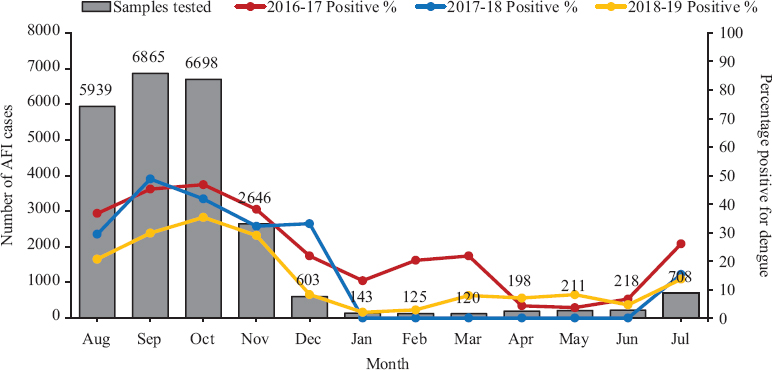
- Month-wise distribution of acute febrile illness cases with average dengue positivity (dengue NS1 and/or immunoglobulin M). AFI: acute febrile illness.
Figure 2 depicts the changing prevalence of dengue serotypes over the course of three years. In 2016, DENV-1 was the predominant serotype (n=111, 61.3%), followed by DENV-2 (n=34, 18.8%), DENV-4 (n=24, 13.3%) and DENV-3 (n=12, 6.6%). But in 2017, DENV-2 became the dominant strain (n=206, 78.3%) followed by DENV-4 (n=26, 9.9%), DENV-3 (n=19, 7.2%) and DENV-1 (n=12, 4.6%). DENV-2 remained as a majority (n=92, 79.3%) in 2018, followed by DENV-1 (n=15, 12.9%), DENV-3 (n=3, 2.6%) and DENV-4 (n=0). In addition, 6 (5.2%) cases of co-infection were detected with multiple serotypes in 2018 (3 cases of DENV-1 with DENV-2, 2 cases of DENV-2 with DENV-4 and 1 case of DENV-2 with DENV-3 and DENV-4).
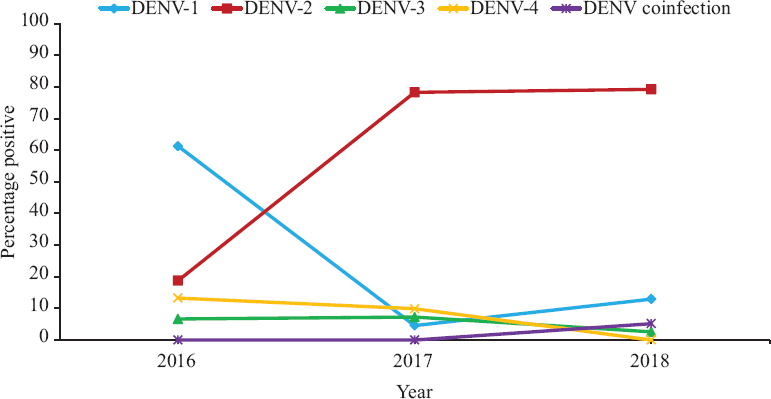
- Dengue serotype distribution over the years (2016-2018).
Fig. 3 shows the distribution of the positivity rate of dengue cases according to postal zip codes in Kolkata and adjoining areas plotted as a heat map.
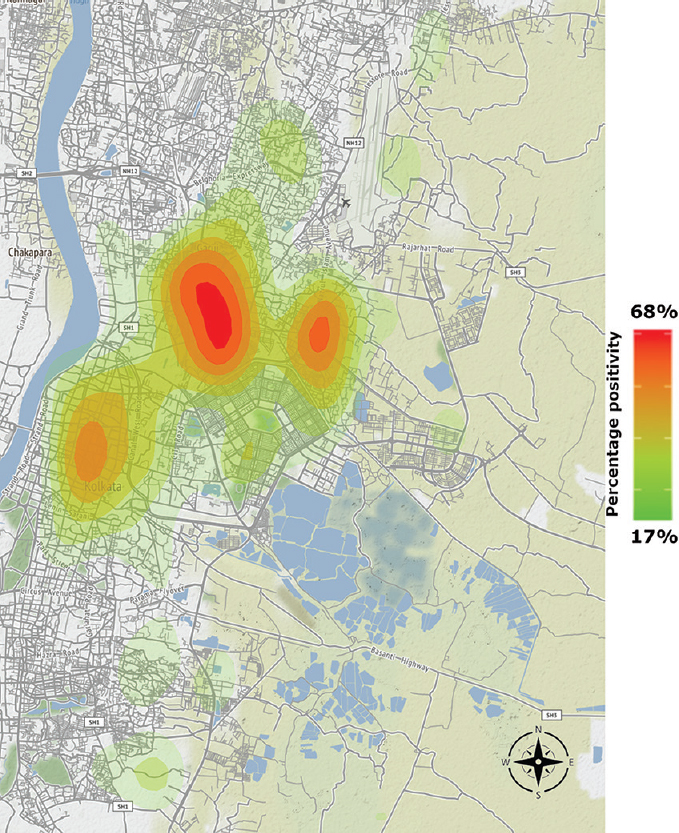
- Heat map showing the distribution of dengue cases in and around Kolkata during 2016-2019.
Discussion
Kolkata, a metropolitan city, and its suburban areas spread across the adjacent districts host all the factors necessary for being an ideal geographical setup for dengue vector ecology and disease transmission dynamics23,24. The proportion (38.3%) of laboratory-confirmed cases of dengue seen in this study is higher than that reported from other regions of India except Northeast25. The highest dengue positivity was seen in patients belonging to 20-29 yr of age group, followed by those in 30-39 yr of age. These results are similar to other reports from India12,15,20. These groups comprise the bulk of the workers engaged in daily outdoor activities for livelihood and construction site labourers, thus most susceptible for mosquito bites. However, there is no strong correlation between age and dengue positivity.
Currently, physicians and clinical laboratories recommend screening of dengue by NS1 antigen up to five days of illness and by dengue IgM from six days onwards26. However, this study showed that the duration of fever has little to do with the choice of test. It could be implied that irrespective of the duration of illness, as reported by the patient, both dengue NS1 and dengue IgM testing should be performed to rule out dengue. However, it must also be stressed that patients often have difficulty in recalling the exact day of onset of fever and frequently the patients may fail to distinguish between fever and malaise. In such situations too, it is prudent enough to test for both dengue NS1 and IgM.
The high dengue positivity alongside an increase in the number of AFI cases during the months of monsoon and post-monsoon season in this region is consistent with other studies from India9,12,25. The high load of dengue positive cases in the months of August–November should be an indicator for resource allocation and the level of preparedness required for dengue diagnostic and healthcare setups during these months. The presence of dengue positivity in the other months, though comparatively low, highlights the need for year-round dengue awareness and a mosquito control programme is required to combat the disease.
Kolkata and surrounding areas have seen a probable dengue virus serotype switch from DENV-1 in 2016 to DENV-2 in 2017. Such serotype switches could have profound clinical implications – antibodies against one serotype might be ineffective against a different serotype and render a huge population susceptible to severe dengue infection with a different serotype, through various mechanisms such as antibody-dependent enhancement and memory cross-reactive T cells.27,28. Dengue serotype co-infections have been noted in 2018 which were absent in 2016-2017. Previous studies from other States of India have also reported co-infection with multiple dengue serotypes10,14,15. The improved sensitivity of real-time RT-PCR employed in 2018 compared to conventional nested PCR used for serotyping in the earlier years might be a possible reason for no detection of co-infection during 2016-2017.
The spatial plotting of the dengue positivity rate according to the zip codes highlights the region-wise clustering of the cases. Such data give an idea of dengue disease burden in the respective area. This can be an invaluable tool in assessing the need for targeted interventions, in determining how to allocate optimally the limited resources available for dengue control and in evaluating the impact of such activities in the long run7.
The study has certain limitations. The laboratory record system had no information about the severity of dengue and the patient outcome. Hence, correlation of disease severity with dengue serotype could not be established. The samples received at VRDL-NICED were referred from a few tertiary care hospitals and outbreaks. The data on dengue testing done at other government and private healthcare setups catering to the population of the districts included in the study were not available. Hence, the precise estimate of incidence could not be calculated. Instead, calculation of the proportion of positivity was done using the number of cases of AFI tested as the denominator. Missing information regarding the age of 870 cases was another limitation of this study, as this group had maximum (48.6%) dengue positivity.
To conclude, the findings of the present study indicate the importance of dengue as a cause of AFI in the region evident by the high proportion of dengue positivity, co-circulation of multiple serotypes as well as predominant serotype switch. There is a need for testing both dengue NS1 and dengue IgM to rule out the disease irrespective of the duration of fever along with routine serotype monitoring. Region-wide mapping of dengue cases helps implementing targeted awareness spreading campaigns and such geo-mapping can pave the road for identifying potential dengue vaccine clinical trial sites in the future. Further research is required to study the evolutionary sustainability of one strain over another.
Acknowledgment:
Authors acknowledge the Department of Health Research (DHR), ICMR-National Institute of Virology, Pune, and Government of West Bengal to carry out this study.
Financial support & sponsorship: The study was funded by DHR/ICMR VRDL and Government of West Bengal.
Conflicts of Interest: None.
References
- Dengue serotype-specific differences in clinical manifestation, laboratory parameters and risk of severe disease in adults, Singapore. Am J Trop Med Hyg. 2015;92:999-1005.
- [Google Scholar]
- Current perspectives on the spread of dengue in India. Infect Drug Resist. 2014;7:337-42.
- [Google Scholar]
- Global strategy for dengue prevention and control 2012–2020 2012:43.
- Dengue guidelines for diagnosis, treatment, prevention and control. 2009;Vol 1:160.
- 2014. Dengue and severe dengue, vol 117. Geneva: WHO Fact Sheet; :1-4. Available from:https://www.who.int/mediacentre/factsheets/fs117/en/index.htm
- The first major outbreak of dengue hemorrhagic fever in Delhi, India. Emerg Infect Dis. 1999;5:589-90.
- [Google Scholar]
- Dengue burden in India:Recent trends and importance of climatic parameters. Emerg Microbes Infect. 2017;6:e70.
- [Google Scholar]
- Occurrence of concurrent infections with multiple serotypes of dengue viruses during 2013-2015 in northern Kerala, India. Peer J. 2017;5:e2970.
- [Google Scholar]
- Changing pattern of dengue virus serotypes circulating during 2008-2012 and reappearance of dengue serotype 3 may cause outbreak in Kolkata, India. J Med Virol. 2016;88:1697-702.
- [Google Scholar]
- Dengue Fever outbreak in Delhi, North India:A clinico-epidemiological study. Indian J Community Med. 2015;40:135-8.
- [Google Scholar]
- Molecular typing of dengue virus circulating in Kolkata, India in 2010. J Trop Med. 2012;2012:960329.
- [Google Scholar]
- Co-circulation of all four dengue virus serotypes:First report from Odisha. Indian J Med Microbiol. 2017;35:293-5.
- [Google Scholar]
- Correlation of clinical severity and laboratory parameters with various serotypes in dengue virus:A hospital-based study. Int J Microbiol. 2020;2020:6658445.
- [Google Scholar]
- Establishment of a network of laboratories for managing epidemics and natural calamities (VRDL). Available from:https://dhr.gov.in/schemes/establishment-network-laboratories-managing-epidemics-and-natural-calamities
- Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol. 1992;30:545-51.
- [Google Scholar]
- Clinical evaluation of an in-house-developed real-time RT-PCR assay for serotyping of dengue virus. Arch Virol. 2020;165:2311-5.
- [Google Scholar]
- Development of a multiplex real-time RT-PCR assay for simultaneous detection of dengue and chikungunya viruses. Arch Virol. 2015;160:323-7.
- [Google Scholar]
- Emergence of dengue virus type 1 and type 3 as dominant serotypes during 2017 in Pune and Nashik regions of Maharashtra, Western India. Infect Genet Evol. 2018;66:272-83.
- [Google Scholar]
- Modeling and prediction of dengue occurrences in Kolkata, India, based on climate factors. Int J Biometeorol. 2020;64:1379-91.
- [Google Scholar]
- Mosquito control:Can it stop zika at source? 2015:1-8.
- Epidemiology of dengue fever in India, based on laboratory surveillance data, 2014-2017. Int J Infect Dis. 2019;84S:S10-4.
- [Google Scholar]
- Handbook for clinical management of dengue 2012:114.
- Current understanding of the pathogenesis of dengue virus infection. Curr Microbiol. 2021;78:17-32.
- [Google Scholar]






