Translate this page into:
Detection of micro-metastasis using cytokeratins (AE1/AE3) in haematoxylin & eosin-stained N0 lymph nodes of oral squamous cell carcinoma
For correspondence: Dr Gargi Mila Sinha, Department of Oral and Maxillofacial Pathology, SDM Dental College & Hospital, Dharwad 580 009, Karnataka, India e-mail: gargimilasinha@yahoo.com
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Oral squamous cell carcinoma (OSCC) is one of the most common malignancies affecting the head-and-neck region, regional lymph nodes being an important prognostication factor dictating the survival rate. Despite an array of modalities used, clinically, radiographically and routine histopathologically, the detection of micro-metastasis (2-3 mm tumour cell deposits) in the lymph nodes often escapes identification. The presence of few of these tumour epithelial cells in the lymph nodes drastically increases mortality and alters treatment plan. Hence, the identification of these cells is of major prognostic significance for a patient. Thus, the present study was aimed to evaluate and detect the efficacy of the immunohistochemical (IHC) marker [cytokeratin (CK) AE1/AE3] over routine Hematoxylin & eosin (H & E) staining in detecting micro-metastasis in the lymph nodes of OSCC cases.
Methods:
Hundred H & E-stained N0 lymph nodes of OSCC cases treated with radical neck dissection were subjected to IHC with marker AE1/AE3 antibody cocktail for detecting micro-metastasis.
Results:
The IHC marker CK cocktail (AE1/AE3) did not demonstrate any positive reactivity for the target antigen in all the 100 H & E stained lymph node sections evaluated in the present study.
Interpretation & conclusions:
This study was undertaken to check the efficacy of IHC (CK cocktail AE1/AE3) in the detection of micro-metastasis in lymph nodes that are found to be negative in routine H&E stained sections. The findings of this study suggest that the IHC marker AE1/AE3 did not prove to be useful to detect micro-metastasis in this study population.
Keywords
Cytokeratins
immunohistochemistry
lymph nodes
micro-metastasis
oral squamous cell carcinoma
Oral squamous cell carcinoma (OSCC) is the sixth most common cancer across the world, accounting for 90 per cent of total head and neck cancers and five per cent of all malignant tumours worldwide1,2. The status of regional lymph nodes is the most significant prognostic factor in OSCCs of the head and neck region. Although the clinical palpation of the head and neck is still widely used for staging of the neck, newer imaging techniques have been employed for detecting metastasis. Despite using these sophisticated modalities, it might not be possible to detect occult metastasis3. The risk of occult metastasis is high for cancers affecting the oral cavity4, especially in the cervical lymph nodes of level I and II5,6. Given the importance of nodal status in prognosis and treatment planning, accurate staging of cervical lymph nodes becomes most critical.
Histological examination of lymph node remains the gold standard for identification of metastasis7, but the analysis of lymph nodes and its microscopic examination alone using routine haematoxylin & eosin (H & E) stains has its own limitations8. Micro-metastasis, which refers to 2-3 mm tumour cell deposits in the lymph node, can easily escape identification when observed in routine H & E stained sections9.
Cytokeratins (CKs) are intermediate filament proteins specifically expressed by the epithelial cells in a cell type specific and differentiation dependent manner and are not present in the lymph nodes, except in developing metastatic tumour cells, which may be detected by immunohistochemical (IHC) staining by various CK antibody markers10,11. Monoclonal antibody AE1 can recognize acidic subfamilies of CKs, while monoclonal antibody AE3 can recognize basic families of CK. Thus, the combination of these two monoclonal antibodies present in the CK cocktail (AE1/AE3) has been used in our study as it can be used to identify both high molecular weight neutral to basic CKs and low-molecular-weight acidic CKs. Noticeably, the presence of fewer epithelial cells in the lymph nodes drastically reduces mortality and alters treatment plan12,13. Hence, identification of these cells is of major prognostic significance1. It would also identify patients who can benefit from adjunct therapy. Thus, the present study was designed to identify the efficacy of IHC in detection of micro-metastases in the lymph nodes that are negative for metastatic deposits in routine hematoxylin and eosin (H & E) stained sections.
Material & Methods
This study was carried out in the department of Oral & Maxillofacial Pathology, SDM Dental College & Hospital, Dharwad, Karnataka, India. The study consisted of 100 formalin fixed paraffin embedded nodal tissue blocks obtained between December 2017 and January 2019 from 34 cases diagnosed and treated with radical neck dissection for OSCC. The nodal tissue blocks were retrieved from the archives of department of Oral and Maxillofacial Pathology and Microbiology, after approval from the Institutional Review Board (IRB no. 2017/ P/OP/56).
Sample size: The sample size was calculated using G-power software (Heinrich Heine University Dusseldorf, Germany), and calculations were derived from the following formula:
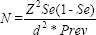
where, α is the alpha error five per cent, z is the z score for said alpha 1.959963985, Se refers to the sensitivity 0.98, d is the clinically significant difference 0.1 and Prev refers to the prevalence 0.1.
Based on the key article by Yoshida et al14, the sensitivity of the technique was represented as 0.98 in Table. Assuming a difference in the sensitivity of 10 per cent, the calculated value computed is 75.29259289. Hence, rounding off, we required a sample size of at least 76 to arrive at a conclusion. Hence, in the present study, 100 formalin fixed paraffin embedded lymph node tissues were included for analysis.
Inclusion and exclusion criteria: Lymph nodes categorized as histologically negative for metastatic foci when stained and observed with routine H & E stain, cervical group of lymph nodes of level I and level II, and ipsilateral level I and II nodes were included in the study. Lymph nodes of level III and above; contralateral nodes of level I and II, patients with a history of previous surgery and recurrence, and nodal tissues with folds or cases with insufficient clinical details were excluded from the study.
Immunohistochemistry (IHC): Clinical details of the patients were collected from institutional patient record database. Paraffin embedded tissue sections of primary tumour tissues of OSCC and histologically established positive nodal tissues of OSCC from the archives constituted our control group. Tissues were subjected for IHC to detect micro-metastasis by staining with primary mouse monoclonal CK cocktail antibody (AE1/AE3) (BIOGENEX, San Ramon, California, USA) according to the manufacturer’s protocol. The presence of a strong globoid brown colour cytoplasmic or membranous staining at the site of target antigen was expected to be a positive result as seen in our control sections. All sections were analyzed under high power (×40) by two independent observers.
Results
Most of the study participants in this study were males, with a male:female ratio of 5.8:1. The age range of the participants was 39-67 yr, with a majority of participants below 50 yr of age. The mean age of the data distribution was around 51 yr. The study participants were grouped according to their age distribution as above and below 40 yr. Majority (n=33, 97.06%) of the participants belonged to above 40 yr of age. About 26 per cent (n=9) of the participants were in the age range of 40-50 yr, while rest of the participants were above 50 yr of age.
In our study samples, histopathological grading was not included as a criterion for selecting samples. The histopathological grading was noted after sample selection. Of the 34 participants, majority (n=26) of cases were well differentiated, followed by moderately differentiated primary tumour (n=6), and few cases were poorly differentiated (n=2) according to the Broder’s system of grading8. There was no difference in the micro-metastatic deposit detection among the groups based on the histological differentiation and grading of the primary tumour. Lymph node sections examined from cases of all three groups were negative for any micro-metastatic deposit.
The clinical staging noted in the majority of our cases (n=22) was stage III, i.e. a majority of cases had tumour sizes greater than 4 cm or had clinically palpable ipsilateral nodes that were fixed and seemed positive for tumour deposit by imaging techniques. Few cases (n=3) who were in stage IVA showed invasion into surrounding structures such as cortical bone, deeper muscles, maxillary sinus or skin surface. Remaining cases (n= 9) were in stage II where clinically metastatic deposit was not noted and had a tumour size >2 cm but <4 cm in greatest dimensions.
The most common site of occurrence of cancer in the study samples was buccal mucosa (n=20, 58.28%), followed by the gingiva-buccal sulcus (n=4, 11.76%), alveolar mucosa (n=3, 8.82%) and tongue (n=3, 8.82%). Rest of the cases were noted in the lip (n=1, 2.94%), floor of the mouth (n=2, 5.88%) and the retromolar trigone area (n=1, 2.94%).
All of the 100 lymph node sections stained by IHC marker CK cocktail (AE1/AE3) were negative (Figs 1 and 2). These did not demonstrate any positive reactivity for the target antigen.
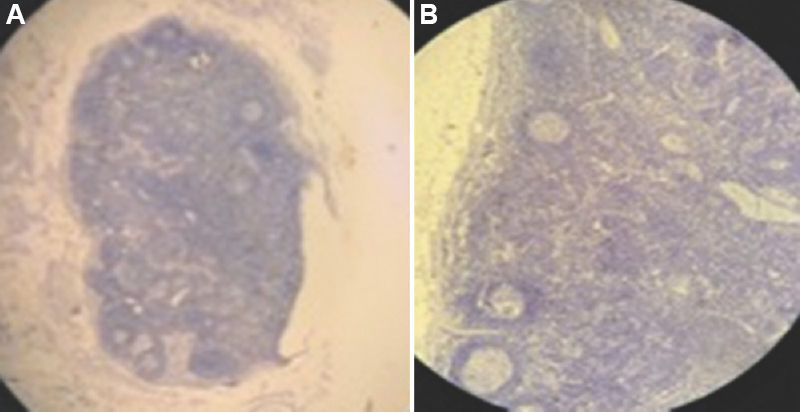
- Photomicrographs showing capsulated lymph nodes with secondary follicles and germinal centres following IHC staining (cytokeratin cocktail, AE1/AE3) at (A) ×4, and (B) ×10 magnification. IHC, immunohistochemistry.
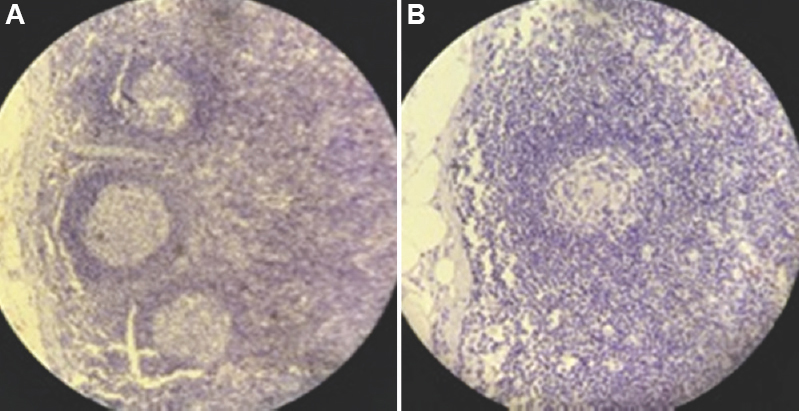
- Photomicrograph showing IHC (cytokeratin cocktail, AE1/AE3) stained lymph nodes with secondary follicles and germinal centres with characteristic absence of micro-metastatic deposit (A) ×20, and (B) ×40 magnification. IHC, immunohistochemistry.
Discussion
Metastasis is one of the major causes of poor prognosis with around 90 per cent of deaths associated with cancer caused via metastatic spread as stated by Hanahan and Weinberg13. Patients who present with metastatic disease or develop metastases even after a successful treatment and eradication of the primary tumour carry a universally grave and poor prognosis14.
A study by Thomsen et al15 showed that the detection of micro-metastasis using special methods, such as serial sectioning and IHC, had upstaged about 30 per cent of patients in their study sample. Data from some other studies that made an attempt to detect micro-metastasis indicate that IHC assessment and molecular analysis of the cervical lymph nodes can be useful to refine the staging system, and a significant percentage of cases found to have micro-metastases would be upstaged5,16-20. Retrospective analyses of cases detected with micro-metastasis have shown that it necessitates the use of adjuvant therapeutic interventions to improve prognosis. Hence, the identification of these cells is of major prognostic significance1.
In the present study, the term micro-metastasis was used to define those metastatic deposits in the lymph nodes that were not evident on routinely stained H & E sections but would be demonstrable by staining with marker antibodies on IHC. These were either single or a group of tumour cells measuring not more than 3 mm in diameter, which were missed on clinical and microscopic examination by routine methods.
In our study sample, majority of the subjects were males which showed a decline in the age of the demographic incidence data compared to older literature reports. This is in accordance with the National Cancer Institute Surveillance, Epidemiology and End Results database21, which provides evidence for an increase in percentage of oral carcinomas that occurred in younger-adult age groups between 35 and 50 yr, from three per cent in 1973 to approximately six per cent in 1993 to 21.11 per cent currently in 2019.
A marked finding to be noted in this study sample was that 100 per cent of cases had associated habits of chewing tobacco in any one of the available commercial preparations. Some of the study subjects along with tobacco chewing habit even had an association with either drinking and/or smoking. We attribute the primary cause for the development of OSCC in all our study participants to the incessant use of these tobacco-related products, which are already known and established potent carcinogens. However, in our literature search, we could not elucidate any direct relationship between the frequency and duration of such habits and the likelihood of establishment of a local or distant metastatic disease.
Studies have demonstrated aggressiveness of the tumour and the presence of metastatic deposits with increase in tumour size and depth22-24. In the present study, although majority of cases were found to be in stage III or above, these were all free from any metastatic deposits including micro-metastatic deposits as confirmed with CK IHC. This shows light that metastatic deposits may not be directly related to the tumour size and depth but to the innate aggressive potential of the tumour cells25 as shown in some of the studies where tumours in stage I and II were found to have micro-metastatic deposits14,16,17. Further, 11 of our cases showed positive lymph nodes by imaging techniques such as contrast computed tomographic scan and magnetic resonance imaging, which were all proved histologically negative. Hence, the accuracy of imaging results to detect metastatic deposit may not be always relied upon26,27, and clinician’s experience along with histopathological final diagnosis should be regarded as a gold standard for the decision of adjuvant treatment choices and surgical approaches.
Histological grading has been thought to be an important diagnostic tool to predict the clinical and biological behaviour of cancer since a long time28. Contrary to this, we did not observe any difference in the micro-metastatic deposit among the groups based on the histological differentiation and grading of the primary tumour.
More recently, contrasting studies that are helpful for those who majorly understand the true biology of these tumour cells have shown contrary results29. A vast majority of the work on the mutant genes and innate potential of the tumour cells in OSCC, the role of tumour microenvironment and host immune response suggest that OSCC is a multifactorial disease and predicting aggressiveness and dissemination of the disease merely on the histopathological grade of differentiation may not be possible14,29,30.
Various studies have reported that oral cancers have the tendency to metastasize from level I to level V in the decreasing order of frequency3,6 . A higher incidence of metastasis positive lymph nodes in level I in studies affirms the fact that nodal involvement is primarily determined by locoregional spread from primary anatomical site5,6.
However, it is interesting to note that in the studies in which the patterns of cervical lymph node metastasis of tongue region were studied, level II was the most commonly involved nodal level4,5. These minor variations in the involvement of the levels of nodes in different studies are mainly influenced by lymphatic drainage of the primary tumour in the anatomical sites.
Sentinel lymph node is commonly used in the evaluation of early staging of tumour and is the nearest lymph node to the lesional site which is affected3,4,14. As stated above, level I lymph nodes have a higher incidence of metastasis due to the fact that nodal involvement is primarily determined by locoregional spread from primary anatomical site, which is the sentinel lymph nodes.
Level II lymph nodes are the lymph nodes belonging to upper jugular group located in the upper third of the internal jugular vein. These lymph nodes are at greatest risk for harbouring metastasis from carcinomas of the oral cavity, nasal cavity, nasopharynx, oropharynx, hypopharynx, larynx and parotid gland. A study done by Nithya et al31 stated that level II lymph nodes are most commonly involved in head-and-neck carcinomas due to proximity of the primary tumour site. Hence, we had included only level I and II lymph nodes in our study sample for this study.
The major strength of this study from a pathologic advantage point was that microscopic study of the regional lymph nodes has proven to be one of the cornerstones of the pathologist’s contribution to the cancer staging enterprise, hence contributing to treatment protocols and altering prognosis. The traditional approach to the examination of regional lymph nodes called for the pathologists to identify each individual node, remove a longitudinal section from the middle of each of those nodes and then cut and stain that section to seek light microscopic evidence of metastatic deposits. This necessity represents an incomplete examination of partial node, with the section examined serving as a proxy for the remainder of the (unexamined) node. However, a major limitation of our study is the inability to use serial/step-serial sections as it is practically impossible and cumbersome in day-to-day practice.
Owing to the importance given in recent times in altering staging, treatment options and overall patient prognosis, it may be proposed that the use of IHC marker CK cocktail (AE1/AE3) for the detection of micro-metastasis routinely for reporting in cases particularly in case of any suspicious areas in routine H & E staining, as it is highly sensitive and specific.
Financial support and sponsorship
The study was funded by the Indian Council for Medical Research (ICMR), New Delhi [grant number: 3/2/June-2017/PG-Thesis HRD (61)].
Conflicts of interest
None.
Acknowledgment:
Authors acknowledge Shrimati Hemalatha Gudagudi, Serveshri Shomshekhar, Vinayak for rendering help with their technical skills for performing the laboratory procedures.
References
- Prognostic implications of node metastatic features in OSCC: A retrospective study on 121 neck dissections. Oncol Rep. 2013;30:2697-704.
- [Google Scholar]
- Clinical and histopathologic independent prognostic factors in oral squamous cell carcinoma: A retrospective study of 334 cases. J Oral Maxillofac Surg. 2008;66:1570-9.
- [Google Scholar]
- The significance of immunohistochemically demonstrated nodal micrometastases in patients with squamous cell carcinoma of the head and neck. Laryngoscope. 2002;112:1970-4.
- [Google Scholar]
- Is it time to develop an 'ultrastaging system'for use in patients with head and neck malignancies? Laryngoscope. 2001;111:185-6.
- [Google Scholar]
- The level of cervical lymph node metastases: Their prognostic relevance and relationship with head and neck squamous carcinoma primary sites. Clin Otolaryngol Allied Sci. 1994;19:63-9.
- [Google Scholar]
- The functional neck dissection for lymph node neck metastasis in oral carcinoma. J Pharm Bioallied Sci. 2012;4:S245-7.
- [Google Scholar]
- Current advances in diagnosis and surgical treatment of lymph node metastasis in head and neck cancer. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2012;11:Doc04.
- [Google Scholar]
- Molecular staging of cervical lymph nodes in squamous cell carcinoma of the head and neck. Cancer Res. 2005;65:2147-56.
- [Google Scholar]
- Disparity in pathologic and clinical lymph node staging in oral tongue carcinoma: Implications for therapeutic decision making. Cancer. 2003;98:508-15.
- [Google Scholar]
- Expression of cytokeratins and additional markers in undifferentiated lymph node metastases of the neck. Anticancer Res. 2000;20:4931-40.
- [Google Scholar]
- Keratins in health and cancer: More than mere epithelial cell markers. Oncogene. 2011;30:127-38.
- [Google Scholar]
- Detection of cervical lymph node micrometastases in patients with squamous cell carcinoma of the oral cavity, pharynx and larynx. Acta Medica (Hradec Kralove). 2015;58:62-5.
- [Google Scholar]
- Immunohistochemical detection of cervical lymph node micrometastases from T2N0 tongue cancer. Acta Otolaryngol. 2005;125:654-8.
- [Google Scholar]
- Sentinel lymph nodes in cancer of the oral cavity: Is central step-sectioning enough? J Oral Pathol Med. 2007;36:425-9.
- [Google Scholar]
- The incidence of lymph node micrometastases in patients pathologically staged N0 in cancer of oral cavity and oropharynx. Oral Oncol. 2002;38:3-5.
- [Google Scholar]
- Detection of nodal micrometastases in head and neck cancer by serial sectioning and immunostaining. Oncology (Williston Park). 1996;10:1221-6.
- [Google Scholar]
- Micrometastasis detection using special stains in nodal tissues of oral squamous cell carcinoma A histochemical study. J Clin Diagn Res. 2016;10:ZC23-6.
- [Google Scholar]
- Occult cervical metastases: Immunoperoxidase analysis of the pathologically negative neck. Otolaryngol Head Neck Surg. 1999;120:713-7.
- [Google Scholar]
- Surveillance, Epidemiology, and End Results Program. Homepage. Available from: https://seer.cancer.gov/
- Clinicopathological prognostic implicators of oral squamous cell carcinoma: Need to understand and revise. N Am J Med Sci. 2013;5:671-9.
- [Google Scholar]
- Mechanisms of invasion in head and neck cancer. Arch Pathol Lab Med. 2015;139:1334-48.
- [Google Scholar]
- Importance of depth of invasion in patients with oral squamous cell carcinoma: A review article. J Orofac Sci. 2018;10:3-6.
- [Google Scholar]
- Effect of tumor microenvironment on pathogenesis of the head and neck squamous cell carcinoma: A systematic review. Mol Cancer. 2019;18:63.
- [Google Scholar]
- Diagnostic accuracy of various methods to detect lymph node metastases in oral squamous cell carcinoma. J Evol Med Dent Sci. 2014;3:6003-10.
- [Google Scholar]
- Does elective neck dissection in T1/T2 carcinoma of the oral tongue and floor of the mouth influence recurrence and survival rates? Br J Oral Maxillofac Surg. 2014;52:590-7.
- [Google Scholar]
- Clinico-pathological prognosticators in oral squamous cell carcinoma: An update. Transl Res Oral Oncol. 2017;2:1-14.
- [Google Scholar]
- Hypoxia and lymphangiogenesis in tumor microenvironment and metastasis. Cancer Lett. 2014;346:6-16.
- [Google Scholar]
- Patterns of cervical metastasis from carcinoma of the oral tongue. World J Surg Oncol. 2003;1:10.
- [Google Scholar]






