Translate this page into:
Polyalanine polymorphism in the signal peptide of Glutathione peroxidase 1 (GPX1) gene & its association with osteoporosis
For correspondence: Dr Richa Ashma, Department of Zoology, Savitribai Phule Pune University, Pune 411 007, Maharashtra, India e-mail: richaashma@unipune.ac.in
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Osteoporosis is a systemic skeletal disease, characterized by a low bone mass leading to increased bone fragility and hence, a greater susceptibility to the risk of fracture. Since age-related oxidative stress is one of the factors that has been implicated in developing low bone mineral density (BMD), leading to osteoporosis, this study wanted to explore the expression of antioxidant enzymes in individuals with osteoporosis. The present study focused on mapping polymorphism in an important antioxidant enzyme glutathione peroxidase 1 (GPx1) among osteoporosis and healthy Asian Indians.
Methods:
Dual-energy X-ray absorptiometry was used to assess BMD of individuals and was classified into normal (n=96) and osteoporotic (n=88) groups. Biochemical parameters such as vitamin D, total oxidant status (TOS), and GPx1 enzyme activity were estimated from plasma samples of recruited individuals. Quantitative real-time qRT-PCR was carried out using GAPDH as an endogenous control. Genomic DNA was isolated from whole blood, and polymorphisms were evaluated by sequencing.
Results:
The BMD was lower in osteoporotic individuals, and further analysis of biochemical parameters indicated significantly low 25-hydroxy vitamin D and GPx1 with higher TOS levels in osteoporotic as compared to healthy individuals. Furthermore, qRT-PCR revealed low expression of GPX1 in osteoporotic individuals. GPX1 sequence analysis of the promoter and two exons revealed the lower frequency of five alanine repeats in the osteoporotic individuals.
Interpretation & conclusions:
In this study, the in silico analysis revealed the lower frequency of five alanine repeats in exon 1 of GPX1 and high TOS to be associated with osteoporosis. However, no polymorphism was found in exon 2 of GPX1 among the two study groups.
Keywords
Glutathione peroxidase 1
osteoporosis
oxidative stress
polyalanine repeat
SNP
total oxidative stress
Osteoporosis is a multifactorial, systemic skeletal disease, characterised by deterioration of bone tissue and low bone mass with a consequent increase in bone fragility and susceptibility to fracture. It is associated with an increase in bone resorption by osteoclasts, a decrease in bone formation by osteoblasts1 and an increase in oxidative stress (OS) within osteoblasts2. More than 61 million Indians have been estimated to have osteoporosis, out of which 80 per cent are reportedly females3. Studies have also reported that older men and women with osteoporosis have lower plasma antioxidants and reduced bone mineral density (BMD)4. Decreased levels of antioxidants are known to elevate the level of reactive oxygen species (ROS) within osteoclasts which may further lead to bone resorption5,6.
Cellular OS refers to the excess production of free radicals compared to the antioxidant capacity of the cell7. The impairment caused by an increased concentration of ROS damages macromolecules such as proteins, lipids and DNA. In addition, it constitutes a stress signal that activates specific redox-sensitive signalling pathways which potentially have protective functions7. Evidence suggests that OS is responsible for the pathophysiology of ageing8 and may participate in the pathogenesis of diabetes9, atherosclerosis10, cancer11 and osteoporosis12. It has been demonstrated that free radicals modulate the differentiation of osteoblasts13 and intervene in bone resorption, thereby promoting osteoclastic differentiation in such a manner that bone resorption increases with OS14,15. One of the key enzymes of the antioxidant defence of a cell is glutathione peroxidase 1 (GPx1) encoded by GPX1 (Gene ID: 2876), located on the p arm of chromosome 3.
GPx1, a soluble selenoprotein (located in mitochondria) is one of the most abundant antioxidant enzymes found in humans. It is ubiquitously expressed in many tissues where it protects cells from OS16. This enzyme uses reduced glutathione as an essential co-substrate and reduces hydrogen peroxide (H2O2) and organic hydroperoxide to water (H2O) and corresponding alcohols16. The GPX1 coding for this enzyme has two exons which together code for 203 amino acids. Two polymorphisms in this gene have been associated with osteoporosis17. One of these is a GCG repeat polymorphism in exon 1 coding for five to seven alanine repeats, a part of signal peptide present from positions 7th to 13th nucleotide17. The second exon consists of CCC/CTC polymorphism coding for proline/leucine at 198th position17. Variation in the number of polyalanine repeats has been reported in Slovenian osteoporotic individuals17. In the same population, polyAla and Pro198Leu polymorphisms, individually and in combination, were found to be associated with BMD and therefore are proposed as useful genetic markers for osteoporosis17. The present study focused on the association of polymorphisms present in the antioxidant gene GPX1 with osteoporosis in the Asian Indian population. Genetic variants in the promoter and the two exons of this gene were screened by sequencing. Furthermore, transcript levels of GPx1 were evaluated in healthy and osteoporotic individuals. The association between biochemical parameters (total oxidant status [TOS], 25-hydroxy vitamin D and GPx1) and GPX1 polymorphism among healthy and osteoporotic groups was also investigated.
Material & Methods
This study was conducted over a period of two years in Pune district of Maharashtra, India, after approval by the Institutes Human Ethics Committee of Saishree Hospital, Pune, which follows the guidelines of the Indian Council of Medical Research. A written consent was obtained from all the participants.
Study design: A priori power analysis was carried out for two-tailed t test using G*Power 3.1.9.2 software, (Heinrich Heine University, Düsseldorf, Germany) with the α-error set at 0.05 (statistical power of 95%), to have large effect size (Ρ=0.50). The total sample size (n) was calculated to be 42 with the allocation ratio N2/N1. Both males and females were recruited for this study and belonged to the age group of 45-75 yr.
Inclusion and exclusion criteria: Before inclusion of the individuals in the study, a stringent exclusion criterion was followed. Individuals visiting multi-speciality Saishree Hospital, Aundh, Pune, as outpatients willing to participate in this study were asked to fill a questionnaire-based history form. Individuals having secondary osteoporosis or any major OS-related diseases such as cardiovascular disease, diabetes mellitus, hyper/hypotension, thyroid deficiency, bone-related diseases such as rheumatoid arthritis, bone fracture were excluded from the study. Personal and family medical histories were also obtained with relevant clinical details such as age, gender, body mass index, ethnicity, physical activity, medications and food habits (such as dietary calcium intake). Individuals with a daily non-vegetarian diet, smokers, alcoholics and those on steroid intake were excluded from the study.
Bone mineral density (BMD) estimation: The BMD was measured from the proximal femoral neck or spine in both study groups using a bone mineral densitometer known as dual-energy X-ray absorptiometry (QDR 4500 C, Hologic, Inc., Bedford, MA, USA). Hologic’s system automatically calibrates by using internal reference standards and scans the spine phantom to confirm system stability and performance and gives maximum accuracy and precision. Assuming a normal distribution of T-scores using estimated BMD, unrelated participants were classified as healthy group (n = 96) with T score > −1.1 standard deviations, or osteoporotic group (n=88) with a T score ≤ −2.5 standard deviation.
Biochemical estimation: Peripheral blood (5 ml) was collected in vials containing EDTA (ethylenediaminetetraacetic acid) or sodium heparin sulphate as an anticoagulant. Plasma was separated from the peripheral blood centrifuged within 2 h of collection and the plasma aliquots were frozen at –80°C until use.
Vitamin D levels were estimated using the COBAS e411 instrument (Roche Diagnostics, Germany) at Genesys Laboratories, Pune, India, following Electro-Chemi Luminescence ImmunoAssay method18. The reference range of vitamin D used for healthy individuals was 20-50 ng/ml. TOS estimation was carried out using Erel’s assay18 and the results were expressed as μmol H2O2/L (Merck Millipore, India). GPx1 enzyme levels were estimated using the Glutathione Peroxidase Activity Colorimetric Assay Kit (Cayman Chemicals, USA) as per the manufacturer’s instructions. The reference range of GPx1 enzyme activity was 5.3-11.5 µmole NADPH oxidised /min/g of Hb19.
RNA extraction and cDNA synthesis: Peripheral blood mononuclear cells (PBMCs) were isolated from 5 ml blood using Histopaque (Sigma-Aldrich, USA), which facilitates the recovery of a large number of viable mononuclear cells by density gradient centrifugation. Isolated PBMCs were washed twice with ice-cold phosphate-buffered saline and then frozen in TRIzol (Invitrogen, Massachusetts, USA) within 3 h of phlebotomy. Total RNA was isolated from the PBMCs of both the study groups according to the protocol developed by Chomczynski20. Isolated RNA was then quantified using a NanoDrop 1000 spectrophotometer (BioSpec-nanodrop, USA). Complementary DNA (cDNA) was synthesised by reverse transcription from total RNA (1 μg) with a Verso cDNA Synthesis Kit (Thermo Scientific, USA).
Quantitative real-time (qRT) PCR: The qRT-PCR was performed (Applied Biosystems, USA) in triplicate using cDNA and gene-specific primers for GPX1 and glyceraldehyde-6-phosphate dehydrogenase (GAPDH) was used as a reference endogenous control. Primer sequences for GPX1 were as follows: forward (5′-CAGTCGGTGTATGCCTTCTCG-3′) and reverse (5′-GAGGGACGCCACATTCTCG-3′) and GAPDH were as follows: forward (5’-GAAGGTGAAGGTCGGAGTCAAC-3’) and reverse (5’-CAGAGTTAAAAGCAGCCCTGGT-3’). PCR reaction was set using 50 ng cDNA, 10 μl SyBr® Green Premix Ex Taq™ II (Tli RNaseH Plus) (Clonetech, Japan) and 10 pmol each of forward and reverse primers in 20 µl reaction volume. For amplification of both genes, the following cycle was used: denaturation at 95°C for 30 sec, annealing at 58°C for 30 sec and extension at 60°C for 1 min. Forty cycles of amplification were performed. ΔΔCt-based fold-change calculations were carried out from raw threshold cycle data.
DNA extraction and sequencing: Genomic DNA was extracted directly from blood samples (700 ml) by standard procedures of phenol–chloroform extraction and ethanol precipitation21. DNA samples were used for PCR-based sequencing for which primers were designed using Primer3 (version 0.4.0)22. The first primer was designed for a part of a promoter sequence (-365 bps) and exon 1 (136 bps) and the second primer for exon 2 (545 bps) of GPX1. Primer sequences for the promoter and exon 1 were as follows: forward (5′-GCCAAACCCCACATCCTAACTCAG-3′) and reverse (5′-CCACATTCTCGATAAGTAGTACCT-3′) and those for exon 2 are as follows: forward (5′-CGTTTCTCTCCTCCTCTTGAC-3′) and reverse (5′-CATCTCGAGGTGGTATTTTCTGTAAG-3′). GAPDH was used as endogenous control12. Aligned DNA sequences were converted to amino acid sequences using six frames of references and the one matching the amino acid sequence of GPx1 protein from the database was selected. Amino acid sequences were aligned for osteoporotic and healthy individuals using ClustalW algorithm (BioEdit software version 7.0, Informer Technologies Inc., Los Angelas. CA, USA).
In-silico analysis: The polyalanine repeat found in GPx1 is a part of the signal peptide, which is cleaved before it is translocated to mitochondria. To check whether variation in the polyalanine repeat number had any effect on signal peptide cleavage, in silico analysis was carried out using Signal P 4.1 servers (http://www.cbs.dtu.dk/services/SignalP). The first 40 amino acids in our amplicons were compared with the reference amino acid sequence from UniProt (GPX1 ID: P07203) in osteoporotic and healthy individuals.
Statistical analysis: Mann-Whitney U test was performed for the biochemical parameters that were not normally distributed and a two-sample t test was carried out for biochemical parameters found to be normally distributed in the study groups using Minitab® 17.1.0 software, Pennsylvania USA. Pairwise correlation coefficient test between the biochemical parameters was performed to check the correlation between GPx1 enzyme activity, 25-hydroxy vitamin D, TOS and BMD among healthy and osteoporotic groups. Since individuals of two groups belonged to a common geographical area, the effects of admixture and mixed ancestry on the allele frequencies could not be ruled out, and hence for inferring population structure, STRUCTURE programme version 2.323 was employed, keeping 10000 burn-in periods and 10000 repetitions. The chromatogram quality of sequence obtained and trace score of the sequences was confirmed using ABI Seq Scanner (ver. 2.0) software (Applied Biosystems, USA). The alignment of the multiple sequences was carried out using ClustalW algorithm of the BioEdit software version 7.0 (Informer Technologies) and sequences were scanned for the presence of single-nucleotide polymorphism (SNPs)24. The presence of polymorphism was verified with the reference sequence of the National Centre for Biotechnology Information and the variation in the alanine repeat number between osteoporotic and healthy individuals was calculated by direct counting12. The reference sequence identifier used for the wild-type gene sequence was NM_001329502.1, Homo sapiens GPX1, transcript variant 3, mRNA. Pearson’s Chi-square test was carried out to assess the significant difference in the distribution of polyalanine repeat variation among healthy and osteoporotic groups. Odds ratio (OR) was estimated using McNemar 2 × 2 contingency test to assess the risk conferred by polyalanine repeat variation towards osteoporosis25. OR of 1.00 denoted no risk associated with that factor while OR more than 1.00 indicated excess risk and OR less than 1.00 a decreased risk26.
Results
Population structure and admixture: The mean alpha value of admixture in this study was estimated to be 0.071, indicating that the individuals of the study groups (both healthy and osteoporotic) belonged to an admixed population, i.e. they were of mixed ancestry (Fig. 1). The genetic distance, FST, calculated between the two populations ranged from 0 to 1.
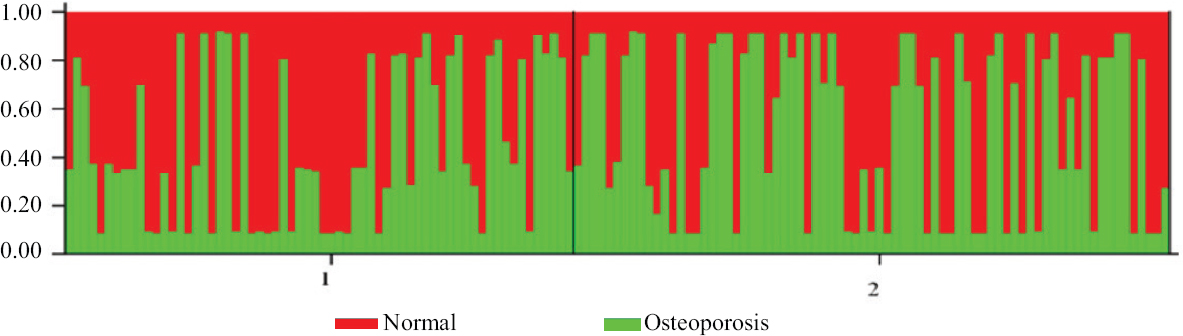
- Bar plot showing admixture for the two population clusters in the sampled population. Red: healthy individuals, Green: osteoporotic individuals. (STRUCTURE programme version 2.3 was employed, keeping 10000 burn-in periods and 10000 repetitions. The analysis is based on SNP allele frequency data of the two study groups for understanding population structure, identifying migrants and admixed individuals).
Analysis of biochemical parameters: Biochemical parameters were analysed for both healthy (mean age = 59 yr; comprising 67.7% of males) and osteoporotic (mean age = 58 yr; comprising 43.2% of males) groups. BMD was found to be lower in osteoporotic individuals as compared to healthy individuals as shown in Table I for age-matched groups belonging to the same geographical area, homogeneous ethnicity and food habits. Likewise, the osteoporotic group had significantly lower circulating 25-hydroxy vitamin D, GPx1 enzyme levels and higher TOS, as compared to a healthy group (Table I).
| Biochemical parameters | 25-OH vitamin D (ng/ml) | TOS (µmol H2O2/l) | GPx1 (µmole/min/g) |
|---|---|---|---|
| Healthy (n=96), T score=−0.16±0.66 | |||
| Mean±SD | 24.78±7.29 | 27.45±18.36 | 0.693±0.078 |
| Median | 24.00 | 23.37 | 0.693 |
| CI for median | 21.775-26.087 | 21.523-32.531 | 0.683-0.707 |
| Osteoporotic (n=88), T score=−3.18±1.25 | |||
| Mean±SD | 18.16±6.90*** | 35.54±9.61 | 0.401±0.032*** |
| Median | 16.93 | 35.80 | 0.409 |
| CI for median | 15.671-20.229 | 25.028-40.571 | 0.398-0.409 |
P ***<0.001 considered as significant. TOS, total oxidative stress; NS, non-significant; SD, standard deviation; CI, confidence interval; 25-OH vitamin D, 25-hydroxy vitamin D; H2O2, hydrogen peroxide
Association analysis of biochemical parameters with osteoporosis risk: Healthy group showed a high GPx1 activity and significantly correlated with high femoral BMD (P=0.026) when compared to osteoporotic group (Table II). However, the osteoporotic study group did not show any significant correlation between GPx1 and femoral BMD (Table II).
| Condition | Biochemical parameters | GPx1 | 25-OH vitamin D | ||
|---|---|---|---|---|---|
| Correlation coefficient | P | Correlation coefficient | P | ||
| Healthy (n=96) | TOS | −0.112* | 0.276 | 0.168 | 0.103 |
| BMD | 0.227 | 0.026 | 0.043 | 0.677 | |
| Osteoporotic (n=88) | TOS | 0.086 | 0.428 | 0.03 | 0.784 |
| BMD | −0.128* | 0.232 | −0.162* | 0.132 | |
*The inverse relationship between biochemical parameters. BMD, bone mineral density; TOS, total oxidative stress; 25-OH vitamin D, 25-hydroxy vitamin D
Analysis of GPx1 transcript and polymorphism: The analysis of GPx1 transcripts by qRT-PCR showed a 3.5-fold decrease in the expression of GPX1 in osteoporotic as compared to healthy individuals (T=2.893, P<0.05) (
Single Nucleotide Polymorphisms (SNPs) in two regions of the GPX1, one spanning part of promoter and exon 1 region and second spanning exon 2 was checked. As per the dbSNP (SNP database), there are 66 SNPs reported in each of these two exonic regions. Of the 132 SNPs reported in dbSNP (https://www.ncbi.nlm.nih.gov/SNP/snp) in the studied exon 1 and 2 regions, four SNPs were detected in our samples (viz., rs17838762, rs1800668, rs3210019 and rs1050450) with a significant difference in their allele frequency distribution (
Polyalanine repeat variations in the first exonic region of GPx1 among the study groups: Compared to the reference sequence, four alanine repeats were found in 37, five in 57, six in two healthy individuals (n=96) and four alanine repeats in 76, five in eight, six in two and seven in two osteoporotic individuals (n=88). Lower frequency of five alanine repeats was found in osteoporotic individuals (0.09) as compared to the healthy group (0.59) (P<0.001). Pearson’s Chi-square test confirmed that variation in five alanine repeats was less prevalent in the osteoporotic group (χ2 = 50.81, df = 1; P<0.001) than the healthy group (Table III). Odds ratio analysis (OR) showed that five alanine repeat variation in GPX1 was associated with excess risk for osteoporosis (OR =14.62, OR >1, 95% confidence interval: 6.35-33.62). Correlation of association Phi (Φ) was estimated to be +0.53, suggesting a positive association between five alanine repeat variations in GPX1 and osteoporosis condition.
| Condition | Prevalence of five alanine repeats | OR | 95% CI | |
|---|---|---|---|---|
| Lower limit | Upper limit | |||
| Healthy (n=96) | 57/96 | 14.62 | 6.35 | 33.62 |
| Osteoporotic (n=88) | 8/88 | |||
Data are represented as McNemar 2×2 contingency test, where χ2=50.81, P<0.0001, Φ=+0.53 represents positive association between alanine repeat variation in GPx1 and osteoporosis condition. OR >1 (14.62) indicates excess osteoporosis risk. OR, odds ratio
In silico analysis of polyalanine repeats revealed that the combined cleavage score (Y-score) was comparable between osteoporotic (0.200) and healthy (0.191) individuals. Signal peptide cleavage site was also observed to be located at the same position at 19th ‘F’ phenylalanine (Fig. 2), as per the reference sequence. Further, the probability of localization of GPx1, predicted by Target P 1.1 servers (http://www.cbs.dtu.dk/services/TargetP), was found to be similar between our amplicons and reference sequence.
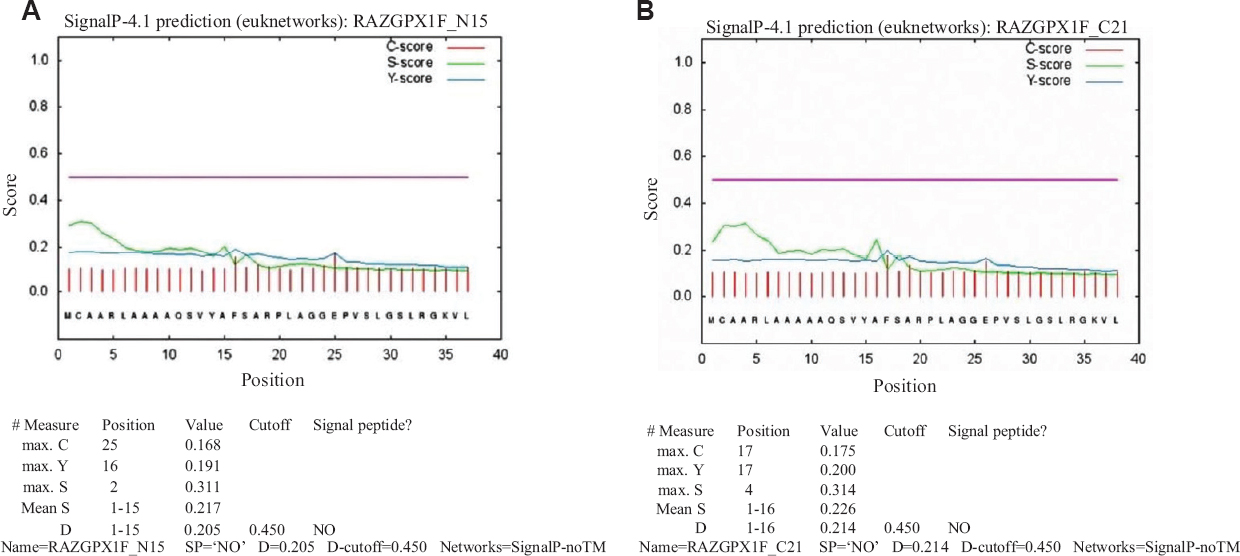
- Signal peptide cleavage site prediction in the protein sequence of (A) healthy, and (B) osteoporosis Individuals. Signal P 4.1 servers were used for the first 40 amino acids in our amplicons to compare with the reference amino acid sequence from UniProt (GPX1 ID: P07203) between osteoporotic and healthy individuals. C-score: raw cleavage site score; S-score: signal peptide score; D-score: Discrimination score; Y-score: Combined cleavage site score that predicts true cleavage. Y-score was comparable amongst osteoporotic (0.200) and healthy (0.191) individuals. GPX1, glutathione peroxidase 1
Discussion
Osteoporosis is an age-related disease with preponderance in females27. Ageing along with OS is involved in the pathogenesis of osteoporosis27. Antioxidant enzymes play a major role in overcoming OS28. Glutathione peroxidase is an important antioxidant enzyme expressed ubiquitously in many tissues29. Different forms of GPx exist, of which GPx1 localises in both cytoplasm and mitochondria and functions in the reduction of H2O2 using glutathione as a reductant29.
In the present study, age-matched healthy and osteoporotic individuals of homogenous ethnicity (Asian Indian Origin) belonging to the same geographical area, food habit and lifestyle were evaluated for population admixture. The admixture model for population ancestry was evaluated based on allele frequencies which indicated the degree of admixture within the two populations23. The model for correlated allele frequencies states that the allele frequencies between the two population clusters may be similar due to migration or recent shared ancestry23. High FST typically indicates a high degree of differentiation among the populations. However, in this study, a low mean value of FST (0.3191 ± 0.047) was obtained which indicated a recent common ancestry due to high gene flow and heterozygosity in the studied population. The two groups were compared for BMD, vitamin D, TOS and GPx1 activity. A significantly lower BMD, vitamin D and GPx1 levels and higher TOS was observed in the osteoporotic group as compared to healthy individuals supporting our hypothesis that OS contributes to the occurrence of osteoporosis. Our results are in corroboration with the previous studies4,5,12,13,30, which indicated a significant increase in the values of plasma TOS and OS index and a decrease in total antioxidant status in patients with post-menopausal osteoporosis than those of healthy individuals. Moreover, similar to our findings, these studies also reported a significant negative correlation between OS index, TOS and BMD estimated from the femoral neck region and spine4,5,12,13,30. Correlational studies using biochemical parameters suggested that the high level of GPx1 activity was significantly associated with high femoral BMD in healthy individuals, as compared to osteoporotic individuals. In addition, a 3.5-fold decrease in the expression of GPX1 and a significant decrease in plasma GPx1 activity were observed in osteoporotic individuals corroborating their involvement in the development of osteoporosis.
In the present study, polymorphism in GPX1 was screened to check for the possible association with lower enzyme activity in osteoporotic individuals. GPx1 being a cytosolic protein requires translocation to mitochondria via signal peptide. The reference peptide sequence of GPX1 consists of seven alanine repeats from positions 7th to 13th constituting part of the signal peptide17. A previous study on the Slovenian population showed that polymorphisms polyAla and Pro198Leu of the GPX1, individually and in combination, were associated with BMD and hence can be used as genetic markers for bone disease17. They found the highest values of BMD in 7/7 Ala group followed by individuals having 5/5 genotype and the lowest BMD values in the group carrying 6/6 genotype which implies that 7/7 Ala group might protect against osteoporosis. In the present study, we found the lower frequency of five alanine repeats in GPX1 of the osteoporotic group as compared to a healthy group. Pearson’s Chi-square test determined that five alanine repeat variation in GPX1 was associated with excess osteoporosis risk. This demonstrates that genetic variability in GPX1 and low GPx1 enzyme expression with high TOS is involved in the development of osteoporosis. Bioinformatics analysis of the signal peptide of GPx1 was carried out to understand its cleavage site and subcellular localization to mitochondria in osteoporotic and healthy individuals. In silico analysis revealed that signal peptide cleavage site did not change in either study group implying that variation in the number of alanine repeats in the N-terminal region of GPx1 protein sequences, did not affect cleavage of a signal peptide. Furthermore, Signal P 4.1 analysis confirmed that subcellular localization of GPx1 to mitochondria was also not influenced; indicating that deletion of the alanine residues from the signal peptide in the osteoporotic group may not influence subcellular localization of the protein to mitochondria. However, these findings need to be confirmed experimentally through biological and functional evidence in future studies. Moreover, we did not find Pro198Leu polymorphism (rs758910873) in the GPX1 which is contrary to a previous report17 or other SNPs reported in dbSNP (https://www.ncbi.nlm.nih.gov/SNP/snp).
The present study was not without certain limitations. This study could not infer causality between the biochemical parameters and osteoporosis. Although the healthy and osteoporotic groups belonged to the same environment, similar ethnicity and lifestyles, larger sample size will be required to increase the significance of the present association analysis.
Overall, in this study of Asian Indian population, low GPX1 expression, high TOS and lower frequency of five alanine repeats was observed in the osteoporotic group which was found to be associated with excess osteoporosis risk. However, in addition to GPX1, there is a need to study the genetic variants of coding and non-coding regions of other antioxidant genes and their isoforms to understand their importance in OS-induced pathogenesis of osteoporosis. These results further emphasise our understanding of genetic variation in the pathogenesis of osteoporosis.
Supplementary Fig. 1
Supplementary Fig. 1 Bar graph of qRT-PCR of GPX1 from PBMCs of healthy and osteoporosis individuals. RNA was isolated from PBMCs of healthy and osteoporotic individuals and cDNA synthesis was carried out. cDNA was subjected to qRT-PCR for GPX1 expression analysis. Statistical analysis using unpaired Student's t test. Error bars indicate mean ± SEM. P* <0.05. GPX1, glutathione peroxidase 1; qRT-PCR, quantitative real-time polymerase chain reaction; PBMCs, peripheral blood mononuclear cells; SEM, standard error of the meanSupplementary Fig. 2
Supplementary Fig. 2 Polyalanine repeat variation in (A) healthy and (B) osteoporotic individuals. DNA sequencing and sequence analysis were done using BioEdit software version 7.0. The results are expressed as the aligned sequence of amino acids and variation of polyalanine repeat shown for the individuals among healthy (a) and osteoporotic groups (b). Rows include amino acid sequences of individuals and columns are the specific amino acid position.Supplementary Fig. 3
Supplementary Fig. 3 Pro198Leu polymorphism in exon 2 of GPX1 in (A) healthy, and (B) osteoporotic individuals. The aligned nucleotide sequences were converted to amino acid sequence and variation of proline to leucine shown for the individuals amongst healthy (a) and osteoporotic groups (b) was analysed. Rows include amino acid sequences of individuals and columns are the specific amino acid position. A significant difference was not found across proline to leucine among osteoporotic and healthy individuals. GPX1, glutathione peroxidase 1.Acknowledgment:
The authors acknowledge volunteers and patients for their active participation in this study.
Financial support & sponsorship: The study was funded by the Department of Biotechnology, Government of India, and RUSA (Rashtriya Uchchtar Shiksha Abhiyan), Savitribai Phule Pune University, Pune. Author (SG) and AS received Junior Research Fellowship from University Grant Commission and department of Biotechnology, New Delhi, respectively.
Conflicts of Interest: None.
References
- Pathogenesis of osteoporosis:Concepts, conflicts, and prospects. J Clin Invest. 2005;115:3318-25.
- [Google Scholar]
- FoxO1 is a positive regulator of bone formation by favoring protein synthesis and resistance to oxidative stress in osteoblasts. Cell Metab. 2010;11:147-60.
- [Google Scholar]
- Prevalence and related risk factors of osteoporosis in peri-and postmenopausal Indian women. J Mid-Life Health. 2011;2:81-5.
- [Google Scholar]
- The association between oxidative stress and bone mineral density according to menopausal status of Korean women. Obstet Gynecol Sci. 2015;58:46-52.
- [Google Scholar]
- Association of oxidative stress with postmenopausal osteoporosis and the effects of hydrogen peroxide on osteoclast formation in human bone marrow cell cultures. Calcif Tissue Int. 2010;87:226-35.
- [Google Scholar]
- Role of mitochondrial reactive oxygen species in osteoclast differentiation. Ann N Y Acad Sci. 2010;1192:245-52.
- [Google Scholar]
- An integrated view of oxidative stress in aging:Basic mechanisms, functional effects, and pathological considerations. Am J Physiol Regul Integr Comp Physiol. 2007;292:R18-36.
- [Google Scholar]
- Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944-8.
- [Google Scholar]
- Roles of oxidative stress and redox regulation in atherosclerosis. J Atheroscler Thromb. 2009;16:532-8.
- [Google Scholar]
- New insights on oxidative stress in cancer. Curr Opin Drug Discov Devel. 2009;12:240-5.
- [Google Scholar]
- Superoxide dismutase 2 polymorphisms and osteoporosis in Asian Indians:A genetic association analysis. Cell Mol Biol Lett. 2015;20:685-97.
- [Google Scholar]
- Effects of reactive oxygen species (ROS) on antioxidant system and osteoblastic differentiation in MC3T3-E1 cells. IUBMB Life. 2007;59:27-33.
- [Google Scholar]
- Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-kappaB. Biochem Biophys Res Commun. 2004;314:197-207.
- [Google Scholar]
- A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood. 2005;106:852-9.
- [Google Scholar]
- Functional variants in the glutathione peroxidase-1 (GPx-1) gene are associated with increased intima-media thickness of carotid arteries and risk of macrovascular diseases in Japanese type 2 diabetic patients. Diabetes. 2004;53:2455-60.
- [Google Scholar]
- The antioxidant enzyme GPX1 gene polymorphisms are associated with low BMD and increased bone turnover markers. Dis Markers. 2010;29:71-80.
- [Google Scholar]
- A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem. 2004;37:112-9.
- [Google Scholar]
- Age-related changes in antioxidant enzyme activities and lipid peroxidation in lungs of control and sulfur dioxide exposed rats. Free Radic Res. 2001;34:621-7.
- [Google Scholar]
- Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156-9.
- [Google Scholar]
- Molecular cloning:A laboratory manual, Vol 16. Plainview. New York: Cold Spring Harbor Laboratory Press; 1989. p. :66.
- [Google Scholar]
- Documentation for structure software :Version 2.3.4, Vol 6. Available from:http://pritch.bsd.uchicago.edu/soft ware/readmestructur.e2.pdf
- BioEdit:A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95-8.
- [Google Scholar]
- Genetic association studies:Design, analysis and interpretation. Brief Bioinform. 2002;3:146-53.
- [Google Scholar]
- Overestimation of risk ratios by odds ratios in trials and cohort studies:alternatives to logistic regression. Cmaj. 2012;184:895-9.
- [Google Scholar]
- From estrogen-centric to aging and oxidative stress:A revised perspective of the pathogenesis of osteoporosis. Endocr Rev. 2010;31:266-300.
- [Google Scholar]
- Total oxidative/anti-oxidative status and relation to bone mineral density in osteoporosis. Rheumatol Int. 2008;28:317-21.
- [Google Scholar]






