Translate this page into:
Role of computed tomography angiography in the evaluation of haemoptysis in children: Decoding the abnormal vessels
For correspondence: Dr Priyanka Naranje, Department of Radiodiagnosis and Interventional Radiology, All India Institute of Medical Sciences, New Delhi 110 029, India e-mail: priyanka11sh@gmail.com
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Haemoptysis in children is potentially life-threatening. In most cases, the bleeding arises from the systemic circulation, and in 5-10 per cent of cases, it arises from the pulmonary circulation. The role of computed tomography angiography (CTA) in this setting is important. This study was undertaken (i) to study the role of single-phase split-bolus dual energy contrast-enhanced multidetector row CTA (DECTA) in the evaluation of haemoptysis in children; (ii) to analyze the patterns of abnormal vascular supply in the various aetiologies encountered.
Methods:
A retrospective study of 86 patients who underwent split bolus DECTA for the evaluation of haemoptysis was performed. Final diagnoses were categorized as normal computed tomography, active tuberculosis (TB), post-infectious sequelae, non-TB active infection, cystic fibrosis (CF), non-CF bronchiectasis, congenital heart disease (CHD), interstitial lung disease, vasculitis, pulmonary thromboembolism and idiopathic pulmonary haemosiderosis. Abnormal bronchial arteries (BAs) and non-bronchial systemic collateral arteries (NBSCs) were assessed for number and site and their correlation with underlying aetiologies.
Results:
A total of 86 patients (45 males, age from 0.3 to 18 yr, mean 13.88 yr) were included in the study; among these only two patients were less than five years of age. The most common cause of haemoptysis was active infection (n=30), followed by bronchiectasis (n=18), post-infectious sequelae (n=17) and CHD (n=7). One hundred and sixty five abnormal arteries were identified (108 BA and 57 NBSC), and were more marked in bronchiectasis group.
Interpretation & conclusions:
Active infections and bronchiectasis are the most common causes of haemoptysis in children. While post-infectious sequelae are less common, in patients with haemoptysis, the presence of any abnormal arteries correlates with a more frequent diagnosis of bronchiectasis. NBSCs are more common in post-infectious sequelae and CHD.
Keywords
Bronchial artery
bronchiectasis
computed tomography angiography
haemoptysis
paediatric
tuberculosis
Haemoptysis in children is potentially life-threatening, both on its own and due to the disorders causing it, and therefore requires a thorough evaluation of the airways, pulmonary parenchyma, and pulmonary as well as systemic vessels123. Multidetector row computed tomographic angiography (MDCTA) is a useful non-invasive imaging modality in this regard, as it simultaneously visualizes all the above structures and provides information which helps in diagnosis as well as guiding further management. In most cases, the bleeding arises from the systemic circulation, and in 5-10 per cent of cases, it arises from the pulmonary circulation14. Compared to adults, the common aetiologies of haemoptysis in children differ in their site of involvement, onset and chronicity; and may differ in the patterns of abnormal vessels recruited by the diseased lung23.
To the best of our knowledge, no study, thus far, looked for the distribution of abnormal vessels according to the underlying aetiology in paediatric haemoptysis. Therefore, this study was undertaken to study, (i) the role of single-phase split-bolus dual energy contrast-enhanced multidetector row computed tomography angiography (DECTA) in the evaluation of haemoptysis in children; and (ii) to analyze the patterns of abnormal systemic vascular supply in the various groups, which would be relevant to subsequent management of haemoptysis.
Material & Methods
Subjects: This was a single centre, retrospective, observational study and was undertaken after its approval by the Institutional Ethics Committee of the All India Institute of Medical Sciences, New Delhi. The radiology database was reviewed using the picture archiving and communication system to identify all patients up to 18 yr of age, who underwent DECTA for evaluation of haemoptysis as per our institutional protocol between March 2015 and February 20184. A total of 86 patients met the inclusion criteria, and were included in the study. Informed consent for the procedure was obtained from all patients at the time of admission.
Computed tomography (CT) acquisition protocol: DECTA was performed on dual-source, dual-energy 2×128-slice Computed Tomography (CT) scanner, Siemens Somatom Definition Flash (Forchheim, Germany) at 80 and 140 kVp with 64 × 0.6 mm collimation. CT was acquired from the thoracic inlet to the lower border of L2 vertebra, in order to visualize inferior phrenic and celiac arteries. Patients were administered intravenous non-ionic iodinated contrast at a dose of 1.5 ml/kg body weight, maximum of 60 ml, with 400 mg iodine/ml (Iomeprol, Iomeron 400, Bracco, Milano, Italy). The contrast was administered using a pressure injector (Medrad Stellant) Version 105.0_SH with an automated injection protocol where three-fourth of contrast administered at 3-5 ml/s, followed by one-fourth contrast at 1.5-3 ml/s, followed by saline (volume equal to one-fourth contrast) at 1.5-3 ml/s. In most patients (>5 yr), the desired flow rate was achieved. In children <5 yr, the flow rates were adjusted according to the size of cannulas. In patients who were unable to cooperate, the procedure was performed under general anaesthesia or sedation, as deemed appropriate. Scanning was started by automated bolus triggering using a circular ROI – at ascending aorta, threshold HU of 100 HU and scan delay time of five seconds. DECT was used considering its advantages such as acquisition at similar radiation doses when compared to regular CT, generation of low KVp images improving the contrast resolution of images with reduced volume of intravenous contrast requirements. Viewing high KVp images reduced the beam hardening and contrast streak artifacts.
Image processing: Mixed KVp images (40% of 140 KVp and 60% of 80 KVp) were reconstructed in the axial planes and studied on mediastinal- and lung-window settings. Evaluation in multiple planes was done on SYNGOVIA Diagnostic workstation Version VB10B-HF06. High-resolution lung window images were also viewed. CT angiogram was evaluated using thick MIP (maximum intensity projection) images generated from the low KVp dataset. Two reviewers (A.S.B and P.N. with 20 and 7 yr of experience, respectively) evaluated the scans consensually.
Data collection:
Cause of haemoptysis: The final diagnosis was made by consensus on the basis of clinical, imaging and laboratory findings. The diagnoses were categorized under the following headings; normal CT, active tuberculosis (TB), post-infectious sequelae, non-TB active infection, cystic fibrosis (CF), non-CF bronchiectasis, congenital heart disease (CHD), interstitial lung disease, vasculitis (Takayasu’s arteritis), pulmonary thromboembolism and idiopathic pulmonary haemosiderosis. Patients having two possible causes were included in both categories. For descriptive purposes, age in years of the study population was stratified into 0-<2, 2-<5, 5-<10, 10-<15 and 15-<18 yr. For statistical analysis, the above-mentioned aetiologies were clubbed into six groups, namely normal, active infection (TB + non-TB active infection), post-infectious sequelae, bronchiectasis (CF + non-CF), CHD and others (rest of the causes).
Assessment of vessels: Bronchial arteries (BAs) were defined as arteries entering the lung from the pulmonary hilum and coursing parallel to the bronchi. BAs were termed orthotopic when they originated between the superior endplate of the T5 vertebral body and the inferior endplate of the T6 vertebral body; and ectopic if these arose elsewhere. BAs were considered abnormal if (i) these were hypertrophied (>2 mm at origin) or (ii) if these were non-hypertrophied but tortuous and leading to the pathological area. Any non-bronchial systemic collateral artery (NBSC) was considered abnormal if it was hypertrophied as compared to the contralateral side and led into the pathological area. The abnormal arteries were assessed for their number and site. Pulmonary arteries and veins were assessed for any visible abnormality.
Statistical analysis: Data tabulation and its subsequent analyses were performed using the SPSS 21.0 software (SPSS Inc., Chicago, IL, USA). The variables of the groups were analyzed using Fisher’s exact test (for analysis of normal CT, sequelae, bronchiectasis, CHD, ectopic BA) and in rest, Chi-square test (for analysis of active infection or other causes) as applicable according to the sample number. A P<0.05 was considered significant.
Results
Demographics: There were 86 patients (45 males: age: 0.3 to 18 yr; mean 13.88±3.67 yr, median 15 yr) who met the inclusion criteria. Most of our patients were in the 11-15 yr age group.
Cause of haemoptysis: Age-wise occurrence of all of the causes is depicted in Table I. The most common cause of haemoptysis in the study group was active infection (Fig. 1), which was seen in 30 patients (21 TB and 9 non-TB), followed by bronchiectasis in 18 (7 CF, 11 non-CF). No cause could be identified on CT in nine patients. Among seven CHDs encountered, there were three cases of tetralogy of Fallot (TOF), two transposition of great vessels (TGA), one proximal interruption of the pulmonary artery (PIPA) and one case of ventricular septal defect with right upper lobe agenesis.
| Etiology | Age groups in (yr) | |||||
|---|---|---|---|---|---|---|
| <2 | 2-<5 | 5-<10 | 10-<15 | 15-<18 | Total (%) | |
| Normal CT | 1 | 1 | 5 | 2 | 9 (10.3) | |
| Active TB | 1 | 5 | 15 | 21 (24.1) | ||
| Non-TB active infection | 1 | 1 | 6 | 1 | 9 (10.3) | |
| Post-infectious sequelae | 10 | 7 | 17 (19.6) | |||
| CF | 5 | 2 | 7 (8.04) | |||
| Non-CF bronchiectasis | 3 | 5 | 3 | 11 (11.4) | ||
| CHD | 3 | 3 | 1 | 7 (8.04) | ||
| ILD | 1 (HP) | 1 (LCH) | 2 (2.2) | |||
| Vasculitis | 1 | 1 (1.1) | ||||
| PTE | 1 | 1 | 2 (2.2) | |||
| IPH | 1 | 2 | 3 (3.4) | |||
| Other causes | DAH: 1 | CPAM: 1; Sequesteration: 1 Scimitar syndrome: 1 | CCPA: 1; DAH: 1 | 6 (6.8) | ||
Nine patients had two causes of hemoptysis e.g., CPAM with active infection; and have been included in both the aetiological groups. CT, computed tomography; TB, tuberculosis; CF, cystic fibrosis; CHD, congenital heart disease; ILD, interstitial lung disease; HP, hypersensitivity pneumonitis; LCH, langerhan’s cell histiocytosis; PTE, pulmonary thromboembolism; IPH, idiopathic pulmonary hemosiderosis; DAH, diffuse alveolar hemorrhage; CPAM, congenital pulmonary airway malformation; CCPA, chronic cavitary pulmonary aspergillosis
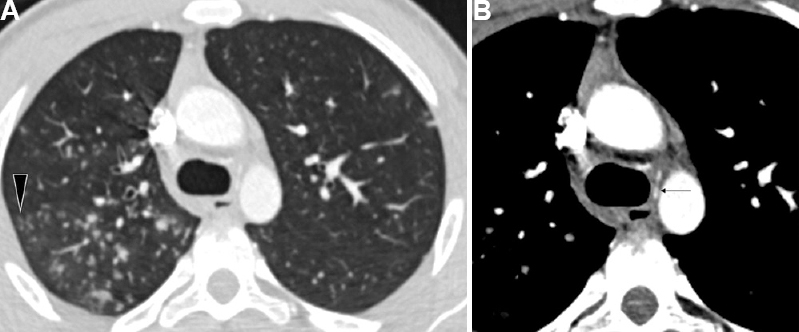
- Active tubercular infection in a 16 yr old boy presenting with haemoptysis. (A) CT lung window images show multiple tree-in-bud nodules (arrowhead). (B) DECTA (mediastinal window) revealed no hypertrophied bronchial arteries. A normal caliber common bronchial artery trunk could be identified (arrow). DECTA, dual energy contrast enhanced multidetector row computed tomography angiography; CT, computed tomography.
Comparison of patients with the presence or absence of abnormal arteries: Of the 86 children, 56 (65.1%) had detectable abnormal arteries. Among these 56 patients, 31 had enlargement of BAs alone, 25 had enlargement of both BAs and NBSCs and no patient had NBSCs alone. Patients with and without abnormal arteries did not show any difference in the age or sex distribution. A total of 165 abnormal arteries were identified (mean 1.91, median 2), out of which 108 were bronchial (mean 1.25) and 57 were ectopic (mean 0.66). Among the bronchial vessels, 99 were eutopic (mean 1.15) and nine ectopic (mean 9). Number of abnormal arteries identified per patient ranged from 0 to 7, with 30, 15, 12, 13, 4, 5, 4 and 3 patients having 0, 1, 2, 3, 4, 5, 6 and 7 arteries, respectively In patients with abnormal vessels present, the average number of abnormal arteries per patient was 2.89 (median 3).
Comparison of presence or absence of abnormal arteries and correlation with causes of haemoptysis: In patients with no identifiable abnormal arteries, the most common finding was active infection (43.3%), followed by a normal CT scan (30%). In patients with identifiable abnormal arteries, the most common finding was active infection (33.9%), followed by bronchiectasis (32.1%). The overall distribution of aetiologies of haemoptysis was different in the two groups, the difference being significant. Bronchiectasis was significantly more common in patients with abnormal arteries (P=0.002) (Table II).
| Cause | Abnormal arteries absent (n=30) | Abnormal arteries present (n=56) |
|---|---|---|
| Normal *** | 9 | 0 |
| Active infection | 11 | 19 |
| Sequelae | 3 | 14 |
| Bronchiectasis*** | 0 | 18 |
| CHD | 1 | 6 |
| Others** | 9 | 5 |
P **<0.01, ***<0.001. CHD, congenital heart disease
Comparing causes of haemoptysis in the abnormal bronchial artery (BA) only group; and abnormal bronchial artery+non-bronchial systemic collaterals (BA+NBSC) group: In the BA-only group, the most common cause of haemoptysis was active infection (41.9%), followed by bronchiectasis (38.7%). In the BA + NBSC group, the most common cause was post-infectious sequelae (40%) (Fig. 2). Analysis for individual causes revealed that, patients in the abnormal BA+NBSC group had less frequent bronchiectasis, and more frequent post-infectious sequelae as compared with those with BA’s only; and the difference was statistically significant (P=0.03 and 0.02, respectively) (Table III).
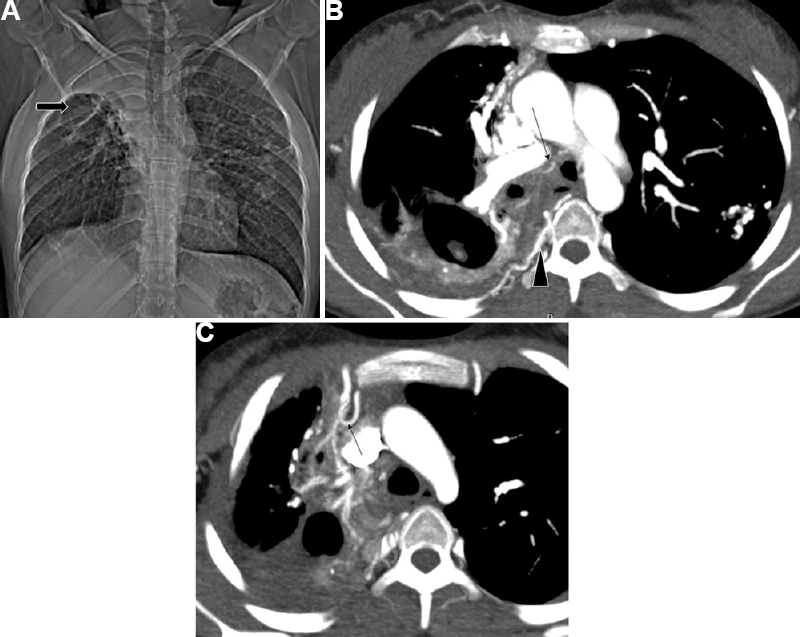
- Post-infectious sequelae in a 16 yr old girl in a case of treated tuberculosis. (A) CT topogram – Volume loss with fibrocavitary changes in right upper zone (arrow) with fibrocalcific changes in the left lung. (B and C) DECTA shows the same along with hypertrophied bronchial arteries (arrow), multiple posterior intercostal arteries (arrowhead) and branches from the right internal mammary artery (arrow in c) (non-bronchial systemic collaterals).
| Etiologies of hemoptysis | BA only | BA+NBSC |
|---|---|---|
| Post-infective sequelae* | 4 | 10 |
| Congenital heart disease | 1 | 5 |
| Bronchiectasis* | 12 | 3 |
| Active infection | 13 | 5 |
P *<0.05. BA, bronchial arteries; NBSC, non-bronchial systemic collaterals
Ectopic bronchial arteries (BAs): A total of nine abnormal ectopic BAs were identified in eight patients. Eight of these arteries arose from the subclavian arteries, and one from the arch of the aorta. Among these eight patients, bronchiectasis was present in five (62.5%), post-infectious sequelae in two (25%), CHD and TB in one (12.5%) each. One patient had both bronchiectasis and post-infectious sequelae. Bronchiectasis was more common in the abnormal ectopic BAs group, as compared to the rest of the patients with haemoptysis (16.66%) (Fig. 3). The difference being significant (P=0.0089).
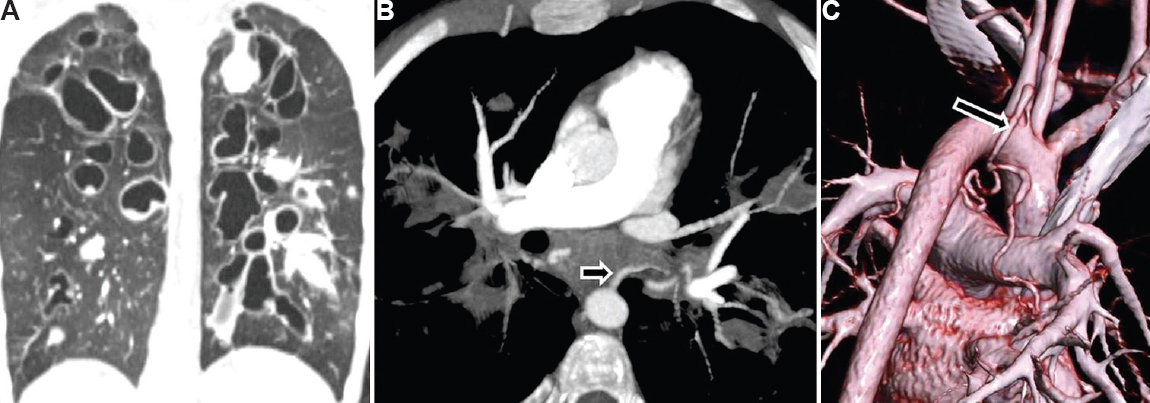
- Cystic fibrosis with bronchiectasis in a 15 yr old boy presenting with moderate haemoptysis. (A) CT lung window shows bilateral central tubular/varicose bronchiectasis with multiple mucoceles. (B) DECTA shows dilated left bronchial artery (arrow). (C) Volume rendered image shows dilated common bronchial artery (arrow) arising from arch of aorta just adjacent to origin of left subclavian artery. The child underwent bronchial artery embolization for control of haemoptysis.
Pulmonary arteries (PAs): Pulmonary artery (PA) abnormalities were found in 10 patients (11.6%). Four patients had narrowed PA due to hypoplasia/aplasia (n=2; 2.3%) or pulmonary thromboembolism (n=2; 2.3%). These patients had abnormal BAs (n=4) and NBSCs (n=6), resulting in the haemoptysis; and PA was not the source of bleeding (Fig. 4). Rasmussen’s aneurysm was encountered in two patients. Four patients also had pulmonary arterial hypertension (PAH) due to underlying cardiac or pulmonary causes.
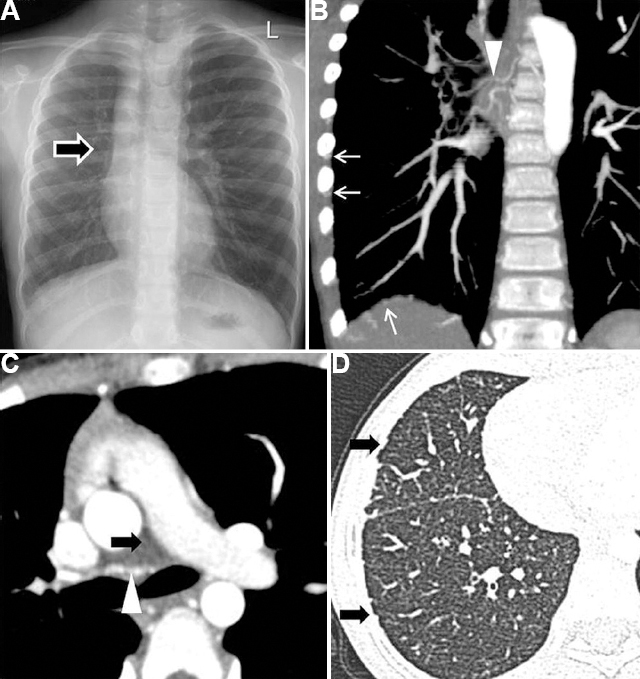
- Aplasia of pulmonary artery/proximal interruption of the right pulmonary artery in a 10 yr old girl presenting with haemoptysis. (A) CXR- Small right hemithorax with absent right hilar opacity (arrow). (B and C) DECTA - Absent right PA (black arrow) and multiple hypertrophied bronchial (arrowhead) and non-bronchial systemic collaterals from inferior phrenic and intercostal arteries (white arrow). (D) Lung window shows the irregularity of the subpleural regions and fissures due to hypertrophied systemic collaterals supplying the lung (arrows).
Discussion
The incidence of haemoptysis in the paediatric population, although less common than adults, is difficult to determine with accuracy since it may go unnoticed, as younger children may swallow the sputum and are unable to provide a clear history, or cooperate with the physical examination. Most episodes of haemoptysis in children are mild and self-limiting. Life-threatening haemoptysis in children is generally defined as a 24 h volume of >8 ml/kg2.
In our study group of 86 patients, the four most common causes observed were active infection, bronchiectasis, post-infectious sequelae and CHD. Together, these four accounted for occurrence in approximately 80 per cent of our patients. In about 10 per cent of our patients, no cause could be identified, despite exhaustive clinical and laboratory workup. These patients were labelled as idiopathic. The distribution of cases in our study is consistent with a recent meta-analysis, which also showed active infections as the most common cause of haemoptysis in children3. However, the subsequent causes differ in order and magnitude. This is likely due to the predominance of single-institution-based studies with varied demographics of the patients in the meta-analysis, spanning developed and underdeveloped countries across continents.
Infectious processes such as necrotizing pneumonia, tracheobronchitis or infected bronchiectasis lead to the destruction of lung parenchyma and erosion of blood vessels, resulting in haemoptysis. All active infections (bacterial, mycobacterial, fungal or parasitic) clubbed together accounted for approximately one-third of the study patients. This is in concordance with available literature, from developing countries3. The mean age of patients with active infection in the present study group was 14.7 yr. The mean number of abnormal bronchial, NBSC and total abnormal arteries were 1.03, 0.55 and 1.58, respectively. In this study, active TB was the most common cause among the active infections as well as overall. It was, however, present mainly in older children, majority of them being >15 yr of age.
Bronchiectasis, particularly due to CF, has been reported as the most common cause of haemoptysis in literature from developed countries, accounting for as many as 65 per cent of patients in one study5. In such patients, chronic inflammation and hypoxia were found to trigger the proliferation of the BAs. Inflammation and mechanical stresses have been found to weaken the walls of these friable and hypertrophied submucosal arteries, which then bleed into the airway67. Besides this, secondary infections can also trigger haemoptysis. In the present study, most of the patients with bronchiectasis were 11-15 yr. Further, bronchiectasis was the commonest cause of haemoptysis in patients between six and 10 yr of age. Two of these patients had underlying PCD with situs inversus. The mean number of abnormal bronchial, NBSC and total abnormal arteries was 2.41, 0.29 and 2.7, respectively, per patient.
The next largest group was of post-infectious sequelae in the form of fibro-parenchymal opacities, cavities, atelectasis, architectural distortion and traction bronchiectasis. This is in contrast to adults, where post-infective sequelae are the predominant cause of haemoptysis, especially in TB endemic regions1. Three of these patients also had associated aspergillomas. Most of these patients were between 11 and 15 yr of age and in them, it was the predominant cause of haemoptysis. The mean number of abnormal bronchial, NBSC and total abnormal arteries was 1.64, 1.29 and 2.9, respectively.
CHD causes haemoptysis due to pulmonary arterial hypertension, pulmonary venous hypertension or by causing pulmonary oligemia, which results in systemic collateralization8. Such collaterals tend to arise from the descending thoracic aorta, subclavian artery, intercostal and BAs. They supply the terminal respiratory unit and, unlike typical bronchial vessels, do not necessarily course along with the bronchial tree9. In the present study, CHD was the fourth most common cause of haemoptysis overall and most common causes of haemoptysis in patients aged 6-10 yr together with bronchiectasis. Of the seven patients of CHD, six had abnormal arteries and all of them had both BA and NBSC hypertrophy; as all of these patients had a CHD which resulted in pulmonary oligemia. In case no lung parenchymal abnormality is found, children with haemoptysis should undergo an echocardiography10.
In the present study 165 abnormal arteries were identified in 56 patients and none were identified in 30 (34.8%) of our patients. Each of these 56 patients had at least one abnormal BA, with or without additional BA or NBSC. In this study, the total number of arteries recruited increased with the increasing age of the patient, mirroring the changing aetiologies in different age groups. Systemic supply to the lung normally accounts for less than one per cent of the cardiac output but these vessels may enlarge in response to pulmonary insults to accommodate as much as 30 per cent of the cardiac output in some conditions such as chronic thromboembolic pulmonary hypertension111213.
Patients with no abnormal arteries present showed no difference in demographics from those with abnormal arteries. However, overall disease distribution showed a significant difference between the two groups. Patients who had abnormal arteries had a significantly higher prevalence of bronchiectasis, while a normal CT was significantly more prevalent in patients with no abnormal arteries. Active infection remained the most common cause in both groups1415.
Patients with abnormal NBSCs invariably had associated BA hypertrophy. We compared the disease distribution of the BA only and BA with NBSC groups and found the difference to be significant. Bronchiectasis was significantly more common in the BA-only group, while post-infectious sequelae were common in the BA+NBSC group. NBSCs have been reported in 40-62 per cent of patients undergoing BAE16171819. This study reports a smaller percentage of NBSCs than reported in literature, mainly because these studies consisted predominantly of adults with post-infectious sequelae.
Pulmonary artery is responsible for haemoptysis in 5-10 per cent of cases, and suboptimal opacification during CT will lead to a false-negative study in such cases. The application of a split bolus protocol permits consistent adequate visualization of both vascular trees with a lower contrast and radiation dose4. This radiation dose reduction is especially important in the paediatric population. The use of dual-energy acquisition enables the generation of low Kv images, which further improve the perceived luminal contrast enhancement. Khalil et al20 reported a series of 13 adult cases of pulmonary arterial source in severe haemoptysis, of which five had pseudoaneurysm, three had PA aneurysm and five had pulmonary arterial branches in the inner wall of the thick wall cavity. The study demonstrated PA abnormalities in 11.6 per cent of patients; and PA abnormalities contributing to haemoptysis (i.e. Rasmussen’s aneurysm, PTE, PA hypoplasia and PA agenesis) in 6.9 per cent, while the rest had PAH. This distribution is similar to the reported distribution in adults where PA source is responsible for haemoptysis in 5-10 per cent of cases, with Rasmussen’s aneurysm as the most common cause.
Overall, the highest number of abnormal systemic arteries was found in children with CHD, followed by post-infectious sequelae. No other study in literature comparing aetiology with the mean number of arteries in paediatric haemoptysis. Other vascular causes of haemoptysis reported in literature include pulmonary arteriovenous malformations, Dieulafoy’s lesions and fistulae; however, these are rare in the paediatric age group2122 and no such cases were identified in the present study.
This study was not without limitations. It was a single centre study from a tertiary care institute which serves as a referral centre for paediatric pulmonology and interventional radiology, and therefore may have overestimated the prevalence of some conditions. Some of the patients with trauma, chronic foreign-body aspiration and bronchial tumours who presented with haemoptysis had already undergone NCCT or CECT outside the institute, and in these, a repeat DECT was not performed due to radiation dose concerns.
In conclusion, children have a different etiological profile of haemoptysis as compared to adults, with active infections and bronchiectasis being the most common cause, while post-infectious sequelae are less common. Haemoptysis is more frequent in older children and rare below five years of age. In patients with haemoptysis, the presence of any abnormal arteries correlates with a presence of an abnormal CT diagnosis of bronchiectasis, and the presence of no abnormal arteries correlates with the presence of a normal CT. The type of abnormal vessels also differs according to aetiology; BAs, including ectopic BA, are more predominant in bronchiectasis, whereas NBSCs (invariably associated with BAs) are more frequent with other aetiologies such as post-infectious sequelae and CHDs. PA abnormalities though less frequent than systemic artery abnormalities, need careful evaluation to guide proper management, especially when embolization or surgery is considered.
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- Haemoptysis and bronchial artery embolization in children. Paediatr Respir Rev. 2008;9:95-104.
- [Google Scholar]
- Etiologies of hemoptysis in children:A systematic review of 171 patients. Pediatr Pulmonol. 2017;52:255-9.
- [Google Scholar]
- Single-phase split-bolus dual energy computed tomography angiography for evaluation of hemoptysis:A novel application. J Thorac Imaging. 2018;33:366-76.
- [Google Scholar]
- Pediatric bronchiectasis:No longer an orphan disease. Pediatr Pulmonol. 2016;51:450-69.
- [Google Scholar]
- A novel approach to the diagnosis and treatment of hemoptysis in infants:A case series. Pediatr Pulmonol. 2018;53:1504-9.
- [Google Scholar]
- Aortopulmonary collaterals in single-ventricle congenital heart disease:How much do they count? Circ Cardiovasc Imaging. 2009;2:171-3.
- [Google Scholar]
- An interventionalist's guide to hemoptysis in cystic fibrosis. Radiographics. 2018;38:624-41.
- [Google Scholar]
- Chronic thromboembolic pulmonary hypertension –Still evolving. Glob Cardiol Sci Pract. 2020;2020:e202011.
- [Google Scholar]
- Massive hemoptysis:Prediction of nonbronchial systemic arterial supply with chest CT. Radiology. 2003;227:232-8.
- [Google Scholar]
- Bronchial artery embolization for massive hemoptysis:Long-term follow-up. Asian Cardiovasc Thorac Ann. 2015;23:55-60.
- [Google Scholar]
- Bronchial artery embolization to control hemoptysis:Comparison of N-butyl-2-cyanoacrylate and polyvinyl alcohol particles. Radiology. 2013;269:594-602.
- [Google Scholar]
- Bronchial artery embolization for hemoptysis:A retrospective observational study of 344 patients. Chin Med J (Engl). 2015;128:58-62.
- [Google Scholar]
- Bronchial and nonbronchial systemic artery embolization in management of hemoptysis:Experience with 348 patients. Int Sch Res Notices. 2013;2013:1-7.
- [Google Scholar]
- Severe hemoptysis of pulmonary arterial origin:Signs and role of multidetector row CT angiography. Chest. 2008;133:212-9.
- [Google Scholar]
- Recurrent hemoptysis:A bronchial Dieulafoy's lesion in a pediatric patient. Ann Otol Rhinol Laryngol. 2021;130:528-31.
- [Google Scholar]
- A rare cause of hemoptysis in children;bronchial artery to pulmonary artery fistula in healthy child. J Pulmonol Res Rep. 2021;3:1-4.
- [Google Scholar]






