Translate this page into:
Diethylcarbamazine citrate-fortified salt for lymphatic filariasis elimination in India
Expert Review Committee
Chairperson: Dr V.M. Katoch
Members: Drs C.S. Pandav, K.N. Panicker, Rajib Dasgupta, Vasantha Muthuswamy, P. Jambulingam, Y.K. Gupta, Patrick Lammie, Nilanthi Renuka De Silva
For correspondence: Dr Manju Rahi, Division of Epidemiology & Communicable Diseases, Indian Council of Medical Research, New Delhi 110 029, India e-mail: drmanjurahi@gmail.com
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Lymphatic filariasis (LF) is a vector-borne neglected tropical disease, causing permanent disability. The disease is debilitating and widespread, leading to tremendous productivity and economic loss. The Government of India (GOI) prioritized the elimination of LF through the annual mass drug administration (MDA) programme in 2004 and continued with a single dose of diethylcarbamazine citrate (DEC), 6 mg/kg of body weight, plus albendazole annually over a period of 5-6 years. The GOI had set the target to achieve LF elimination by 2015 and now by 2030. The progress so far has been suboptimal. Much remains to be done as about 84 per cent of the total 328 endemic districts are still under MDA. The major challenge in implementing MDA is poor compliance. It is necessary to have a feasible alternative strategy addressing the above challenge to achieve the desired goal of LF elimination. At this juncture, a well-researched approach, i.e. the use of DEC-fortified salt, also advocated by the World Health Organization, as a unique form of MDA, is proposed. As per this strategy, a low dose of DEC (0.2% w/w) is added to the cooking salt at the manufacturing facility of iodized salt and consumed by the LF-endemic communities for about two years. Many examples of successful use of this strategy for LF elimination in small- and large-scale trials have been documented in India and several other endemic countries in the world. Implementing DEC–iodine-fortified salt is a safe, less expensive, more efficient and prompt approach for achieving the elimination of LF in India. Adverse effects are none or minor and self-limiting. The DEC-fortified salt strategy can easily piggyback on the existing countrywide deployment of iodized salt under the National Iodine Deficiency Disorders Control Programme (NIDDCP), which has achieved a great success in reducing iodine-deficiency disorders such as hypothyroidism. This existing robust programme can be leveraged to launch DEC-fortified salt for the community. If implemented appropriately, this strategy will ensure the complete cessation of LF transmission within two years from its introduction. If the said strategy is implemented in 2022, it is expected that India will be able to achieve the LF elimination by 2024, much before the global target of 2030.
Keywords
DEC-fortified salt
elimination
India
lymphatic filariasis
strategy
Lymphatic filariasis (LF) is a parasitic infection caused by thread-like worms, Wuchereria bancrofti, Brugia malayi and Brugia timori1. Many species of several mosquito genera transmit these parasites, viz. Anopheles, Aedes, Culex and Mansonia2. Adult parasites are primarily found in the lymphatic system of humans and produce several thousands of microfilariae (MF) released in the bloodstream. The mosquito vector picks up the MF when feeding on an infected human host. They undergo further development in the mosquito host to become infective larvae. When this infected mosquito takes the next blood meal, the infective larvae find their way into another human and develop to become adults. In this way, the life cycle of the parasite continues3. As per 2020 estimates, 863 million people were living with at the risk of LF infection in 50 countries3. At least 36 million people were suffering from chronic disease manifestations and among them, 25 million men were affected with hydrocele and 15 million with lymphoedema3.
In 1997, the World Health Organization (WHO) via the World Health Assembly Resolution, WHA 50.29, pledged to eliminate LF as a public health problem by 20203.
Lymphatic filariasis (LF) burden in India
In India, over 670 million people are at risk of infection by W. bancrofti and B. malayi parasites in 272 districts. Infection due to W. bancrofti is widespread in 21 States and Union Territories, contributing to about 99.4 per cent of the total cases4. The remaining cases are due to B. malayi, being limited to small pockets in six endemic States. About 0.48 million cases of lymphoedema and 0.18 million cases of hydrocele are line-listed in the endemic districts of the country4.
LF is a cause of disability and social stigma. The patients suffer from severe emotional stress. While this disease does not cause mortality, the severe morbidity among the infected individuals is a significant public health concern.
National Programme for Elimination of LF
The Government of India (GOI) prioritized the elimination of LF through the annual mass drug administration (MDA) programme with two drugs, a single dose of diethylcarbamazine citrate (DEC), 6 mg/kg of body weight, along with albendazole (DA) over a period of 5-6 years. This campaign is the MDA pillar of the elimination of LF programme under the National Vector-Borne Disease Control Programme (NVBDCP now NCVBDC: National Centre for Vector-Borne Diseases Control) since 2004, and so far, 17 rounds of MDA have been conducted. The National Health Policy 2002 spelt out the target of LF elimination by 2015 and later extended it twice to 2021 and now to 20304. In 21 districts, ivermectin has been added to the MDA and now is being called an IDA strategy.
All the 272 endemic districts received more than five rounds of MDA. During 2001-2018, a total of 672.72 million doses of either DEC alone or DA were delivered. Of these, only 471.03 million doses were reportedly consumed5. The reported treatment coverage ranged from 65 to 89 per cent5. By 2020, as many as 98, 87 and 42 endemic districts have cleared 1st, 2nd and 3rd transmission assessment survey5, respectively, and stopped MDA (Figure). By 2019, 0.49 million lymphoedema and 0.15 million hydrocele cases were identified through line-listing in endemic districts5. The patients are provided with the recommended minimum care package to alleviate their suffering and improve their quality of life. A total of 141,202 hydrocelectomies were performed, thereby improving the condition of the affected people5.
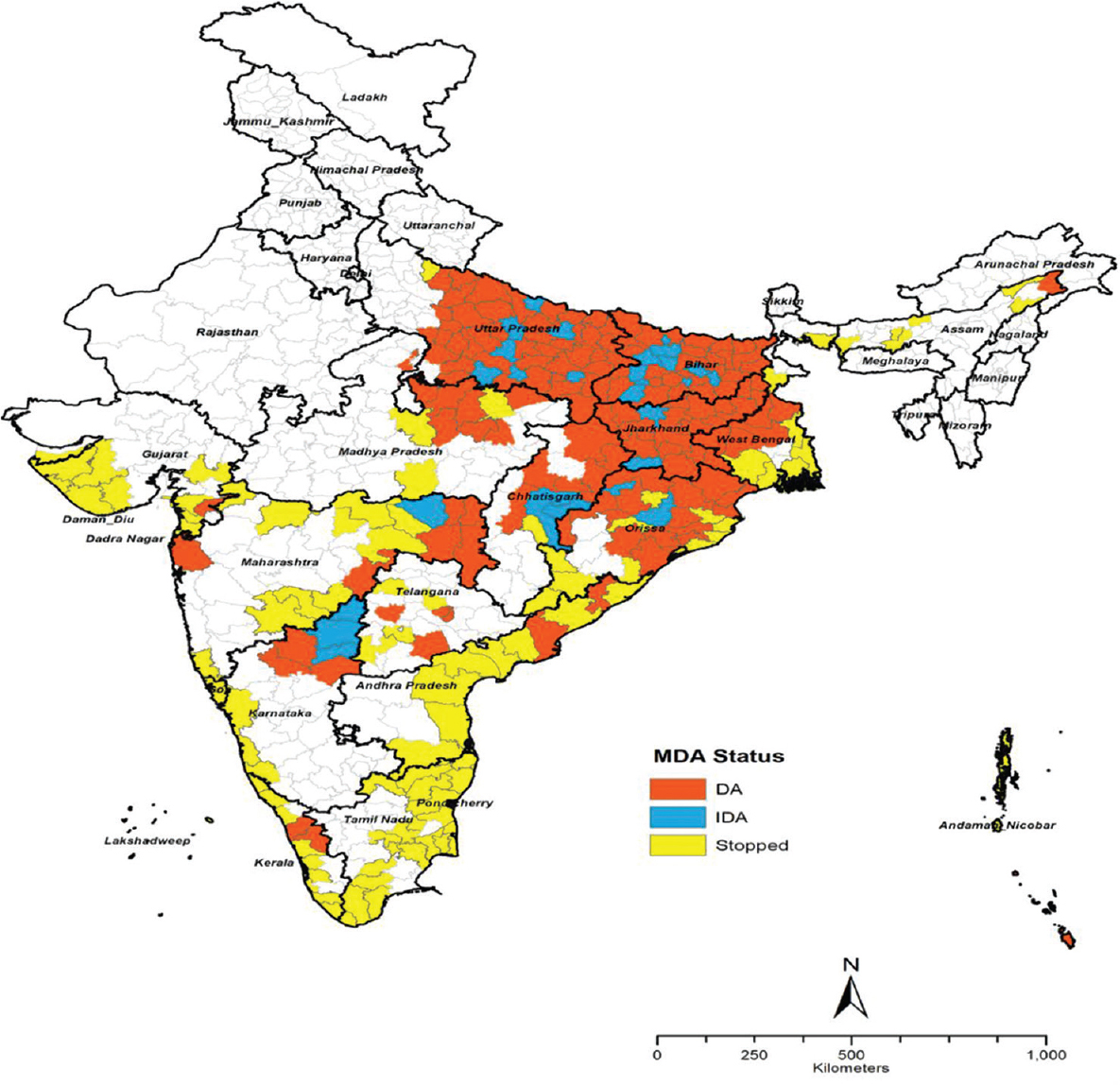
- Lymphatic filariasis endemic districts in India indicating their MDA (mass drug administration) status as on 2021. DA, districts with DEC and albendazole; IDA, districts with ivermectin, DEC and albendazole; Stopped, districs where the MDA stopped.
Problem statement
Although interruption of LF transmission requires 5-6 rounds of DA or 3-4 rounds of IDA with a minimum coverage of 65 per cent each time, it was observed that in two-thirds of districts under MDA, the LF transmission continued despite 15 rounds of MDA6. The suboptimal coverage was attributed to poor community perception, fear of adverse events, the number of antifilarial tablets, the non-availability of people at the time of MDA and population migration6. The extended period of MDA necessitates continued programmatic activities at substantial recurring costs. Further, the DA/IDA MDA ineligible population comprising pregnant women, young children and severely ill individuals remain out of the ambit of MDA, leading to continued LF transmission.
DEC-fortified salt as an adjunct strategy
Due to the impediments mentioned above, the target fixed for LF elimination was deferred twice in the past. Therefore, the need for an adjunct strategy in addition to the ongoing MDA with IDA/DA has become imperative to achieve the desired goal. In the present scenario, DEC-fortified salt appears to be the most viable and proven solution, based on the evidence generated in India and other countries7. As per the DEC-fortified salt strategy, a low dose of DEC (0.1-0.4% w/w) is added to the regular cooking salt consumed by the endemic communities for 12-24 months. DEC-fortified salt strategy is a simple, inexpensive, prompt and efficient way to eliminate LF. This strategy has many success stories from small and large community-based trials in India and other endemic countries89101112.
Pharmacokinetics: DEC gets metabolized in the liver rapidly, and diethylcarbamazine-N-oxide (an inactive form) is the metabolite13. There is no resultant toxic metabolic element. DEC has an elimination half-life of eight hours8. It is mainly excreted through the renal route (>95%) and the remaining through the faeces.
Adverse drug reactions: Adverse reactions to DEC are primarily due to the killing of MF and adult worms and not as a reaction to DEC per se8. DEC tablets (standard dose: 6 mg/kg body weight) often cause acute adverse reactions in people with high MF loads and/or adult worms. These adverse reactions are generally transient and self-limiting14.
Efficacy of DEC-fortified salt: The efficacy of DEC-fortified salt is well proven by the evidence generated by pilot studies carried out in India and other countries. The studies have shown that DEC-fortified salt reduces microfilaraemia significantly. The administration of DEC-fortified salt is simple, rapid, safe, inexpensive, efficient, prophylactic and practical for filariasis elimination815. The following evidence from India and other countries shows that LF elimination can be successfully achieved by implementing the DEC-fortified salt strategy.
Experience in India
Limited community-scale trials in India with DEC-fortified salt at the concentration ranging from 0.1 to 0.4 per cent over 3-12 months with complete coverage led to the reduction of MF rate ranging from 86 to 100 per cent161718192021. These are described below.
Pilot-scale studies:
Hill tribe settlements in Kerala: It is noteworthy that 0.2 per cent (w/w) DEC-fortified salt when provided for one year (1981-1982) to the Brugian filariasis-affected population of about 1380 in three isolated hill tribe settlements in Kerala led to the complete reduction of MF prevalence and also infection in vectors, thereby showing complete cessation of LF transmission16. In this trial, the average total consumption of DEC-fortified salt was 7.25 g/person/annum. Before the programme started, the MF rates ranged from three to eight per cent in the three study villages, and the mean MF counts ranged from seven to 14 MF/20 µl of blood. After one year of trial with DEC-fortified salt, no MF was detected in the study population, and entomological indices showed that the transmission was interrupted entirely17.
Cherthala in Kerala: In another major initiative, DEC-fortified salt (0.2% w/w) was introduced commercially in the Cherthala region of Kerala, endemic for Brugian filariasis in 199618. There was a significant reduction in the density of MF among the treated individuals. The duration required for the clearance of MF ranged from nine to 28 wk. The MF carriers developed adverse reactions, which were minor and self-limiting. However, no endemic normal reported any side effect18.
Islands of Andaman: During 2016, a two-arm study in the islands of Andaman was conducted, wherein one arm had annual MDA with DA in 14 villages, and the other arm had both annual MDA-DA and DEC-fortified salt (0.2% w/w) in 12 villages for one year19. With coverage of more than 90 per cent of households with DEC-fortified salt, the MF prevalence reduced from 2.3 to 0.14 per cent, whereas in the MDA alone arm, the MF prevalence reduced from 1.26 to 0.74 per cent. One year after the intervention, in the first arm (DA+DEC-fortified salt), all the 14 villages had <1 per cent MF rate and therefore achieved elimination, whereas four out of 12 villages continued to have MF rate above one per cent in the second arm (in which DA was used alone).
Large-scale trials: Large-scale implementation of DEC-fortified salt in programme mode was conducted in Karaikkal district of Puducherry Union Territory (UT)20 and Lakshadweep islands21, which significantly reduced MF prevalence.
Karaikkal in Puducherry: In Puducherry UT, DEC-fortified salt (0.1-0.2% w/w) programme was implemented by the then National Filaria Control Programme (NFCP) in the Karaikkal district for about four years during 1982-198620. This programme was one of the largest trials with DEC-fortified salt in programmatic mode, covering a population of 119,978 in the country, with the highest per capita consumption of DEC (13.3 g) over the entire intervention period. The quality and content of DEC in the salt were monitored every week in randomly selected sites. Initially, 0.15 per cent DEC-fortified salt was distributed for eight months and 0.2 per cent for the subsequent 38 months. This 46-month regimen of DEC-fortified salt resulted in a reduction of 97 per cent in MF rate and 72 per cent in disease rate20.
Islands of Lakshadweep: In the nine endemic islands of Lakshadweep, the DEC-fortified salt programme was implemented in 1976 by the local NFCP unit in a phased manner for 27 months with a DEC concentration ranging from 0.1 to 0.15 per cent (w/w)21. The DEC-fortified salt was distributed via private country boats to the inhabitants (population: 26,000) and also by the local administration using ships from mainland India. The average per capita total consumption of DEC was 12 g, ranging from 6.7 to 15.0 g in all these islands throughout the intervention. The MF prevalence reduced from 4.4 to 0.9 per cent in the second year of the administration of DEC-fortified salt. The vector infection rate also decreased from 0.7 to 0.07 per cent by the end of two years. The DEC-fortified salt was found to be safe, acceptable, economical and effective in decreasing the MF prevalence and density21.
Experience from other countries
Studies in Tanzania, Haiti, China and other countries with the DEC concentration ranging from 0.1 to 0.56 per cent administered over 5-12 months showed 92-100 and 96-100 per cent reduction in MF prevalence and densities, respectively222324.
Tanzania: The study in Tanzania assessed the DEC-fortified salt (0.33% w/w) strategy and three other DEC methods, i.e. the standard dose, semi-annual dose and low monthly dose. The results showed a microfilaria clearance rate of 92 per cent in the DEC-fortified salt arm, and when observed after a year of intervention, the MF clearance was by 99.9 per cent. DEC-fortified salt sustained a reduction in microfilaraemia for a longer duration than DEC tablets22.
Haiti: In the first-ever large study in 1966-1967, cooking salt fortified with 0.34 per cent DEC was provided in food to 1000 inmates of a prison in Haiti for a maximum of 415 days23. This study concluded that (i) DEC-fortified salt was acceptable to the inmates, (ii) there was no alteration of the taste of food, (iii) there were no ill effects even when it was continued for over 10 months, and (iv) it withstood cooking temperatures and produced its expected therapeutic effect in reducing the W. bancrofti MF prevalence by 95 per cent.
China: In China, the fortified salt with varying concentrations of DEC (0.24, 0.33, 0.38-0.56 and 0.5-1% w/w) was given to the communities over a period of 4-6 months. In all these studies, post-one-year intervention, the reduction in MF prevalence ranged from 94 to 100 per cent and MF density ranged from 87 to 100 per cent24.
Papua New Guinea: Comparison was made between DEC-fortified salt (0.2% w/w) and a single DEC dose (6 mg/kg) as MDA, in two communities in Papua New Guinea with baseline antigen prevalence of 55 and 71 per cent, respectively25. The salt was provided monthly to the community free of cost for a year. DEC-fortified salt was found to be more efficacious than single-dose DEC tablets25.
Guyana: The programme in Guyana was unique, where DEC-fortified salt was part of the national elimination programme. Community awareness, mobilization and engagement activities were done as preparatory activities, which paved the way to the successful launch of the salt in 2003. A huge demand was created, and quick sales of the DEC-fortified salt was recorded initially. Still, the programme suffered later because of the short supply of fortified salt, less restrictive regulatory mechanism and lack of partnership with salt producers10. Three factors that proved critical for a successful deployment included: (i) a permissive regulatory mechanism that facilitated the availability of DEC-fortified salt as a food product instead of a drug, (ii) collaboration with salt producers and importers using regular marketing channels for DEC-fortified salt introduction and sales, and (iii) creation of sustained consumer demand for DEC-fortified salt by social marketing. These could serve as a lesson for the other countries aiming to introduce DEC-fortified salt aiming to accelerate LF elimination10.
Safety
In all the large community-based field trials in India and other countries, all categories of the endemic communities were given the DEC-fortified salt without any exclusion criteria, and it was shown to be safe, cheap and effective26. Further, it was interesting to note specifically from a pilot trial conducted in China27, where the entire population (23.7 million) was administered with DEC-medicated salt and its impact on pregnant women (85), nursing women (99) and several chronically ill patients (61 cases) was observed. No abnormality was detected among 234 infants and newborns (males 122, females 112) nursed and borne by mothers who consumed DEC-fortified salt. Bodyweight, height, sitting height, chest and abdomen circumference, teeth and growth milestones were normal27. There was no accumulation of the drug following prolonged intake, and no chronic toxicity, abortifacient or teratogenic effects were reported28. These results showed that DEC-fortified salt was safe for the entire population, including pregnant women, lactating mothers, infants and chronically ill patients. A study by Freeman et al23 using salt with the double fortification of iodine and DEC showed that it could effectively interrupt the transmission of LF without eliciting severe side effects, indicating its safety.
Collateral benefits: DEC has anthelmintic activity against intestinal worms such as Trichuris trichiura2930, hookworms31 and Ascaris lumbricoides3032. DEC is found effective for the treatment of tropical pulmonary eosinophilia caused by a hypersensitivity reaction to filarial parasites8.
Need for a policy change in the elimination of Lymphatic Filariasis strategy in India
Until 2004, DEC alone was administered, and then, albendazole was added with the hope that it would enhance community compliance which did not happen33. Further, a Cochrane review has shown that albendazole, either alone or in combination with DEC or ivermectin, does not have any additional effect in the reduction of MF prevalence or density, antigenaemia and adult worms up to 12 months post-treatment34. The overwhelming focus on the easy-to-use form of DEC as tablets, the established modalities of the MDA programme, besides the albendazole availability on donation, led to neglecting of the DEC-fortified salt as an alternative strategy, regardless of its advantages and the success stories in many parts of India, China, and elsewhere. Thus, the DEC-fortified salt remained an underutilized intervention and was not scaled up, especially in India. Notwithstanding the well-established safety and efficacy profile of DEC, the non-implementation of this strategy possibly reflects a combination of perceived regulatory and implementation challenges of rolling out a drug into a food commodity for day-to-day use on a countrywide scale.
The Public Health Managers had limited experience working with private partners on a countrywide scale in the production, supply chain, stocking, distribution, public consumption and quality assurance at each step to implement DEC-fortified salt strategy. Given the country’s commitment to accelerate the LF elimination, the major stakeholders started looking at the various available options to achieve the goal of LF elimination in a short period. They are prompted to consider using the DEC-fortified salt which is aligned with the global WHO strategy.
Proposed DEC-fortified salt strategy for implementation in India
Based on the research evidence and experience gained from various trials with DEC-fortified salt, conducted over several decades in India and other countries, also leveraging the successful experience of the iodized salt strategy in the country, the DEC-fortified salt strategy is formulated and proposed here for implementation in India. The ultimate goal is to achieve LF elimination within the shortest possible time. The technology for double fortification with DEC and iodine is standardized and straightforward, and several prominent players (public and private) are already engaged in the production of iodized salt.
Studies conducted in India used DEC-medicated salt with a concentration ranging from 0.1 to 0.26 per cent were found to be safe and effective11,17-21. Further, in many of the study sites in India, the DEC concentration used was 0.2 per cent (w/w)16171819. Therefore, a 0.2 per cent (w/w) DEC concentration is proposed for fortification of the salt in the present strategy. Studies on the use of DEC-fortified salt with DEC concentrations ranging from 0.1 to 0.4 per cent DEC (w/w) have been shown to be safe and effective7.
Targeted area approach versus universal coverage approach in the implementation of DEC-fortified salt
The main debate in the roll-out of the DEC-fortified salt is whether to implement it in the known LF endemic areas or follow a universal coverage approach. The benefits of the targeted approach are (i) ease of production and distribution, (ii) the lesser burden of implementation, supervision, monitoring and evaluation, and (iii) exclusion of areas considered as non-endemic for LF to save resources. The benefits of universal coverage are: (i) that persons who move or migrate across the country and between the endemic and non-endemic areas are automatically covered, (ii) un-surveyed areas and low-endemic areas (with <1.0% MF rate) will also be covered, and (iii) issue related to suboptimal coverage is unlikely, as the entire population of the country (including the pregnant women, children and chronically ill individuals) will be consuming the food using cooking salt fortified with DEC passively. So far, there has been no report of adverse reactions in pregnant women, nursing women, children and severely ill patients262728.
Thus, the benefits of universal coverage are expected to outweigh the benefits of the targeted approach in several ways. Further, the country has the necessary infrastructure to meet the demand of the DEC-fortified salt as it depends on the existing facilities for iodized salt production and its supply chain. Furthermore, the double-fortified salt (iodine + DEC) will reach every individual in every nook and corner of the country, ensuring complete coverage and accelerating LF elimination.
The decision on the targeted versus universal approach can be deliberated upon by the National Programme and Ministry of Health and Family Welfare (MoHFW), involving all partners and stakeholders to reach a consensus, based on scientific evidence and logistical feasibility.
Implementation of adjunct strategy
A series of steps are involved in the process of implementation of this new strategy. A prerequisite for the successful implementation of DEC-fortified salt is the motivation of the community through the mass media to accept the medicated salt. Political commitment, government policy decisions and the stakeholders’ support will significantly facilitate the success of this campaign. Such campaigns should cover the entire geographical area of the country. Careful preparation of the infrastructure and logistics for such campaigns is also essential.
While implementing a programme of this dimension, barriers at bureaucratic, technocratic, political and social levels are anticipated. These bottlenecks need to be addressed by concerned stakeholders using evidence-based advocacy tools. Past country experiences indicate that a permissive regulatory environment is necessary for successful DEC-fortified salt strategy implementation. In this regard, the country’s regulatory authorities should be taken on board, right from policy formulation till implementation. Another critical issue is that DEC-fortified salt strategy needs to establish solid partnerships with salt producers and DEC suppliers. The system of iodized salt manufacturing is already in place in the country, which can be leveraged for executing this strategy. Social marketing is another critical issue to be taken into consideration. Appropriate advocacy tools also need to be developed and disseminated through various social media. A polite, consistent and convincing response to the ‘naysayers’ is essential at every stage to ensure the desired compliance of all the concerned.
Among the elements to be considered in the infrastructure are manufacturers of iodized salt and DEC, procurement in the quantities required, the supply line, the places, and methods of distribution. At this point, it is essential to ensure the smooth transition, i.e. ending of the supplies of iodized salt, which has been made mandatory to the entire country35 and switching over to the iodine-DEC double-fortified salt (switch strategy). Commercial channels already used for iodized salt in the goitre control can be used for piggybacking DEC fortification, transportation and distribution.
The ongoing MDA programme needs to be continued in the endemic areas along with DEC–iodine-fortified (DECIDE) salt, as this would accelerate the elimination further22. The scaling up of this proposed strategy in a vast country like India may take one year and may require one more year for an effective implementation of this strategy. Therefore, it is proposed to stop this strategy by the end of the second year of its implementation, following the existing monitoring and evaluation guidelines.
Financial implications of DEC-fortified salt strategy
The cost comparison between the current DA-based MDA strategy and the proposed DEC-fortified salt strategy is essential for its adoption. Therefore, the cost of double-fortified salt is compared with that of the existing DA-based MDA. The programme costs are estimated by considering the duration necessary for each strategy to reach the WHO recommended one per cent MF prevalence threshold for LF transmission interruption. The computer-simulated model determined the time, assuming a baseline prevalence of about 15 per cent36.
The cost of drugs for each round of MDA with DA is estimated as ₹ 23 per capita, and for seven years, it is ₹ 161 (estimation based on per capita cost of the drug and distribution in the MDA strategy). Therefore, the total cost of drugs for MDA for the endemic population of 450 million works out to ₹ 7250 million. The cost of DEC and fortification of salt per Kg is ₹ 4.50 and ₹ 45 per capita for two years. The cost of DEC-fortified salt for India’s entire population (1330 million) for two years will be ₹ 5720 million against ₹ 7250 million incurred for DA-MDA, and thus the cost-saving would be ₹ 1530 million. Here, drug delivery and distribution costs under MDA are not included. The cost of DEC-fortified salt is expected to be 1.3 times lesser than the cost of drugs for MDA.
Operational costs involve the cost of DEC and its fortification (which form a small proportion), advocacy, and awareness campaign. In contrast, the cost of transportation and distribution is taken care of as it is proposed to make available in the open market.
For initial 2-3 yr, deployment of DECIDE salt as an adjunct strategy will add to the financial burden of the GOI, but the cost will be compensated by the subsequent number of MDA rounds saved once the LF is eliminated in the areas where DECIDE salt would be consumed.
Monitoring and evaluation
Monitoring and evaluation are integral components of programme implementation. The purpose of coverage evaluation is to determine what percentage of households consumed DEC-fortified salt and whether the salt used in the household contained the recommended concentration of DEC. This can be done by conducting household surveys for sampling. The programme’s impact can be assessed by periodically (at three-month intervals) determining filarial infections in humans and mosquito vectors from the selected sentinel (endemic areas) and random sites (endemic and non-endemic/un-surveyed areas), preferably by an independent assessment team.
The impact assessment of the adjunct strategy may be necessary at least for five years after the cessation of the programme to ascertain the sustenance of the achievement accomplished.
Conclusion
The LF elimination programme launched in 2004 with the strategy of annual MDA of a combination of DEC and albendazole was to be administered for five years with 65 per cent coverage to achieve the set goal. However, it is being continued for the past 17 yr (initially as DA, and subsequently IDA in many districts), because of suboptimal results, mainly due to inadequate coverage/compliance. This necessitated a proven alternative/adjunct strategy that can ensure adequate coverage to achieve the goal of LF elimination in a shorter time. Common cooking salt fortified with a low dose of DEC (0.2% w/w) has been proven safe and effective in reducing the MF load in the communities in India as well as in several LF-endemic countries.
The DEC-fortified salt strategy can be run on the existing countrywide deployment of iodized salt under the National Iodine Deficiency Disorders Control Programme. Implementing this strategy is a straightforward, faster, safer, less expensive, more efficient and practical approach for achieving LF elimination in India. All the infected individuals who consume DEC-fortified salt will become infection-free by the end of two years of its introduction in the communities and ensure the complete cessation of LF transmission. Adverse effects are none or minor and self-limiting. The collateral benefits like relief from the tropical pulmonary eosinophilia and intestinal helminthic worms could also be anticipated while eliminating LF as a public health problem in the country.
Acknowledgment
The authors acknowledge the help of Dr Hari Kishan K. Raju, Sr. Technical Officer, ICMR-VCRC, in the preparation of the map and Dr V.M. Chandrasekhar, Professor, Hangal Shri Kumareshwar College of Pharmacy, Bagalkot for his inputs related to toxicology and pharamacology.
Financial support & sponsorship: This study was supported by the ICMR, New Delhi, India.
Conflicts of Interest: None.
References
- Strategies and tools for controlling/eliminating lymphatic filariasis. Bull World Health Organ. 1997;75:491-503.
- [Google Scholar]
- Lymphatic filariasis: A handbook of practical entomology for eliminating national lymphatic filariasis elimination programmes. Geneva: WHO; 2013.
- Lymphatic filariasis:Fact sheet. Available from: https://www.who.int/news-room/fact-sheets/detail/lymphatic-filariasis
- National Health Mission. National Centre for Vector Borne Diseases Control. Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India. Filaria. Available from: https://nvbdcp.gov.in/index1.php?lang=1&level=1&sublinkid=5777&lid=3691
- [Google Scholar]
- Department of Health and Family Welfare, Ministry of Health and Family Welfare, Government of India. Annual Report 2020-21. Available from: https://main.mohfw.gov.in/sites/default/files/Annual%20Report%202020-21%20English.pdf
- Coverage of, and compliance with, mass drug administration under the programme to eliminate lymphatic filariasis in India:A systematic review. Trans R Soc Trop Med Hyg. 2014;108:538-49.
- [Google Scholar]
- DEC-fortified salt for the elimination of lymphatic filariasis:A manual for program managers. Available from: https://www.gaelf.org/sites/gaelf/files/content/attachments/2019-05-14/saltmanual.pdf
- Lymphatic filariasis:The disease and its control, fifth report of the WHO Expert Committee on Filariasis. Geneva: WHO; 1992.
- Diethylcarbamazine salt in the control of lymphatic filariasis. Am J Trop Med Hyg. 1994;50:655-62.
- [Google Scholar]
- Unfulfilled potential:Using diethylcarbamazine-fortified salt to eliminate lymphatic filariasis. Bull World Health Organ. 2007;85:545-9.
- [Google Scholar]
- Diethylcarbamazine (DEC)-medicated salt for community-based control of lymphatic filariasis. Cochrane Database Syst Rev. 2007;2007:CD003758.
- [Google Scholar]
- Control of bancroftian filariasis by cooking salt medicated with diethylcarbamazine. Bull World Health Organ. 1967;37:405-14.
- [Google Scholar]
- The effect of variations in urinary pH on the pharmacokinetics of diethylcarbamazine. Br J Clin Pharmacol. 1981;12:807-12.
- [Google Scholar]
- Raju KHK, Jambulingam P. Lymphatic filariasis in India: Epidemiology and control measures. J Postgrad Med. 2010;56:232-8.
- [Google Scholar]
- Prophylactic effect of diethylcarbamazine on Wuchereria bancrofti filariasis. J Commun Dis. 1987;19:128-35.
- [Google Scholar]
- Control of Brugia malayi filariasis with common salt medicated with DEC in some hill-tribe settlements of Kerala. Indian J Med Res. 1984;79:600-3.
- [Google Scholar]
- Efficacy of diethylcarbamazine medicated salt in interrupting Brugia malayi transmission in hill tribe settlements in Kerala State. J Commun Dis. 1992;24:16-9.
- [Google Scholar]
- Efficacy of diethylcarbamazine-medicated salt for microfilaraemia of Brugia malayi. Natl Med J India. 1997;10:275-6.
- [Google Scholar]
- Elimination of diurnally subperiodic Wuchereria bancrofti in Andaman and Nicobar Islands, India, using mass DEC-fortified salt as a supplementary intervention to MDA. Parasitol Res. 2020;119:1467-83.
- [Google Scholar]
- Control of bancroftian filariasis by salt medicated with diethylcarbamazine citrate. J Indian Med Assoc. 1986;84:1-3.
- [Google Scholar]
- Control of bancroftian filariasis with common salt medicated with diethylcarbamazine in Lakshadweep. Indian J Med Res. 1981;73:865-73.
- [Google Scholar]
- Mass diethylcarbamazine chemotherapy for control of bancroftian filariasis through community participation:Comparative efficacy of a low monthly dose and medicated salt. Trans R Soc Trop Med Hyg. 1996;90:74-9.
- [Google Scholar]
- A community-based trial for the control of lymphatic filariasis and iodine deficiency using salt fortified with diethylcarbamazine and iodine. Am J Trop Med Hyg. 2001;65:865-71.
- [Google Scholar]
- Filariasis Research Group. Follow-up survey of the long-term effects of DEC medicated salt in the control of filariasis bancrofti (author's translation) Chin J Prev Med. 1979;13:200-3.
- [Google Scholar]
- Efficacy of mass single-dose diethylcarbamazine and DEC-fortified salt against bancroftian filariasis in Papua New Guinea six months after treatment. P N G Med J. 2000;43:213-20.
- [Google Scholar]
- A rational approach to the control of filariasis in India. Natl Med J India. 1993;6:114-6.
- [Google Scholar]
- Control of lymphatic filariasis in China. Geneva: WHO; 2003.
- Lymphatic filariasis:forth report of the WHO expert committee on filariasis. Geneva: WHO; 1984.
- Efficacy of albendazole and its combinations withivermectin or diethylcarbamazine (DEC) in the treatment of Trichuris trichiura infections in Sri Lanka. Ann Trop Med Parasitol. 1999;93:501-4.
- [Google Scholar]
- A comparison of the efficacy of single doses of albendazole, ivermectin, and diethylcarbamazine alone or in combinations against Ascaris and Trichuris spp. Bull World Health Organ. 2003;81:35-42.
- [Google Scholar]
- Impact of two rounds of mass drug administration using diethylcarbamazine combined with albendazole on the prevalence of Brugia timori and of intestinal helminths on Alor Island, Indonesia. Filaria J. 2005;4:5.
- [Google Scholar]
- Efficacy of DEC against Ascaris and hookworm infections in school children. Trop Med Int Health. 2001;6:739-42.
- [Google Scholar]
- Albendazole for mass drug administration to eliminate lymphatic filariasis. Lancet Infect Dis. 2006;6:684-5.
- [Google Scholar]
- Albendazole alone or in combination with microfilaricidal drugs for lymphatic filariasis. Cochrane Database Syst Rev. 2019;1:CD003753.
- [Google Scholar]
- Mean population salt consumption in India:A systematic review. J Hypertens. 2017;35:3-9.
- [Google Scholar]
- Prospects for elimination of bancroftian filariasis by mass drug treatment in Pondicherry, India:A simulation study. J Infect Dis. 2003;188:1371-81.
- [Google Scholar]






