Translate this page into:
Direct-acting antiviral agents decrease haemoglobin A1c level in patients with diabetes infected with hepatitis C virus: A systematic review & meta-analysis
For correspondence: Dr Kamolyut Lapumnuaypol, Department of Internal Medicine, Albert Einstein Medical Center, PA, USA e-mail: lapumnuaypol.k@gmail.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Several epidemiologic studies have demonstrated that type 2 diabetes mellitus (T2DM) is more prevalent in patients infected with hepatitis C virus (HCV), and the eradication of HCV has been shown to decrease the risk of T2DM. This meta-analysis was undertaken to see if treatment with direct-acting antiviral (DAA) agents would improve glycaemic control among HCV-infected patients with T2DM .
Methods:
A systematic review was conducted using MEDLINE and EMBASE databases since inception to February 2018. Eligible studies must be cohort studies that recruited HCV-infected patients with T2DM and received DAA therapy. The studies must report the change of haemoglobin A1c (HbA1c) level (before vs. after DAA therapy). Patients who achieved sustained virologic response (SVR) were included in the meta-analysis. The mean HbA1c level and standard deviation of participants were extracted from each study to calculate the mean difference (MD). Pooled MD was then calculated using the random effects model.
Results:
Four cohort studies with 2648 patients were included. Among HCV-infected T2DM patients who achieved SVR with DAA agents, the mean HbA1c level after treatment was significantly lower than the mean HbA1c level before treatment, with the pooled MD of −0.50 per cent (95% confidence interval, −0.66 to −0.34, I2 = 77%). The main limitation of this study was the lack of comparison groups. Therefore, it could not be concluded that the observed decreased HbA1c level was a direct result of DAA therapy.
Interpretation & conclusions:
Treatment with DAA agents was found to be associated with a significant reduction of post-treatment HbA1c level compared with pre-treatment HbA1c level among T2DM patients who achieved SVR.
Keywords
Diabetes mellitus
direct-acting antiviral agents
haemoglobin A1c
hepatitis C virus
meta-analysis
Hepatitis C virus (HCV) infection is a common chronic infection worldwide. It is estimated that over 185 million patients around the world have been infected with HCV, with genotype 1 being the most common genotype1. Approximately 15-20 per cent of HCV-infected patients develop cirrhosis2. HCV infection is also a risk factor for hepatocellular carcinoma, independent of cirrhosis3.
Type 2 diabetes mellitus (T2DM) is a major public health problem that affects over 400 million people globally4. Several epidemiologic studies have demonstrated that T2DM is more prevalent among HCV-infected patients5678, and eradication of HCV has been shown to decrease the risk of development of T2DM910, which is probably a result of impaired glucose tolerance and insulin resistance caused by interference of the normal glucose metabolism by HCV infection11. The exact mechanisms as to how the infection can alter the normal glucose metabolism remain unclear, but are likely to be a combination of direct viral oxidative effects, cytokines, chemokines, derangement of metabolic hormones and other immune-mediated reactions12.
The direct-acting antiviral (DAA) agents have been shown to increase the rate of sustained virologic response (SVR) in HCV infection. The newer regimens are also simpler and are associated with significantly fewer harmful adverse effects131415. DAA therapy may improve glycaemic control in HCV-infected patients who have T2DM, but the data are limited. The current systematic review and meta-analysis was conducted to explore the benefit of DAA therapy on haemoglobin A1c (HbA1c) level in patients with T2DM infected with HCV.
Material & Methods
Search strategy: Two authors (KL and CT) independently searched for potentially relevant studies in MEDLINE and EMBASE databases since inception to February 2018, using the search strategy that comprised of the terms for DAA agents. No language limitation was applied. References of the selected articles were also manually reviewed for additional eligible studies.
Inclusion and exclusion criteria: Studies that were eligible for this meta-analysis must be cohort studies (either retrospective or prospective) that recruited HCV-infected patients who also had T2DM and received DAA therapy for HCV infection. The studies must report the outcome of interest, that is, change of mean HbA1c level (before vs. after DAA therapy). Only patients who achieved SVR were included in the meta-analysis. The same two authors (KL and CT) independently reviewed and assessed the eligibility of the retrieved articles. Any disagreement was resolved through a conference with all authors. Eligible studies were further reviewed for their quality using the NIH Quality Assessment Tool for Before-After (Pre-Post) Studies With No Control Group16.
Data extraction: A standardized case record form was used to collect the following characteristics from each study: name of the first author, study location, year of publication, study design, baseline characteristics of participants, the method used to diagnose HCV infection and T2DM, DAA regimens and mean HbA1c level and standard deviation (SD) of participants before and after treatment.
Statistical analysis: The mean HbA1c level and SD of the participants were extracted from each study to calculate the mean difference (MD). If the included study provided the median and interquartile range (IQR) instead of mean and SD, the mean was estimated from the median, whereas SD was estimated from IQR/1.3517. Pooled MD was then calculated by combining MDs of each study using the random effects model17. The heterogeneity of effect size estimates across the studies was quantified using the Q statistic and I2. A value of I2 of 0-25 per cent indicates insignificant heterogeneity, 26-50 per cent low heterogeneity, 51-75 per cent moderate heterogeneity, and 76-100 per cent high heterogeneity18. Data analysis was performed using Review Manager 5.3 software from the Cochrane Collaboration (London, United Kingdom).
Results
Search results: A total of 9458 articles were retrieved from MEDLINE and EMBASE databases, in which 2127 duplicate articles were identified and removed, leaving 7331 articles for the title and abstract review. A total of 7316 articles were excluded at this stage as these did not fulfil the eligibility criteria, leaving 15 studies for full-text review. After full-text review, five studies were excluded because those did not report the outcome of interest and six studies were excluded because those included both patients with T2DM and normal but did not report the data on changes of mean HbA1c level in the T2DM subgroup. Finally, four eligible cohort studies (one prospective cohort study and three retrospective cohort studies) with a total of 2648 patients met the eligibility criteria and were included in the meta-analysis19202122. The study review and selection process are depicted in Fig. 1.
- Literature search and review process.
Characteristics of the included studies: The methodology and basic characteristics of the four included studies are summarized in the Table. Two studies were conducted in Egypt and the other two were conducted in the United States. The majority of participants had HCV genotypes 1 and 4. The DAA agent regimens used in the studies were sofosbuvir/ledipasvir, sofosbuvir/simeprevir, sofosbuvir/ribavirin, sofosbuvir/ribavirin/pegylated interferon, sofosbuvir/ledipasvir/ribavirin or boceprevir/ribavirin/pegylated interferon, paritaprevir/ritona vir/ombitasvir, dasabuvir monotherapy and sofosbuvir/daclatasvir with or without ribavirin for 12 wk.
| Characteristics | Abdel Alem et al19 | Dawood et al20 | Hum et al21 | Stine et al22 |
|---|---|---|---|---|
| Country | Egypt | Egypt | The United States | The United States |
| Study design | Retrospective cohort study | Prospective cohort study | Retrospective cohort study | Retrospective cohort study |
| Year of publication | 2017 | 2017 | 2017 | 2017 |
| Number of participants | 65 | 378 | 2180 | 25 |
| Participants | Cases were patients with chronic HCV infection who also had DM. Cases must receive DAA therapy and achieve SVR. Cases were recruited from the Kasr Al-Ainy Viral Hepatitis Center, Cairo, Egypt | Cases were patients with chronic HCV infection who also had DM. Cases must receive DAA therapy and achieve SVR. Cases were recruited from the outpatient clinics of Menoufia University Hospital from February to December 2016 | Cases were patients with chronic HCV infection who also had DM. Cases must receive DAA therapy and achieve SVR. Cases were identified from the VA Cooperate Data Warehouse which covered 167 VA medical centres and 875 clinics across the country | Cases were patients with chronic HCV infection who also had DM. Cases must receive DAA therapy and achieve SVR. Cases were identified from the database of the University of Virginia from May 2013 to April 2016 |
| Diagnosis of DM | Presence of diagnosis of DM in medical records plus current use of oral hypoglycaemic agent or insulin or twice-fasting glucose level ≥126 mg/dl (7 mmol/l) in medical records | Diagnosis of DM was made by medical record review plus direct interview of the patients | Presence of ICD-9 codes for type 2 DM (ICD-9 codes 250.00-250.92) recorded at least twice together with either a measurement of HbA1c of>6.5% or an active prescription of antidiabetic medication over the 12 months prior to treatment with DAA | Presence of ICD-9 codes for type 2 DM in medical records plus measurement of HbA1c of >6.5%, prescriptions of antidiabetic medications or a fasting glucose of >200 mg/dl |
| Diagnosis of chronic HCV infection | Presence of anti-HCV antibodies and HCV RNA | Presence of anti-HCV antibodies and HCV RNA | DAA is used as a surrogate for chronic HCV infection | Presence of anti-HCV antibodies and HCV RNA |
| HCV genotype | Genotype 4 100% | Genotype 4 100% | Genotype 1 99.3% | Genotype 1 80.8% |
| Genotype 4 0.7% | Genotype 2 15.3% | |||
| Genotype 3 3.9% | ||||
| Direct-acting antiviral agent regimen | Sofosbuvir/simeprevir, sofosbuvir/ledipasvir or sofosbuvir/daclatasvir with or without ribavirin for 12 wk | Sofosbuvir/daclatasvir with or without ribavirin for 12 wk | Sofosbuvir/ledipasvir, paritaprevir/ritonavir/ombitasvir, dasabuvir monotherapy or sofosbuvir/simeprevir | Sofosbuvir/ledipasvir, sofosbuvir/simeprevir, sofosbuvir/ribavirin, sofosbuvir/ribavirin/pegylated interferon sofosbuvir/ledipasvir/ribavirin or boceprevir/ribavirin/pegylated interferon |
| Outcome measurement | Pre-treatment HbA1c level at wk 0 compared with HbA1c level at 24 wk after the end of treatment | Pre-treatment HbA1c level at wk 0 compared with HbA1c level at 12 wk after the end of treatment | Pre-treatment HbA1c level within 12 months before the initiation of DAA compared with HbA1c level at 3 to 15 months after treatment | Pre-treatment HbA1c level at wk 0 compared with HbA1c at level 12 wk after the end of treatment |
| NIH Quality Assessment Tool16 | Fair | Fair | Fair | Fair |
HCV, hepatitis C virus; DM, diabetes mellitus; DAA, direct-acting antiviral; SVR, sustained virologic response, ICD, International Classification of Diseases; HbA1c, haemoglobin A1c; RNA, ribonucleic acid; NIH, national institutes of health
HbA1c level was measured before the treatment with DAA therapy and then after completion. The duration from completion of DAA therapy to the measurement of HbA1c level varied between studies (12 wk in two studies)2022, 24 wk in one study19 and 3-15 months in one study21. All studies were of fair quality based on the NIH Quality Assessment Tool.
Effects of direct-acting antiviral agents on HbA1c level: Among HCV-infected T2DM patients who achieved SVR, the mean HbA1c level after treatment with DAA agents was significantly lower than the mean HbA1c level before the therapy, with the pooled MD of −0.50 per cent (95% confidence interval, −0.66 to −0.34). The heterogeneity of this meta-analysis was high with an I2 of 77 per cent (Fig. 2).
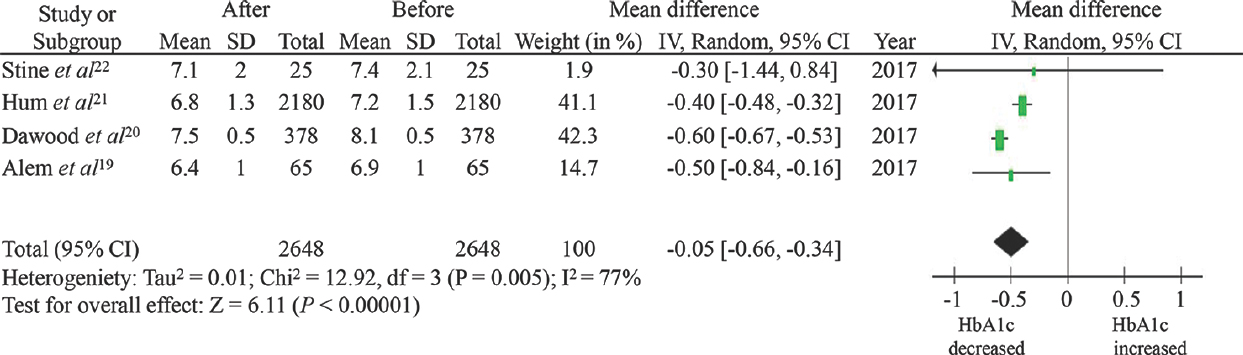
- Forest plot assessing the effect of direct-acting antiviral therapy on haemoglobin A1c level.
Discussion
It was found that among those who achieved SVR, DAA therapy was associated with lower post-treatment HbA1c level, with an average absolute decrease of 0.5 per cent across the studies. The exact mechanisms behind this observation are not known with certainty, but there are some possible explanations.
Eradication of HCV may help to restore normal glucose homeostasis as HCV infection is known to disrupt the normal glucose metabolism through insulin resistance and inflammatory cascades. One possible mechanism of insulin resistance associated with HCV infection involves the Pi3K/AKT pathway where phosphorylation after insulin signalling is crucial for the inhibition of gluconeogenesis in the liver23. HCV core protein was found to directly degrade insulin receptor substrates 1 and 2 via increased expression of tumour necrosis factor-alpha and reduced cytokine signalling, which would ultimately lead to decreased downstream phosphorylation of the Pi3K/AKT pathway24. One study found that eradication of HCV could increase the expression of insulin receptor substrates 1 and 2, which would lead to an improvement in insulin signalling and restoration of glucose homeostasis25. In fact, this glycaemic benefit is not unique to DAA therapy as previous studies have also demonstrated an improved glycaemic control among patients who achieved SVR by non-DAA agents. For instance, a large controlled study found a significant reduction of insulin resistance following SVR among HCV-infected patients who received interferon treatment26. Similar findings were also observed in other smaller studies of interferon-based therapy2728.
It is known that the use of ribavirin is associated with an increased risk of haemolytic anaemia29 and any processes that lead to the destruction of red blood cells can decrease the level of HbA1c30. Therefore, it could be possible that the apparent lower level of HbA1c among patients who received ribavirin-containing regimen was not associated with an improved glycaemic control. Nonetheless, two studies1920 included in the current meta-analysis also measured pre- and post-treatment fasting blood glucose level and found a lower post-treatment fasting blood glucose level among those who achieved SVR compared with their pre-treatment fasting blood glucose level, suggesting that the lower HbA1c level was an indicator of improved diabeties control.
The current study had several limitations related to its descriptive nature as the data included were from those who received DAA therapy and achieved SVR without a comparison group. Therefore, It could not be concluded that the observed decreased HbA1c level was a result of DAA therapy. It is possible that co-interventions, such as antidiabetic medications and lifestyle modification, were responsible for the improved glycaemic control. Similarly, it could not be known that the decreased HbA1c level was specific to DAA therapy or was associated with HCV eradication from any anti-HCV treatment. The analysis also had a high between-study heterogeneity which was probably due to difference in the baseline characteristics of the patients in the included studies, viz., background population, DAA regimen, HCV genotypes, co-intervention and duration of the study.
In conclusion, this systematic review and meta-analysis found that treatment of HCV infection with DAA agents was associated with a significant reduction of post-treatment HbA1c level compared with pre-treatment HbA1c level among T2DM patients who achieved SVR. The main limitations were the descriptive nature of the study and the high heterogeneity. Future randomized controlled studies are, therefore, needed.
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- Global distribution and prevalence of hepatitis C virus genotypes. Hepatology. 2015;61:77-87.
- [Google Scholar]
- Hepatitis C virus infection as a risk factor for hepatocellular carcinoma in patients with cirrhosis. A case-control study. Ann Intern Med. 1992;116:97-102.
- [Google Scholar]
- Worldwide trends in diabetes since 1980: A pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387:1513-30.
- [Google Scholar]
- Glucose abnormalities in patients with hepatitis C virus infection: Epidemiology and pathogenesis. Diabetes Care. 2006;29:1140-9.
- [Google Scholar]
- Hepatitis C virus infection and the development of type 2 diabetes in a community-based longitudinal study. Am J Epidemiol. 2007;166:196-203.
- [Google Scholar]
- Virus C hepatitis and type 2 diabetes: A cohort study in southern Italy. Am J Gastroenterol. 2013;108:1108-11.
- [Google Scholar]
- Sustained virological response reduces incidence of onset of type 2 diabetes in chronic hepatitis C. Hepatology. 2009;49:739-44.
- [Google Scholar]
- Sustained virological response correlates with reduction in the incidence of glucose abnormalities in patients with chronic hepatitis C virus infection. Diabetes Care. 2006;29:2462-6.
- [Google Scholar]
- Hepatitis C virus infection and type 1 and type 2 diabetes mellitus. World J Diabetes. 2014;5:586-600.
- [Google Scholar]
- Hepatitis C virus infection: Molecular pathways to metabolic syndrome. Hepatology. 2008;47:2127-33.
- [Google Scholar]
- Benefit-risk assessment of new and emerging treatments for hepatitis C: Focus on simeprevir and sofosbuvir. Drug Healthc Patient Saf. 2014;6:37-45.
- [Google Scholar]
- Impact of direct acting antiviral therapy in patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;64:1224-31.
- [Google Scholar]
- HCV direct-acting antiviral agents: The best interferon-free combinations. Liver Int. 2014;34(Suppl 1):69-78.
- [Google Scholar]
- National Heart Lung, and Blood Institute, Study Quality Assessment Tools. Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- Selecting studies and collecting data. In: Higgins JPD, Thomas J, eds. Cochrane handbook for systematic reviews of interventions. Chichester (UK): John Wiley & Sons; 2008. p. :176.
- [Google Scholar]
- Improvement of glycemic state among responders to sofosbuvir-based treatment regimens: Single center experience. J Med Virol. 2017;89:2181-7.
- [Google Scholar]
- Factors associated with improved glycemic control by direct-acting antiviral agent treatment in Egyptian type 2 diabetes mellitus patients with chronic hepatitis C genotype 4. Diabetes Metab J. 2017;41:316-21.
- [Google Scholar]
- Improvement in glycemic control of type 2 diabetes after successful treatment of hepatitis C virus. Diabetes Care. 2017;40:1173-80.
- [Google Scholar]
- Effect of treatment with direct acting antiviral on glycemic control in patients with diabetes mellitus and chronic hepatitis C. Ann Hepatol. 2017;16:215-20.
- [Google Scholar]
- Hepatitis C eradication with sofosbuvir leads to significant metabolic changes. World J Hepatol. 2016;8:1557-63.
- [Google Scholar]
- Assessing cardiovascular risk in hepatitis C: An unmet need. World J Hepatol. 2015;7:2214-9.
- [Google Scholar]
- Effect of sustained virological response to treatment on the incidence of abnormal glucose values in chronic hepatitis C. J Hepatol. 2008;48:721-7.
- [Google Scholar]
- Viral clearance is associated with improved insulin resistance in genotype 1 chronic hepatitis C but not genotype 2/3. Gut. 2012;61:128-34.
- [Google Scholar]
- Clearance of HCV improves insulin resistance, beta-cell function, and hepatic expression of insulin receptor substrate 1 and 2. Am J Gastroenterol. 2007;102:570-6.
- [Google Scholar]
- Reduction of insulin resistance with effective clearance of hepatitis C infection: Results from the HALT-C trial. Clin Gastroenterol Hepatol. 2010;8:458-62.
- [Google Scholar]
- Hemolytic anemia induced by ribavirin therapy in patients with chronic hepatitis C virus infection: Role of membrane oxidative damage. Hepatology. 2000;31:997-1004.
- [Google Scholar]
- What do we need beyond hemoglobin A1c to get the complete picture of glycemia in people with diabetes? Int J Med Sci. 2012;9:665-81.
- [Google Scholar]






