Translate this page into:
Relationship between type of smokeless tobacco & risk of cancer: A systematic review
For correspondence: Dr Sanjay Gupta, Division of Cytopathology, ICMR-National Institute of Cancer Prevention & Research, I-7, Sector-39, Noida 201 301, Uttar Pradesh, India e-mail: sanjaydr17@hotmail.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Causative linkages of smokeless tobacco (SLT) use with oral potentially malignant disorders and cancers of oral cavity, oesophagus and pancreas have been reported. Published meta-analyses have provided pooled risk estimates for major cancers caused by SLT, both on global and regional levels. This systematic review was aimed at summarizing the available studies on occurrence and mortality risk of common cancers due to various SLT products.
Methods:
PubMed and Google Scholar databases were systematically searched from 1985 till January 2018 for observational studies on SLT and cancer. The included studies were evaluated and data were extracted and reviewed.
Results:
The review included 80 studies providing 121 risk estimates for various cancers. Majority of the studies from South-East Asian Region (SEAR) and Eastern Mediterranean Region (EMR) showed a significant positive association of SLT use with oral [odds ratio (OR) ranging from 1.48 to 27.4] and oesophageal cancers (OR between 2.06 and 12.8), while studies from European Region (EUR) reported a positive association with pancreatic cancer (OR between 1.6 and 2.1). Cancer-related mortality was evaluated in a few reports with higher risk of mortality for lung (OR between 2.0 and 9.1), cervical (OR 2.0) and prostate (OR 2.1) cancers. A wide variation was noted in the association of various cancers and specific SLT products based on their nature, methods of use and inherent toxicity. The majority of chewing tobacco products displayed higher risk for oral and oesophageal cancers while the same was not observed for snus.
Interpretation & conclusions:
This review emphasizes on the significantly positive association of SLT use with oral and oesophageal cancers in SEAR and EMR and pancreatic cancer in EUR. Mortality estimates for SLT-associated cancers need further analysis. Risk analysis for cancers of other sites in SLT users also requires multicentric well-designed studies.
Keywords
Cancer
mortality
occurrence
oesophagus
oral
pancreas
pharynx
smokeless tobacco
Smokeless tobacco (SLT) consumed orally or nasally has been in use for as long as other forms of tobacco. Research studies conducted over years have shown linkage of SLT use with oral potentially malignant disorders and cancers of oral cavity, oesophagus and pancreas along with possible contributory role in cardiovascular disease, hypertension, peptic ulcer and foetal morbidity and mortality1.
SLT products are known to contain more than 30 carcinogens, including tobacco-specific N-nitrosamines (TSNAs), nitrite, nitrate and heavy metals such as nickel, cadmium and chromium2. The levels of these carcinogens vary widely among the SLT products consumed in different countries. The additives used in these products leading to changes in toxicity and associated health risks also differ in various geographic regions. This hinders the comparability of results of various studies evaluating the health effects of SLT use3.
A conceptual model of SLT-associated carcinogenesis postulates that carcinogens present in SLT products are ingested and processed, leading to metabolic activation of carcinogens. The carcinogens cause formation of DNA adducts and subsequent mutations in K-ras, p53 and other genes, leading to uncontrolled cell growth. Other changes, including chronic local inflammation, oxidative stress and formation of reactive oxygen species, may also contribute to tumour promotion4. Mechanisms such as activation of Akt and protein kinase A lead to reduced apoptosis and increased angiogenesis and cellular transformation. Apart from TSNAs, other compounds present in SLT products such as polycyclic aromatic hydrocarbons and areca nut may also contribute to causation of cancer in SLT users. Epigenetic pathways, such as promoter methylation of tumour-suppressor genes leading to unregulated proliferation, are also speculated to be involved in SLT-related carcinogenesis5.
Summary risk estimates of cancer occurrence have shown a higher risk of oral cancer [risk ratio (RR) 3.43, 95% confidence interval (CI) 2.26-5.19], pharyngeal cancer (2.23, 95% CI 1.55-3.20) and oesophageal cancer (2.17, 95% CI 1.70-2.78) in SLT users6. However, regional variation in this risk has also been demonstrated. Risk for mortality due to cancers of upper aerodigestive tract (UADT), stomach and uterine cervix has also been shown to be significantly higher with SLT use7. This systematic review was undertaken to summarize the available studies (categorized into WHO-defined Regions) on cancer occurrence as well as mortality risk in users of SLT products.
Material & Methods
A systematic literature search was conducted in PubMed and Google Scholar databases for articles on SLT-associated cancers published since 1985 till January 2018 using the search terms ‘smokeless tobacco,’ ‘chewing tobacco,’ ‘snus,’ ‘snuff,’ ‘khaini,’ ‘gutka,’ ‘toombak,’ ‘shammah,’ ‘tuibur’ and ‘cancer’ or ‘neoplasm.’ The PRISMA guidelines were followed8. The flow chart shows the search strategy used (Figure). Cross-references of all included articles were also examined for additional studies.
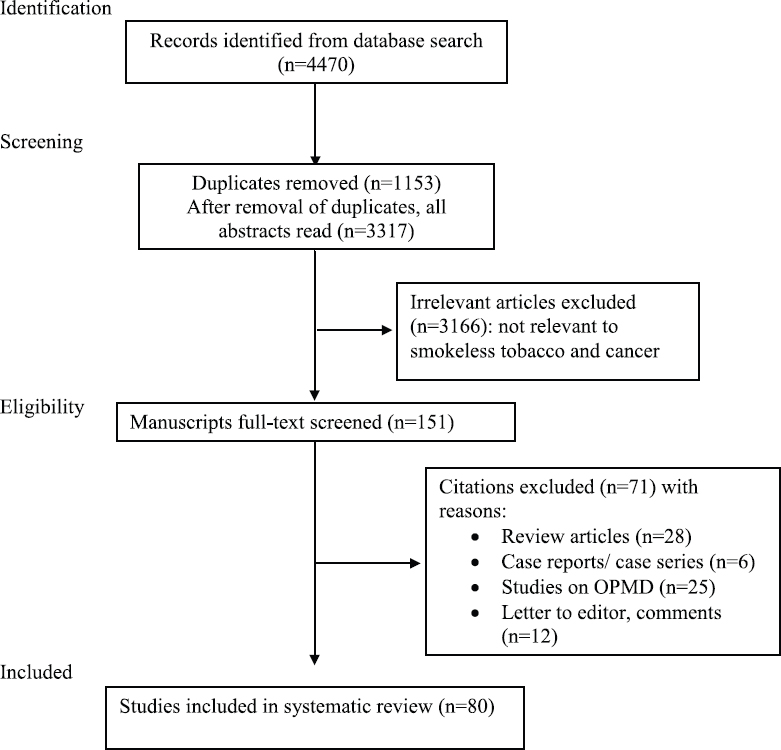
- Flow chart showing search strategy for studies on association of SLT with cancer. OPMD, oral potentially malignant diseases.
Inclusion criteria: (i) Articles published in English language or published in other languages with summary having detailed results available in English; (ii) Case-control or cohort studies including any age group and either or both gender and total sample size of at least 100; (iii) Exposure variable: SLT in one of its various forms; (iv) Outcome: Cancer of oral cavity, nasal cavity, pharynx, larynx, oesophagus, stomach, lung, uterine cervix, breast, prostate, urinary bladder, kidney, penis, brain, skin, colon and rectum; leukaemia/ lymphoma, multiple myeloma; sarcoma; and (v) Risk estimate: Estimates for combined exposure or individual SLT products were extracted. Gender-wise estimates were noted, where available.
Exclusion criteria: Case series, case reports, letters or reviews, reports of only precancerous lesions, duplicate data, and reports of chewable products without tobacco were excluded.
Data extraction: Each article was subjected to quality assessment by two authors. Data regarding type of study, location, sample size, publication year, exposure variable, outcome definition and risk estimates with 95 per cent CIs were extracted for risk of occurrence and mortality and compared. Any disparities were resolved by deliberations and final decision was reached by mutual consensus. Risk estimates were also recorded for different SLT products, wherever available.
Results
The initial search yielded 4470 articles, of which 80 studies providing 121 risk estimates for various cancers were included in this review. Of these, 47 were conducted in WHO South-East Asian Region (SEAR, 46 in India, 1 in Indonesia), 12 in European Region (EUR), 11 in American Region (AMR), eight in Eastern Mediterranean Region (EMR) and two in African Region (AFR). No studies were retrieved from Western Pacific Region (WPR).
Smokeless tobacco (SLT) and cancer occurrence risk
Oral cancer: Risk of occurrence of oral cancer has been extensively assessed for the association with SLT; 33 studies (22 from SEAR, 5 EMR, 3 EUR, 2 AFR and 1 AMR) were retrieved in the selected time period (Table I). Majority of these studies have been case-control (28 of 33) while only five (three from SEAR and two from EUR) were cohort studies.
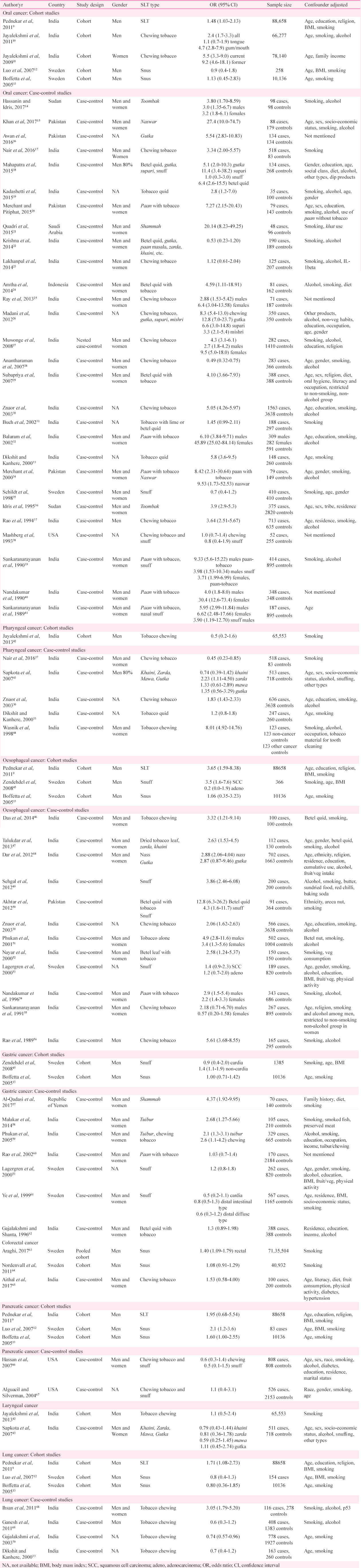
Cohort studies: Of the five cohort studies evaluating risk of oral cancer in SLT users, all three from SEAR91011 showed a significant positive association with SLT intake while both studies from EUR1213 did not show this positive association. Of the four studies mentioning SLT product, two studies evaluating risk of oral cancer in snus users did not find an increased risk of occurrence of oral cancer1213 while both the studies evaluating risk with tobacco chewing reported higher risk of oral cancer in chewers1011. Four of these five studies adjusted for smoking as a confounding factor.
Case-control studies: Nineteen (19) case-control studies were retrieved from SEAR, of which 16 reported a significant positive association with the use of SLT products17181924252627293031323337394041 while the remaining three did not concur with this association222328. The single studies from EUR35 and AMR38 did not detect any significant positive association of oral cancer with SLT use. All five studies from EMR1516202134 and two from AFR1436 demonstrated significantly higher risk of oral cancer in SLT users.
Seven studies gave separate estimates for males and females, and found significantly higher risk of oral cancer both in male and female SLT users14252732394041. Some studies demonstrated a higher risk of cancer in female users [odds ratio (OR) ranging between 3.2 and 45.89] compared to males in the same study (OR ranging from 2.7 to 9.33).
There were 30 estimates mentioning the type of SLT product - 22 on chewing products, five on snuff, two on toombak and one on naswar. One study evaluated the risk of oral cancer with naswar as well as the use of paan with tobacco. Of the 22 studies assessing risk of oral cancer with chewing tobacco products, 15 specified the product including gutka, betel quid, paan with tobacco, zarda, khaini and mishri. Fourteen studies reported a significant positive association between oral cancer and SLT product while one study did not find similar association22. The remaining seven studies mentioned only tobacco chewing in the exposure variable without specifying the product type; of these, four demonstrated significantly higher risk of oral cancer in chewers while three did not find any similar association. Both the studies including toombak users and two estimates for risk of oral cancer in naswar users reported significant positive association14153436. Snuff was evaluated in five studies; two found significantly higher risk of oral cancer in users4041 while three studies did not report similar risk183538. Of the 28 case-control studies, eight did not adjust for smoking as a confounding variable.
Cancer of pharynx (excluding nasopharynx): Six studies (Table I) were found for risk of occurrence of pharyngeal cancer (all from SEAR173033424344) in SLT users. There was one cohort study42 while the rest five were case-control in design1730334344. All these studies evaluated this association with chewing tobacco. Three studies did not report significant association with SLT use173342 while two showed positive association3044. In the study by Sapkota et al43, positive association was found only with zarda while the same was not true for khaini, mawa and gutka. Six of seven studies were adjusted for smoking.
Oesophageal cancer: Risk of oesophageal cancer in SLT users has been evaluated in 15 studies (11 from SEAR930464748495152545556, three EUR134553 and one EMR50). Only three were cohort91345 while the rest 12 were case-control studies304647484950515253545556. Of the cohort studies, one report each from SEAR and EUR showed significant positive association between SLT use and oesophageal cancer945. The third study from EUR did not find an increased risk of oesophageal cancer in snus users13.
Nine of ten case-control studies from SEAR demonstrated a higher risk of oesophageal cancer in SLT users304647484951525456 while one study did not report any similar risk55. The study from EMR reported a significant positive association between SLT use and oesophageal cancer50 while the report from EUR53 did not find a positive association. Ten studies evaluated chewing tobacco - six specifying the product including zarda, khaini, gutka, betel quid, tobacco alone or paan with tobacco. Of these six studies, five found significantly higher risk of oesophageal cancer in tobacco users while one did not report similar association with gutka though this study found positive association of nass chewing and oesophageal cancer48. On the other hand, three studies evaluated snuff; two of these (from SEAR49 and EMR50) revealed significantly higher risk of oesophageal cancer in snuff users while the study from EUR53 did not report similarly higher risk of cancer. Smoking was adjusted as a confounding variable in 14 studies while alcohol was adjusted in only nine studies (Table I).
Gastric cancer: Of the nine studies included, four were conducted in SEAR58596062, four in EUR13455361 and one in EMR57, as depicted in Table I. Of these, two were cohort studies1345 while seven were case-control in design535758596162. Of the cohort studies, the report by Zendehdel et al45 showed significant positive association of cancer of non-cardia part of stomach with SLT use while the same was not found for cancers in the cardia region. The other cohort study did not find increased risk of gastric cancer in snus users13. Among the case-control studies, report from EMR (shammah users)57 and those from SEAR evaluating the effect of tuibur intake5859 reported a significantly higher risk of gastric cancer. However, the studies including users of chewing tobacco (shammah, paan with tobacco, betel quid) or snuff did not reveal significantly positive association with gastric cancer53606162.
Colorectal cancer: Three studies (one pooled cohort63 one cohort64, and one case-control65) were retrieved evaluating risk of colorectal cancer in SLT users. Of these, only one study with pooled cohort reported a significantly higher risk of rectal cancer in snus users. However, risk of colon cancer was not found to be higher in SLT users in any of the studies (Table I).
Pancreatic cancer: Five studies (two EUR1213, two AMR6667 and one SEAR9) have assessed the risk of risk of occurrence of pancreatic cancer in SLT users (Table I). Three studies were cohort91213 while two were case-control reports6667. Two cohort studies, both from EUR1213, reported significant positive association between snus use and pancreatic cancer. The third cohort study as well as both case-control studies did not find a similar association96667. All the five studies were adjusted for smoking as a confounding factor.
Respiratory cancer: Two studies evaluated association of SLT with laryngeal cancer (both SEAR4243) and both studies (subjects consuming chewing tobacco) reported lack of significant positive association of SLT with cancer of larynx (Table I).
Lung cancer was evaluated in three cohort91213 and four case-control studies33686970. One of the cohort (SLT type not specified9) and one of case-control studies (assessing chewing tobacco68) demonstrated significant positive association of lung cancer with SLT use. The other cohort and case-control studies failed to detect similar association between SLT use and lung cancer (Table I). All the seven studies were adjusted smoking as a confounding variable.
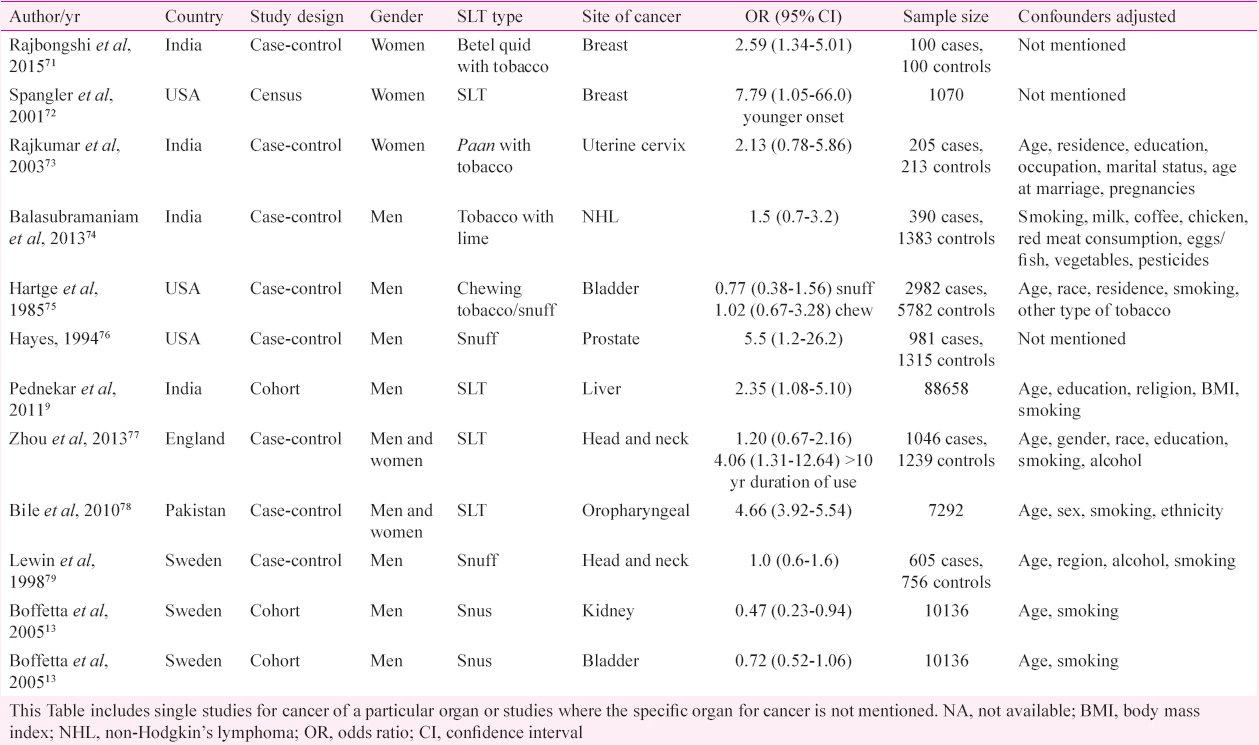
Other cancers: Other neoplasias including breast cancer7172, cervical cancer73, lymphoma74, genitourinary tumours137576 liver9, and others777879 have also been evaluated for their association with SLT use with variable results in sporadic studies (Table II).
Smokeless tobacco (SLT) and cancer mortality
Eight studies providing 19 individual estimates for mortality due to various cancers were retrieved for this review (Table III)8081828384858687. Of these, seven studies provided estimates for digestive tract cancers, three for respiratory, two for combined oral and pharyngeal cancers, two for genitourinary and one each for pharyngeal, upper aero-digestive tract (UADT), breast and cervical cancers. Significantly higher risk of mortality was found for lung (OR ranging from 2.081 to 9.186), cervical (OR 2.0 and 2.2 for urban and rural females, respectively84), prostate (OR 2.1, 95% CI 1.1-4.187) and UADT (OR between 1.9 and 3.884). Due to small number of studies on individual cancer and mortality risk, product-specific assessment was not attempted.

Discussion
SLT products have a worldwide presence in various forms - chewing tobacco in the USA, snuff (snus) in Sweden and mixture of chewing tobacco with other ingredients in developing countries1. Reviews in the mid-1980s as well as the US Surgeon General Report in 1986 concluded that SLT products had negative health implications88. Recent analyses have demonstrated significant morbidity and mortality related to SLT use. One study estimated that globally, 1.7 million disability-adjusted life years (DALYs) were lost and 62,283 deaths were attributed to SLT-associated cancers based on estimated burden of disease figures available for 113 countries6. Another meta-analysis calculated 3.6 million DALYs and 101,004 deaths due to cancers associated with SLT use89. A monograph on SLT and Public Health in India reported that 90 per cent of oral and pharyngeal cancers were caused by tobacco in some form and 50 per cent of these are attributable to SLT90. However, the multitude and heterogeneity of products have raised doubts on these associations. Due to significant differences in composition, production and usage practices of SLT, the levels of most important carcinogens such as TSNA, vary widely across different SLT products91.
A systematic review of health effects of SLT published in 2003 reported significant risk of oral cancers due to betel quid and tobacco chewing in India while studies from the US and Scandinavian countries did not report significant positive association1. Since this review, there have been a few region-specific or cancer-specific systematic reviews and meta-analyses on SLT78992. However, review on association of various cancers with SLT products in a global perspective has not been conducted recently.
Risk of cancer occurrence in SLT users
The present review re-emphasizes the strong association between SLT use and occurrence of oral cancer with risk estimates ranging from 1.48 (1.03-2.13)9 to 27.4 (10.0-74.7)15, especially for studies originating from SEAR. Occasional studies from SEAR did not find significant positive association of oral cancer with SLT use222328. This could partly be attributed to the fewer number of controls in one study22. Studies from EUR, fewer in number compared to those from SEAR, have not found a significant positive association between SLT use and cancer121335. An earlier meta-analysis showed overall 34 per cent higher risk of oral cancer in SLT users although regional variation was evident6. Sinha et al7, in their meta-analysis of Indian studies, gave a risk estimate of 5.67 (3.83-8.40) for oral cancer in SLT users (Table IV).
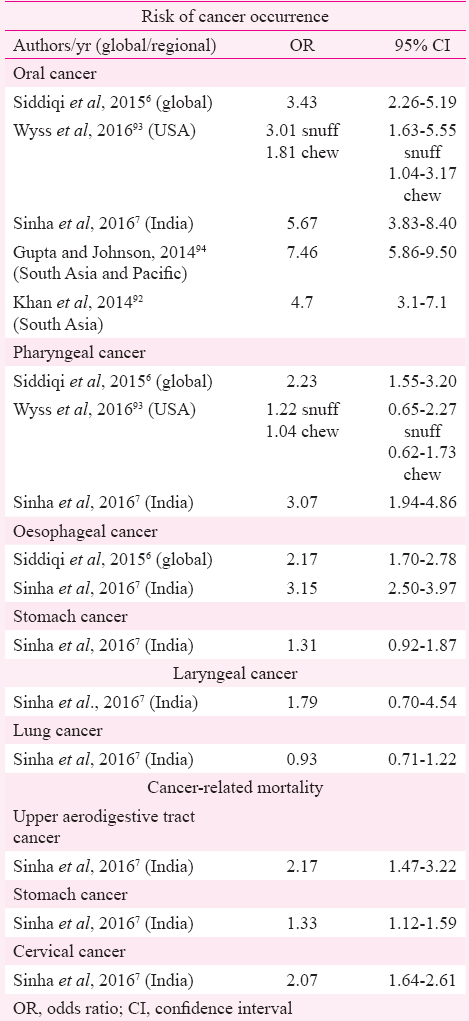
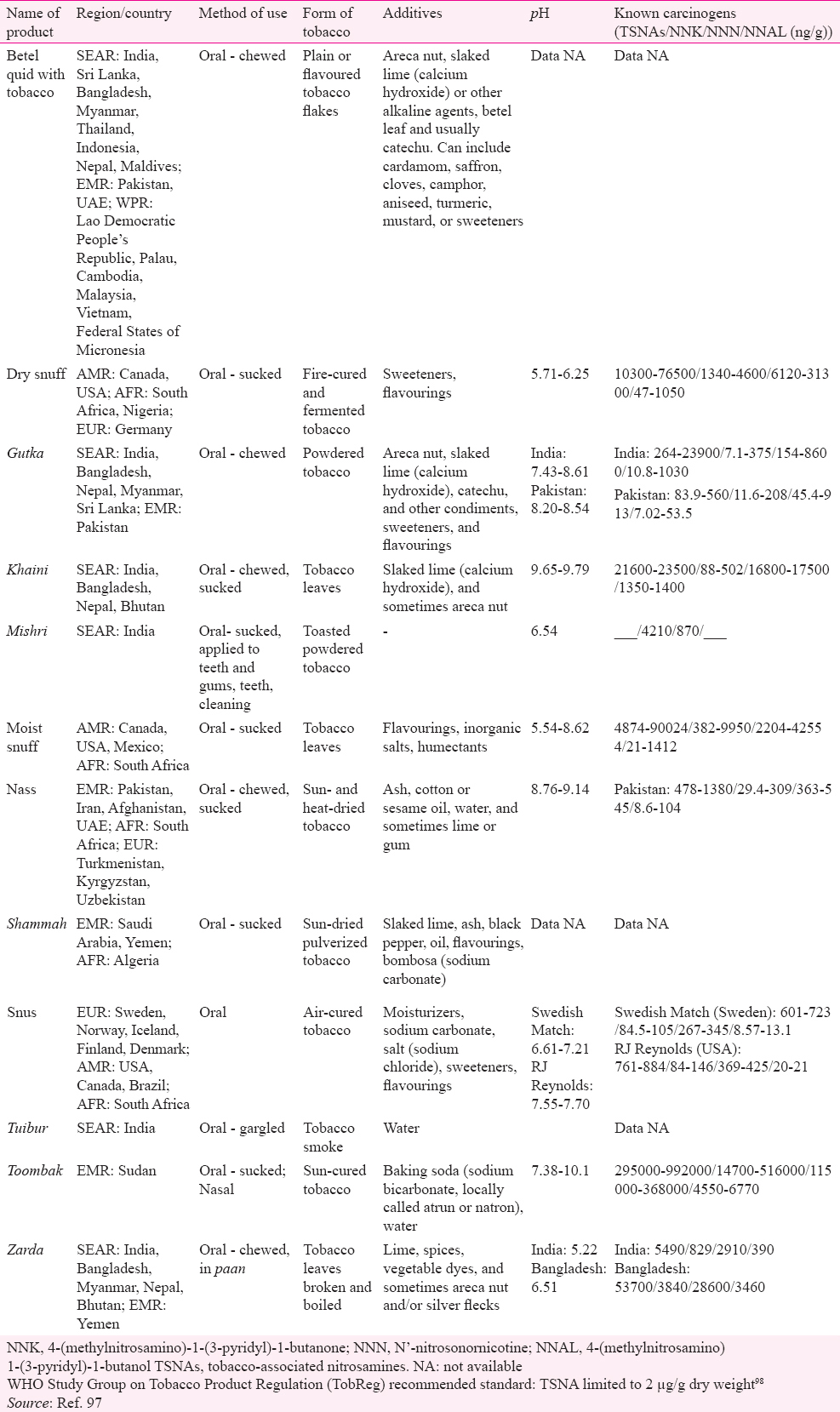
A review of studies from the USA found significantly higher risk of oral cancer with chewing tobacco as well as snuff93. Meta-analysis of studies from South Asia and Pacific concluded increased risk of oral cancer in tobacco chewers (7.46, 5.86-9.50) although need for conducting studies focussing on different types of tobacco and eliciting dose–response relationship was emphasized94. An Indian study has demonstrated a linear dose–response association of oral cancer and chewing tobacco95. This regional variation in risk estimates can partly be explained by the chemical composition of SLT products, especially levels of TSNAs, and their usage practices. The SEAR has the maximum diversity in SLT products as well as their usage methods, varying from chewing tobacco alone to a mixture of tobacco with ingredients such as betel quid and/ or areca nut (both recognized as carcinogens), lime and other such products96. Some products are sucked, gargled/sipped or used as a dentrifice (Table V)97. A review of toxicology of SLT products available in India highlighted the disturbingly high levels of TSNAs in the most popular brands of SLT products such as khaini, zarda and mishri99. Various authors have also detected TSNAs in the saliva of tobacco chewers100101. In addition, mutagenic effects of extracts of SLT products have also been demonstrated102. Formation of micronucleus as a genotoxic effect has been reported in exfoliated buccal epithelial cells from tobacco chewers103. A few studies in the present review reported a higher risk of cancer in female SLT users (OR ranging between 3.2 and 45.89)1432 compared to male users in the same study (OR ranging from 2.7 to 9.33)2739. A previous meta-analysis of studies from India also showed a significantly higher risk of oral cancer in female users (pooled OR 12.09, 95% CI 9.49-15.25) compared to males (5.16, 95% CI 4.49-5.94)7. This difference may be attributed to the behavioural differences in the usage of SLT products between males and females.
Results on association of SLT use and pharyngeal cancer have been conflicting as can be seen from Table I. However, earlier meta-analyses have shown 22 and 30 per cent higher risk of occurrence of pharyngeal cancer in SLT users67. Unlike oral cancer where tobacco is the most important aetiologic agent, pharyngeal cancer, especially oropharyngeal, is causatively linked to human papillomavirus (HPV)104. Synergistic effect of smoking and HPV16 positivity on the causation of head and neck cancer have been demonstrated104 though the same has not been proved for SLT products as yet.
Another significant positive association highlighted is that of oesophageal cancer and SLT products. Majority of studies from SEAR, the single study from EMR and one of two reports from EUR demonstrated positive association of oesophageal cancer with SLT use. A previous global review of SLT-related diseases reported an overall 20 per cent higher risk of oesophageal cancer in SLT users with maximum risk detected in the analysis of studies from EMR and SEAR6. Similar positive association was reported in a meta-analysis of Indian studies7.
Studies on gastric cancer have reported conflicting results with reports from EUR not finding positive association while majority of SEAR and EMR studies demonstrating higher risk of gastric cancer with SLT use. However, a previous meta-analysis of Indian studies did not find significant positive association between gastric cancer and SLT use (1.31, 95% CI 0.92-1.87)7. The association of pancreatic cancer with SLT use has been demonstrated in Scandinavian reports though studies from America have not supported this association1213. The Scandinavian studies have shown this increased risk in SLT users after adjustment for smoking and alcohol use13 or in never-smoking stratum12. Animal model experiments have shown the occurrence of pancreatic adenocarcinoma in rats exposed to TSNAs or their metabolites as well as effect of TSNAs on point mutations in the RAS gene that is implicated in pancreatic carcinogenesis105106. TSNAs have also been documented in human pancreatic juice at higher concentration in smokers compared to non-smokers107. However, the available evidence lacks detailed information regarding the chemical composition of the SLT products consumed in different Regions. Since the toxicity of SLT products differs according to their composition and manufacturing practices, effect of these products in causation of various cancers has been debatable in the studies from different Regions.
The role of SLT use in occurrence of cancers such as colorectal, lung, breast and cervix has not been established beyond doubt as yet and needs further exploration by well-controlled studies.
Cancer-related mortality and SLT use
In comparison with the number of studies evaluating cancer occurrence in SLT users, research into cancer-related mortality with SLT use has been scarce. In the present review, only 19 individual risk estimates were retrieved for mortality of various cancers in SLT users. A previous meta-analysis of SLT-attributable mortality showed significantly higher risk of deaths due to UADT, gastric and cervical cancers in SLT users. Regional variation was noted for mortality outcome of UADT cancer with significant positive association in estimates from SEAR while the same was not true for those from AMR89. However, a limitation of this analysis was the small number of estimates included for each cancer. In addition, mortality estimates were not available from all Regions.
A cohort study from south India on effect of tobacco chewing on cancer mortality did not find significant positive association (1.07, 95% CI 0.94-1.22) after adjustment for age, gender, socio-economic status and dietary variables. However, age-wise evaluation showed detrimental effects on cancer mortality in the middle age group, 40-59 yr (1.26, 95% CI 1.03-1.55)108.
Due to paucity of studies evaluating cancer-related mortality in SLT users, conclusive opinion on cancer-specific, Region-wise or product-related mortality risk for various cancers is currently not possible. Exploring this aspect would need well-designed studies with appropriate adjustment for confounding factors.
Strengths and limitations
The strengths of this review include the wide and comprehensive range of cancers included, thorough literature review and global coverage to the widest extent possible. Cancer sites not considered by previous reviews and meta-analyses were also included in the present review.
However, there were certain limitations also. Many of the observational studies included inadequate descriptions of SLT use as ‘ever or never’ without defining the type of SLT product or estimating the dose-response relationship. Second, biochemical validation of SLT use was not conducted in majority of the studies. Self-reporting of SLT use is fraught with recall bias as well as intentional hiding of facts by the subjects. Such bias can lead to misclassification of subjects as cases or controls, leading to confounding results. A significant limitation of this review was the lack of uniformity of case definition in accordance with the International Classification of Diseases (ICD-10) system, especially for oral cancers. Many studies included in the review failed to mention the case definition criteria. The definition of various outcomes was also not uniform across studies. This was of special concern in the evaluation of studies on mortality since the data from developing countries were usually lacking in the completeness and certification of cause of death. In such a scenario, confounding by other causes of death in a cancer patient could not be excluded with confidence. Absence of studies from WPR limited the evaluation of SLT and cancer association in this Region. From AFR, only two studies evaluating role of toombak in risk of oral cancer were retrieved. Other cancer sites were not examined in AFR for the association with SLT products. Another limitation pertained to countries like India with wide inter-State variations in SLT products. Studies reported from such countries are not distributed uniformly through the country; however, the results are considered to represent the country as a whole.
Conclusion & recommendations for the future
The present review highlights the significant positive association of SLT use with risk of oral and oesophageal cancer in SEAR and EMR. Higher risk of pancreatic cancer in SLT users has been emphasized in studies from EUR. Association of SLT products with cancers of other sites and with cancer-related mortality is still an unresolved issue that requires robust studies from across the globe.
Although association of SLT and oral cancer is well accepted especially for SEAR, further studies with adequate power and control of confounding factors are required from other Regions, as well as for other cancers to establish their association with SLT. The studies should specifically address the product-specific association to enable clear policy decisions and also to refute the claims of tobacco industry regarding relative safety of SLT products as an alternative to quitting for smokers. To address the latter issue, studies also need to include a category of ‘switchers’ in their long-term follow up to obtain real estimates of adverse health consequences of SLT use compared to smoking.
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- Health effects associated with smokeless tobacco: A systematic review. Thorax. 2003;58:435-43.
- [Google Scholar]
- Smokeless tobacco and some tobacco-specific N-nitrosamines. IARC Monogr Eval Carcinog Risks Hum. 2007;89:1-592.
- [Google Scholar]
- Tobacco carcinogenesis. In: Schwab M, ed. Encyclopedia of cancer. Berlin, Heidelberg: Springer Berlin Heidelberg; 2011. p. :3717-9.
- [Google Scholar]
- Global surveillance of oral tobacco products: Total nicotine, unionised nicotine and tobacco-specific N-nitrosamines. Tob Control. 2011;20:e2.
- [Google Scholar]
- Global burden of disease due to smokeless tobacco consumption in adults: Analysis of data from 113 countries. BMC Med. 2015;13:194.
- [Google Scholar]
- Smokeless tobacco-associated cancers: A systematic review and meta-analysis of Indian studies. Int J Cancer. 2016;138:1368-79.
- [Google Scholar]
- Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ. 2009;339:b2535.
- [Google Scholar]
- Association of tobacco habits, including bidi smoking, with overall and site-specific cancer incidence: Results from the Mumbai cohort study. Cancer Causes Control. 2011;22:859-68.
- [Google Scholar]
- Oral cavity cancer risk in relation to tobacco chewing and bidi smoking among men in Karunagappally, Kerala, India: Karunagappally cohort study. Cancer Sci. 2011;102:460-7.
- [Google Scholar]
- Tobacco chewing and female oral cavity cancer risk in Karunagappally cohort, India. Br J Cancer. 2009;100:848-52.
- [Google Scholar]
- Oral use of Swedish moist snuff (snus) and risk for cancer of the mouth, lung, and pancreas in male construction workers: A retrospective cohort study. Lancet. 2007;369:2015-20.
- [Google Scholar]
- Smokeless tobacco use and risk of cancer of the pancreas and other organs. Int J Cancer. 2005;114:992-5.
- [Google Scholar]
- Attribution of oral cancer in the Sudan to toombak dipping. Transl Res Oral Oncol. 2017;2:2057178X1668572.
- [Google Scholar]
- Oral cancer via the bargain bin: The risk of oral cancer associated with a smokeless tobacco product (Naswar) PLoS One. 2017;12:e0180445.
- [Google Scholar]
- Assessing the risk of oral cancer associated with gutka and other smokeless tobacco products: A case-control study. J Contemp Dent Pract. 2016;17:740-4.
- [Google Scholar]
- Squamous cell carcinoma of the upper aerodigestive tract in exclusive smokers, chewers, and those with no habits. Indian J Cancer. 2016;53:538-41.
- [Google Scholar]
- Risk of oral cancer associated with gutka and other tobacco products: A hospital-based case-control study. J Cancer Res Ther. 2015;11:199-203.
- [Google Scholar]
- Analysis of various risk factors affecting potentially malignant disorders and oral cancer patients of central India. J Cancer Res Ther. 2015;11:280-6.
- [Google Scholar]
- Total, direct, and indirect effects of paan on oral cancer. Cancer Causes Control. 2015;26:487-91.
- [Google Scholar]
- Oral squamous cell carcinoma and associated risk factors in Jazan, Saudi Arabia: A hospital based case control study. Asian Pac J Cancer Prev. 2015;16:4335-8.
- [Google Scholar]
- Demographic risk factors, affected anatomical sites and clinicopathological profile for oral squamous cell carcinoma in a North Indian population. Asian Pac J Cancer Prev. 2014;15:6755-60.
- [Google Scholar]
- Association of interleukin-1β -511 C/T polymorphism with tobacco-associated cancer in Northeast India: A study on oral and gastric cancer. Cancer Genet. 2014;207:1.
- [Google Scholar]
- Tobacco (kretek) smoking, betel quid chewing and risk of oral cancer in a selected Jakarta population. Asian Pac J Cancer Prev. 2014;15:8673-8.
- [Google Scholar]
- Clinico-epidemiological profile of oral potentially malignant and malignant conditions among areca nut, tobacco and alcohol users in Eastern India: A hospital based study. J Oral Maxillofac Pathol. 2013;17:45-50.
- [Google Scholar]
- Risk for oral cancer associated to smoking, smokeless and oral dip products. Indian J Public Health. 2012;56:57-60.
- [Google Scholar]
- Role of tobacco smoking, chewing and alcohol drinking in the risk of oral cancer in Trivandrum, India: A nested case-control design using incident cancer cases. Oral Oncol. 2008;44:446-54.
- [Google Scholar]
- Susceptibility to oral cancer by genetic polymorphisms at CYP1A1, GSTM1 and GSTT1 loci among Indians: Tobacco exposure as a risk modulator. Carcinogenesis. 2007;28:1455-62.
- [Google Scholar]
- Assessment of risk factors for oral squamous cell carcinoma in Chidambaram, Southern India: A case-control study. Eur J Cancer Prev. 2007;16:251-6.
- [Google Scholar]
- Independent and combined effects of tobacco smoking, chewing and alcohol drinking on the risk of oral, pharyngeal and esophageal cancers in Indian men. Int J Cancer. 2003;105:681-6.
- [Google Scholar]
- Polymorphism at GSTM1, GSTM3 and GSTT1 gene loci and susceptibility to oral cancer in an Indian population. Carcinogenesis. 2002;23:803-7.
- [Google Scholar]
- Oral cancer in Southern India: The influence of smoking, drinking, paan-chewing and oral hygiene. Int J Cancer. 2002;98:440-5.
- [Google Scholar]
- Tobacco habits and risk of lung, oropharyngeal and oral cavity cancer: A population-based case-control study in Bhopal, India. Int J Epidemiol. 2000;29:609-14.
- [Google Scholar]
- Paan without tobacco: An independent risk factor for oral cancer. Int J Cancer. 2000;86:128-31.
- [Google Scholar]
- Oral snuff, smoking habits and alcohol consumption in relation to oral cancer in a Swedish case-control study. Int J Cancer. 1998;77:341-6.
- [Google Scholar]
- Toombak dipping and cancer of the oral cavity in the sudan: A case-control study. Int J Cancer. 1995;63:477-80.
- [Google Scholar]
- Risk assessment of tobacco, alcohol and diet in oral cancer – A case-control study. Int J Cancer. 1994;58:469-73.
- [Google Scholar]
- Tobacco smoking, alcohol drinking, and cancer of the oral cavity and oropharynx among U.S. veterans. Cancer. 1993;72:1369-75.
- [Google Scholar]
- Risk factors for cancer of the buccal and labial mucosa in Kerala, Southern India. J Epidemiol Community Health. 1990;44:286-92.
- [Google Scholar]
- A population-based case-control investigation on cancers of the oral cavity in Bangalore, India. Br J Cancer. 1990;62:847-51.
- [Google Scholar]
- Tobacco chewing, alcohol and nasal snuff in cancer of the gingiva in Kerala, India. Br J Cancer. 1989;60:638-43.
- [Google Scholar]
- Associations of tobacco use and alcohol drinking with laryngeal and hypopharyngeal cancer risks among men in Karunagappally, Kerala, India – Karunagappally cohort study. PLoS One. 2013;8:e73716.
- [Google Scholar]
- Smokeless tobacco and increased risk of hypopharyngeal and laryngeal cancers: A multicentric case-control study from India. Int J Cancer. 2007;121:1793-8.
- [Google Scholar]
- Tobacco consumption practices and risk of oro-pharyngeal cancer: A case-control study in central India. Southeast Asian J Trop Med Public Health. 1998;29:827-34.
- [Google Scholar]
- Risk of gastroesophageal cancer among smokers and users of Scandinavian moist snuff. Int J Cancer. 2008;122:1095-9.
- [Google Scholar]
- Promoter methylation of MGMT gene in serum of patients with esophageal squamous cell carcinoma in North East India. Asian Pac J Cancer Prev. 2014;15:9955-60.
- [Google Scholar]
- Epigenetic, genetic and environmental interactions in esophageal squamous cell carcinoma from Northeast India. PLoS One. 2013;8:e60996.
- [Google Scholar]
- Hookah smoking, nass chewing, and oesophageal squamous cell carcinoma in Kashmir, India. Br J Cancer. 2012;107:1618-23.
- [Google Scholar]
- Risk factors and survival analysis of the esophageal cancer in the population of Jammu, India. Indian J Cancer. 2012;49:245-50.
- [Google Scholar]
- Chewing areca nut, betel quid, oral snuff, cigarette smoking and the risk of oesophageal squamous-cell carcinoma in South Asians: A multicentre case-control study. Eur J Cancer. 2012;48:655-61.
- [Google Scholar]
- Betel nut and tobacco chewing; potential risk factors of cancer of oesophagus in Assam, India. Br J Cancer. 2001;85:661-7.
- [Google Scholar]
- Nutritional risk factors in esophageal cancer. J Assoc Physicians India. 2000;48:781-7.
- [Google Scholar]
- The role of tobacco, snuff and alcohol use in the aetiology of cancer of the oesophagus and gastric cardia. Int J Cancer. 2000;85:340-6.
- [Google Scholar]
- Importance of anatomical subsite in correlating risk factors in cancer of the oesophagus – Report of a case – Control study. Br J Cancer. 1996;73:1306-11.
- [Google Scholar]
- Risk factors for cancer of the oesophagus in Kerala, India. Int J Cancer. 1991;49:485-9.
- [Google Scholar]
- Tobacco chewing and risk of gastric cancer: A case-control study in Yemen. East Mediterr Health J. 2017;22:719-26.
- [Google Scholar]
- P53 codon 72 polymorphism interactions with dietary and tobacco related habits and risk of stomach cancer in Mizoram, India. Asian Pac J Cancer Prev. 2014;15:717-23.
- [Google Scholar]
- Tobacco use and stomach cancer in Mizoram, India. Cancer Epidemiol Biomarkers Prev. 2005;14:1892-6.
- [Google Scholar]
- A case-control study of stomach cancer in Mumbai, India. Int J Cancer. 2002;99:727-31.
- [Google Scholar]
- Tobacco, alcohol and the risk of gastric cancer by sub-site and histologic type. Int J Cancer. 1999;83:223-9.
- [Google Scholar]
- Lifestyle and risk of stomach cancer: A hospital-based case-control study. Int J Epidemiol. 1996;25:1146-53.
- [Google Scholar]
- Smokeless tobacco (snus) use and colorectal cancer incidence and survival: Results from nine pooled cohorts. Scand J Public Health. 2017;45:741-8.
- [Google Scholar]
- Smoking, snus use and risk of right- and left-sided colon, rectal and anal cancer: A 37-year follow-up study. Int J Cancer. 2011;128:157-65.
- [Google Scholar]
- Colorectal cancer and its risk factors among patients attending a tertiary care hospital in Southern Karnataka, India. Asian J Pharm Clin Res. 2017;10:109.
- [Google Scholar]
- Passive smoking and the use of noncigarette tobacco products in association with risk for pancreatic cancer: A case-control study. Cancer. 2007;109:2547-56.
- [Google Scholar]
- Smokeless and other noncigarette tobacco use and pancreatic cancer: A case-control study based on direct interviews. Cancer Epidemiol Biomarkers Prev. 2004;13:55-8.
- [Google Scholar]
- Multiple analytical approaches reveal distinct gene-environment interactions in smokers and non smokers in lung cancer. PLoS One. 2011;6:e29431.
- [Google Scholar]
- A case-control study of risk factors for lung cancer in Mumbai, India. Asian Pac J Cancer Prev. 2011;12:357-62.
- [Google Scholar]
- Tobacco smoking and chewing, alcohol drinking and lung cancer risk among men in Southern India. Int J Cancer. 2003;107:441-7.
- [Google Scholar]
- Evaluation of female breast cancer risk among the betel quid chewer: A bio-statistical assessment in Assam, India. Nepal J Epidemiol. 2015;5:494-8.
- [Google Scholar]
- Association between smokeless tobacco use and breast cancer among native-American women in North Carolina. Ethn Dis. 2001;11:36-43.
- [Google Scholar]
- Role of paan chewing and dietary habits in cervical carcinoma in Chennai, India. Br J Cancer. 2003;88:1388-93.
- [Google Scholar]
- Case-control study of risk factors for non-Hodgkin lymphoma in Mumbai, India. Asian Pac J Cancer Prev. 2013;14:775-80.
- [Google Scholar]
- Bladder cancer risk and pipes, cigars, and smokeless tobacco. Cancer. 1985;55:901-6.
- [Google Scholar]
- Tobacco use and prostate cancer in blacks and whites in the United States. Cancer Causes Control. 1994;5:221-6.
- [Google Scholar]
- Smokeless tobacco and risk of head and neck cancer: Evidence from a case-control study in New England. Int J Cancer. 2013;132:1911-7.
- [Google Scholar]
- Smokeless tobacco use in Pakistan and its association with oropharyngeal cancer. East Mediterr Health J. 2010;16:S24-30.
- [Google Scholar]
- Smoking tobacco, oral snuff, and alcohol in the etiology of squamous cell carcinoma of the head and neck: A population-based case-referent study in Sweden. Cancer. 1998;82:1367-75.
- [Google Scholar]
- Tobacco associated mortality in Mumbai (Bombay) India. Results of the bombay cohort study. Int J Epidemiol. 2005;34:1395-402.
- [Google Scholar]
- Two large prospective studies of mortality among men who use snuff or chewing tobacco (United States) Cancer Causes Control. 2005;16:347-58.
- [Google Scholar]
- Cancer and mortality among users and nonusers of snus. Int J Cancer. 2008;123:168-73.
- [Google Scholar]
- A longitudinal study of smokeless tobacco use and mortality in the United States. Int J Cancer. 2017;141:264-70.
- [Google Scholar]
- Tobacco chewing and adult mortality: A case-control analysis of 22,000 cases and 429,000 controls, never smoking tobacco and never drinking alcohol, in South India. Asian Pac J Cancer Prev. 2015;16:1201-6.
- [Google Scholar]
- Cigarette smoking, use of other tobacco products and stomach cancer mortality in US adults: The cancer prevention study II. Int J Cancer. 2002;101:380-9.
- [Google Scholar]
- Chronic disease mortality in a cohort of smokeless tobacco users. Am J Epidemiol. 2002;156:730-7.
- [Google Scholar]
- Diet, tobacco use, and fatal prostate cancer: Results from the Lutheran brotherhood cohort study. Cancer Res. 1990;50:6836-40.
- [Google Scholar]
- Health consequences of using smokeless tobacco: Summary of the advisory committee's report to the surgeon general. Public Health Rep. 1986;101:355-73.
- [Google Scholar]
- Global burden of all-cause and cause-specific mortality due to smokeless tobacco use: Systematic review and meta-analysis. Tob Control. 2018;27:35-42.
- [Google Scholar]
- Smokeless tobacco and public health in India. New Delhi: Ministry of Health&Family Welfare, Government of India; 2016.
- [Google Scholar]
- Formation and analysis of tobacco-specific N-nitrosamines. Crit Rev Toxicol. 1996;26:121-37.
- [Google Scholar]
- Smokeless tobacco and oral cancer in South Asia: A systematic review with meta-analysis. J Cancer Epidemiol. 2014;2014:1-11.
- [Google Scholar]
- Smokeless tobacco use and the risk of head and neck cancer: Pooled analysis of US studies in the INHANCE consortium. Am J Epidemiol. 2016;184:703-16.
- [Google Scholar]
- Systematic review and meta-analysis of association of smokeless tobacco and of betel quid without tobacco with incidence of oral cancer in South Asia and the Pacific. PLoS One. 2014;9:e113385.
- [Google Scholar]
- Associations between oral hygiene habits, diet, tobacco and alcohol and risk of oral cancer: A case-control study from India. Cancer Epidemiol. 2017;51:7-14.
- [Google Scholar]
- Burden of smoked and smokeless tobacco consumption in India – Results from the global adult tobacco survey India (GATS-India)- 2009-201. Asian Pac J Cancer Prev. 2013;14:3323-9.
- [Google Scholar]
- National Cancer Institute and Centers for Disease Control and Prevention. In: Smokeless tobacco and public health: A global perspective. NIH publication No. 14-7983. Bethesda, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Institutes of Health, National Cancer Institute; 2014. p. :B5-63.
- [Google Scholar]
- WHO Study Group on Tobacco Product Regulation: Report on the Scientific Basis of Tobacco Product Regulation: Third Report of a WHO Study Group. (WHO Technical Report Series; No. 955) Available from: http://www.who.int/tobacco/global_interaction/tobreg/publications/tsr_955/en/
- [Google Scholar]
- Carcinogenic tobacco-specific nitrosamines are present at unusually high levels in the saliva of oral snuff users in Sudan. Carcinogenesis. 1992;13:1001-5.
- [Google Scholar]
- Localized formation of micronuclei in the oral mucosa and tobacco-specific nitrosamines in the saliva of “reverse” smokers, Khaini-tobacco chewers and gudakhu users. Int J Cancer. 1992;50:172-6.
- [Google Scholar]
- Mutagenicity and carcinogenicity of masheri, a pyrolysed tobacco product, and its content of tobacco-specific nitrosamines. IARC Sci Publ. 1987;84:460-2.
- [Google Scholar]
- Genotoxicity of ‘gudakhu’, a tobacco preparation. II. In habitual users. Food Chem Toxicol. 1992;30:1045-9.
- [Google Scholar]
- Human papillomavirus and oral cancer: The international agency for research on cancer multicenter study. J Natl Cancer Inst. 2003;95:1772-83.
- [Google Scholar]
- Induction of lung and exocrine pancreas tumors in F344 rats by tobacco-specific and areca-derived N-nitrosamines. Cancer Res. 1988;48:6912-7.
- [Google Scholar]
- Mechanisms of smoking-related lung and pancreatic adenocarcinoma development. Nat Rev Cancer. 2002;2:455-63.
- [Google Scholar]
- Identification of tobacco-derived compounds in human pancreatic juice. Chem Res Toxicol. 2002;15:677-85.
- [Google Scholar]
- Effect of tobacco chewing, tobacco smoking and alcohol on all-cause and cancer mortality: A cohort study from Trivandrum, India. Cancer Epidemiol. 2010;34:405-12.
- [Google Scholar]






