Translate this page into:
Immunohistochemical expression of hypoxia-inducible factor-1α in stromal cells of vaginal tissue in post-menopausal women with pelvic organ prolapse
Reprint requests: Dr Ivana Alujević Jakus, Clinical Department of Gynecology & Obstetrics, Split University Hospital Center, Spinciceva 1, 21000 Split, Croatia e-mail: alujevicivana@hotmail.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Pelvic organ prolapse (POP) is a common medical condition that affects adult women of different ages. The support of a normal pelvic floor is the result of complex interactions between ligaments, muscles, connective tissue and vaginal walls. Hypoxia and oxidative stress can reduce protein synthesis in the pelvic muscles that may contribute to muscular atrophy. Hypoxia-inducible factor-1α (HIF-1α) is a transcriptional activator which, expressed in response to hypoxia, activates a number of genes involved in cellular response to hypoxia. However, a potential role of hypoxia and oxidative stress in pathogenesis of POP is not known. This study was aimed to compare the level of HIF-1α immunohistochemical expression in the vaginal stromal cells of postmenopausal women with and without POP.
Methods:
Samples of the vaginal tissue from 120 menopausal women were obtained during surgery, and immunohistochemical expression of HIF-1α was assessed. There were 60 women with POP while 60 women in the control group were without prolapse but with benign gynaecological diseases.
Results:
In post-menopausal women with prolapse, significant differences were observed in the number of HIF-1α-positive stromal cells in the vaginal tissue compared to the control group. There was a significant increase in the number of HIF-1α in the stromal cells of the vaginal tissue in women with prolapse.
Interpretation & conclusions:
Difference in expression of HIF-1α in stromal cells of the vaginal tissue in the post-menopausal women with and without POP suggests that prolonged hypoxia probably has an important role in the aetiopathogenesis of POP.
Keywords
Hypoxia-inducible factor-1α
immunohistochemistry
pelvic organ prolapse
post-menopause
vagina
Pelvic organ prolapse (POP) is a common and debilitating type of pelvic floor dysfunction caused by the dropping of pelvic organs below their normal position. Pelvic floor is a single anatomical and functional unit. Its stability is provided by pelvic bones, muscles of the pelvic diaphragm and those of the urogenital diaphragm, endopelvic fascia and vaginal walls. Pelvic floor integrity is the result of a complex interaction between uterine ligaments, skeletal muscle, connective tissue and vaginal wall12. The most common aetiological factors of POP are vaginal birth, especially that of children heavier at birth, congenital weakness of the pelvic floor muscles and connective tissue, lack of oestrogen (post-menopausal atrophy) and the factors which increase intra-abdominal pressure such as hard physical labour, chronic constipation and chronic obstructive pulmonary disease3456. Due to the loss of pelvic floor supportive structures, hysterectomy constitutes an important factor in the development of certain POP subtypes7. It is estimated that approximately 50 per cent of the women who gave birth suffer from pelvic floor dysfunction, but only 10-20 per cent of those women seek medical treatment34. POP affects women of all ages, but unfortunately, it is often under-reported, under-diagnosed and under-treated. POP is linked to urinary, intestinal and sexual problems in women8. With the increase in life expectancy, it is logical to expect an increase in the frequency of POP, which makes this problem essential for throwing light on its aetiopathogenesis and especially on its possible prevention910.
Hypoxia-inducible factor-1α (HIF-1α) is a transcription factor induced by hypoxia11. There are more than 100 known genes induced by this protein as it binds to their promoter region. Present in all human tissues, it has a major role in a number of physiological responses such as quick responses (erythropoiesis, glycolysis) or long-term effects (angiogenesis). HIF-1α controls erythropoiesis, iron metabolism, angiogenesis and, depending on the conditions, cell proliferation and survival or apoptosis12. HIF-1α has a short half-life (t½ five minutes) and its presence in cells depends on the quantity of oxygen13. Under normoxic conditions, HIF-1α protein dissolves quickly and its presence in cells cannot be proven14. Under hypoxic conditions, HIF-1α exits the cytoplasm, enters the nucleus and dimerizes with HIF-1β, thus creating a transcriptionally active complex11. The importance of HIF-1α is evident in the pathogenesis of numerous diseases, such as cancer, cardiovascular diseases, chronic renal disease and chronic lung diseases1516. For instance, patients suffering from acute coronary syndrome have an increased level of HIF-1α in their myocardium17. Therefore, investigation of HIF-1α activity and its regulation is necessary for therapeutic purposes in numerous pathologies. The aetiopathogenesis of POP has not been fully explained, which prompted us to conduct this research. Presuming hypoxic tissue damage of pelvic floor, we decided to investigate the expression of HIF-1α in vaginal stromal cells. Stromal vaginal cells were investigated due to the fact that vaginal wall intactness is essential for the preservation of pelvic floor stability. In this study, it was hypothesized that there was an increased expression of HIF-1α in stromal cells of the vaginal tissue of post-menopausal women with POP due to hypoxic tissue damage.
Material & Methods
Patient characteristics and tissue collection: Vaginal wall tissue samples were obtained during surgeries from 120 post-menopausal women. There were 60 women each in POP and control groups. Control group included women with other benign gynaecological diseases such as ovarian cysts and uterine myomas. Biopsy samples of 1 cm × 1 cm of the anterior vaginal wall tissue were taken from the same part of the wall in each case i.e. from the part right next to the connection between the anterior vaginal wall and the cervix. POP was assessed using POP-quantification (POP-Q) score18. In this study, women with the POP-Q 4 (complete eversion) were included in the POP group, while women with the POP-Q 0 were included in the control group. All women included in the study were multiparous. Women with any additional diseases, such as diabetes, malignant diseases, pelvic inflammatory disease and endometriosis were excluded from the study. None of the women has ever used hormone replacement therapy or has been smoking cigarettes. Biopsy samples were obtained during vaginal hysterectomy for POP group and abdominal hysterectomy for control group at the department of Gynecology and Obstetrics, University Hospital in Split, Croatia. Each patient has signed written informed consent to participate in the study. Convenience sample criterion of selection was used when choosing our POP and the control groups. The RECORD (REporting of studies Conducted using Observational Routinely- collected health Data) statement guidelines were followed during the study19. Power of the study and effect size were calculated using G*Power statistical power analysis program (version 3.1.9.2., Heinrich Heine University Düsseldorf, Germany). Power of the study was calculated for all variables and effect size for the variables where null hypothesis was rejected. The study was approved by the Research Ethics Committee of University Hospital Center, Split. This study was a part of a larger project which included a whole series of researches pertaining to pelvic floor damage. The study was conducted between 2009 and 2014.
Immunohistochemistry: Tissue samples were fixed in 10 per cent buffered formalin and processed through standard processes in the automatic tissue processor (Shandon Excelsior, Thermo Fisher Scientific, United Kingdom), embedded in paraffin, cut at 4 μm and placed on positive charged slides (Superfrost Plus Adhesion Slides, Thermo Scientific). Immunohistochemistry was performed using the BenchMark ULTRA Automated IHC/ISH slide staining system (Ventana Medical Systems, Inc., USA), using horseradish peroxidase detection system. After tissue deparaffinization for 10 min at 72°C, slides were pretreated with Tris-based buffer for 52 min at 95°C and incubated with 3 per cent H2O2 for four minutes at 36°C to inactivate endogenous peroxidase. Slides were incubated with primary antibody for HIF-1α (SC-10790, Santa Cruz Biotechnology, Dallas, USA) for 92 min at 37°C. Reaction was visualized with a Olympus BX41 Microscope using diaminobenzidine (DAB) chromogen and counterstained with haematoxylin (ULTRAVIEW Universal DAB Detection Kit, Ventana Medical Systems, Inc.). All washes between the various steps were done with phosphate-buffered saline solution. The same immunohistochemical protocol was followed for the negative controls, with the omission of the primary antibodies. Human colon cancer tissues were used as positive controls.
HIF-1α expression was scored by counting 100 stromal cells nuclei in the representative slides of the vaginal wall using Olympus Image Analyzer (magnification ×200). Counting was performed at the hot spots in the fields with the most prominent immunohistochemical reaction. Data were expressed in the form of the number of positive stromal cells nuclei/total number of stromal cell nuclei in the area scored. All immunohistochemical slides were evaluated by two investigators who were blinded to the study group.
Statistical analysis: Data were tested for normality of distribution using the Kolmogorov-Smirnov test. As data were not normally distributed (data not shown), non-parametric Mann–Whitney test was used. All analyses were conducted using SPSS (version 18; SPSS Inc., Chicago, IL, USA), with the significance level set at P<0.05.
Results
There were no significant differences in body mass index, age, duration of menopause, parity or number of abortions between women with and without POP (Table). Women in both the groups went through menopause at least four years earlier. Immunohistochemical analysis showed a significant increase in the number of HIF-1α-positive stromal cell nuclei counted per 100 stromal cells in the vaginal wall of the women with prolapse [7.33±1.26, median=7, interquartile range (IR)=2] in comparison to the control group (2.12±0.99, median=2, IR=2, P< 0.001) (Figs 1 and 2). For the variable HIF-1α, the post hoc compute achieved power was calculated, and it was 1 (effect size d=4.598123). Post hoc achieved power for other variables ranged between 0.07 and 0.17.
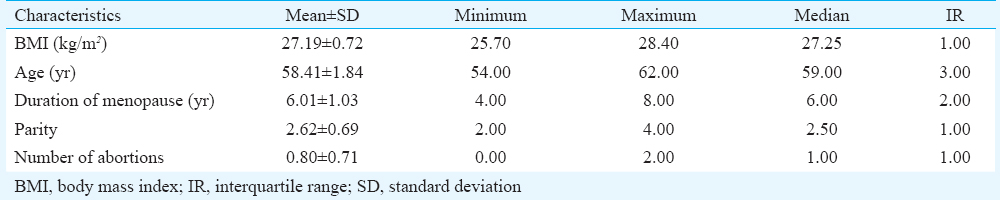
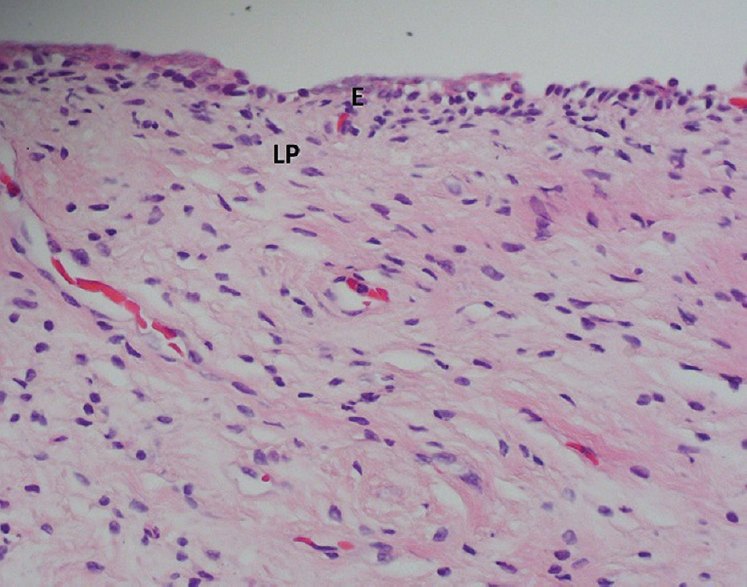
- Vagina, haematoxylin-eosin staining, ×400. E, stratified squamous epithelium; LP, lamina propria.
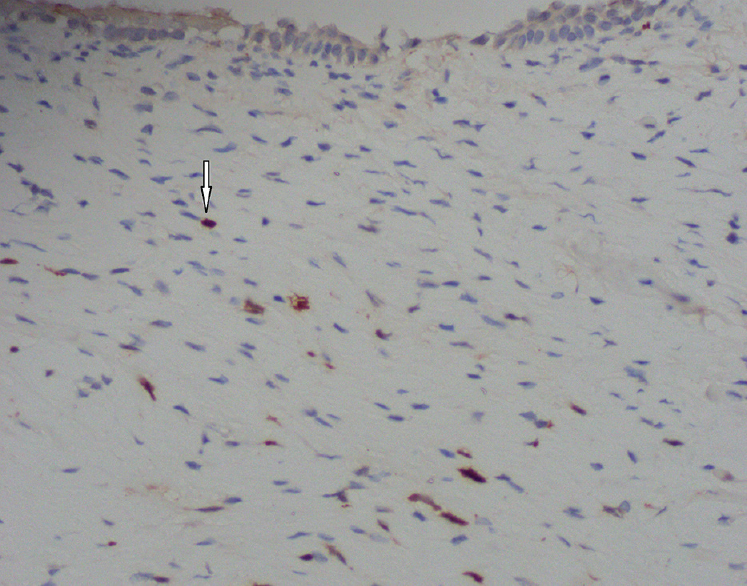
- Vagina, hypoxia-inducible factor-1α protein detection by immunohistochemistry - diaminobenzidine chromogen and haematoxylin counterstained, ×400. Nuclear staining within positive stromal cells (arrow).
Discussion
The results obtained showed a significant difference in expression of HIF-1α transcription factor in response to oxidative stress between women with POP and those in the control group. This has led us to assumption that in the control group, stromal cells of the vaginal tissue are exposed to normoxic conditions. It is evident that HIF-1α, as it binds to the promoter region, can activate a number of genes and thus starts a series of important cell events. Moreover, oxidative stress can inhibit protein synthesis and contribute to muscle atrophy20. Thurmond et al21 have reported that there are structural changes in the prostate of older men in response to hypoxia. Tehrani et al22 have compiled the screening of POP without a physical examination. Their questionnaire-based study examined urinary incontinence following laughing, sneezing or coughing, urinary urgency, feeling pain during defecation and feeling or seeing a bulge in the vagina. Analyzing pelvic floor distress inventory questionnaire, Barber et al23 have singled out the question: ‘Do you usually have a bulge or something that you can see or feel falling out in your vaginal area?’ as the most significant indicator of the disorder. Tan et al24 composed specific questions related to prolapse, which included the questions on urinary splinting, digital assistance for defecation and bulge per vagina. Other investigators2526 have also created their questionnaires for POP screening. All these questionnaires have been of significance in pelvic floor evaluation of healthy patients without clinical examination. Those at risk would be the women who will develop POP and those with an increased risk for developing a pelvic floor defect in the future. Patients with an increased risk could be recognized on time and could undergo prevention or therapy. The wide spectrum of cell processes affected by HIF-1α suggests that it could be clinically significant. Evidence suggests that crosstalk between HIF1-α and aryl hydrocarbon receptor (AhR) modulates the immune response to different signals such as immunological, metabolic and environmental27. For example, tetrachlorodibenzo-p-dioxin (TCDD)-AhR binding acts as a trigger for signalling pathways which lead to impairment of endometrial function28. Although complex, interference between the xenobiotic- and hypoxia-sensing pathways has been elucidated in the study of biphasic role of nuclear receptor coactivator 2 between AhR and hypoxic conditions29.
Various therapeutic processes reducing oxidative stress in cells could significantly contribute to tissue regeneration. One could argue that such preventive processes in cells could improve the quality of the vaginal cavity, thus reducing the risk of POP.
Our study had certain limitations. The parts of vaginal floor from where the samples were taken could be a limitation of this study, given the fact that in the control group vaginal floor was healthy, while in women with POP, there were notable defects. The expression of HIF-1α could vary in different parts of vaginal floor in women with POP, depending on the length of hypoxia in that exact part. The strength of our study was the strict criteria used for the inclusion of patients. Considering the power of the study, although the sample in this study was convenience sample, the only significant variable in the study, HIF-1α showed both strong effect size and high power. The other variables showed no significant difference, thus showing that the POP was associated with expression of HIF-1α. The results of this study showed that HIF-1α expression was related to POP, and in the future studies the other variables influencing the prolapse would also be examined.
Acknowledgment
This study was supported by the Ministry of Science, Education and Sports of the Republic of Croatia (grant no. 021-2160528-0507).
Conflicts of Interest: None.
References
- Changes in connective tissue in patients with pelvic organ prolapse - A review of the current literature. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:461-74.
- [Google Scholar]
- Genitourinary prolapse and joint hypermobility are associated with altered type I and III collagen metabolism. Arch Gynecol Obstet. 2011;283:1081-5.
- [Google Scholar]
- Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501-6.
- [Google Scholar]
- Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166(6 Pt 1):1717-24.
- [Google Scholar]
- Recurrence of vaginal prolapse after total vaginal hysterectomy with concurrent vaginal uterosacral ligament suspension: Comparison between normal-weight and overweight women. Am J Obstet Gynecol. 2016;215:601.e1-601.e4.
- [Google Scholar]
- Surgical management of pelvic organ prolapse in women: A short version Cochrane review. Neurourol Urodyn. 2008;27:3-12.
- [Google Scholar]
- Can pelvic floor muscle training prevent and treat pelvic organ prolapse? Acta Obstet Gynecol Scand. 2006;85:263-8.
- [Google Scholar]
- Conservative management of pelvic organ prolapse. Clin Obstet Gynecol. 2005;48:668-81.
- [Google Scholar]
- Activation of hypoxia-inducible transcription factor depends primarily upon redox-sensitive stabilization of its alpha subunit. J Biol Chem. 1996;271:32253-9.
- [Google Scholar]
- Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394:485-90.
- [Google Scholar]
- Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem. 1997;272:22642-7.
- [Google Scholar]
- Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510-4.
- [Google Scholar]
- HIF-1: Mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol. 2000;88:1474-80.
- [Google Scholar]
- Renal expression of hypoxia inducible factor-1α in patients with chronic kidney disease: A clinicopathologic study from nephrectomized kidneys. Indian J Med Res. 2013;137:102-10.
- [Google Scholar]
- Early expression of angiogenesis factors in acute myocardial ischemia and infarction. N Engl J Med. 2000;342:626-33.
- [Google Scholar]
- Pelvic Organ Prolapse Quantification System (POP-Q) - A new era in pelvic prolapse staging. J Med Life. 2011;4:75-81.
- [Google Scholar]
- The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med. 2015;12:e1001885.
- [Google Scholar]
- Mechanistic links between oxidative stress and disuse muscle atrophy. Antioxid Redox Signal. 2011;15:2519-28.
- [Google Scholar]
- Structural modifications of the prostate in hypoxia, oxidative stress, and chronic ischemia. Korean J Urol. 2015;56:187-96.
- [Google Scholar]
- Screening of the pelvic organ prolapse without a physical examination; (a community based study) BMC Womens Health. 2011;11:48.
- [Google Scholar]
- Can we screen for pelvic organ prolapse without a physical examination in epidemiologic studies? Am J Obstet Gynecol. 2006;195:942-8.
- [Google Scholar]
- Predictive value of prolapse symptoms: A large database study. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:203-9.
- [Google Scholar]
- Epidemiology of prolapse and incontinence questionnaire: Validation of a new epidemiologic survey. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:272-84.
- [Google Scholar]
- A short-form questionnaire identified genital organ prolapse. J Clin Epidemiol. 2005;58:41-6.
- [Google Scholar]
- Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-α. Nat Med. 2015;21:638-46.
- [Google Scholar]
- Correlation between dioxin and endometriosis: An epigenetic route to unravel the pathogenesis of the disease. Arch Gynecol Obstet. 2015;292:973-86.
- [Google Scholar]
- NcoA2-dependent inhibition of HIF-1α activation is regulated via AhR. Toxicol Sci. 2015;148:517-30.
- [Google Scholar]






