Translate this page into:
Temporal trends of intestinal parasites in patients attending a tertiary care hospital in south India: A seven-year retrospective analysis
Reprint requests: Dr. Gagandeep Kang, Wellcome Trust Research Laboratory, Division of Gastrointestinal Sciences, Christian Medical College, Vellore 632 004, Tamil Nadu, India e-mail: gkang@cmcvellore.ac.in
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Intestinal parasitic infections and their associated complications are a major cause of morbidity in the developing world. This retrospective study was done to assess the prevalence of intestinal parasitic infections among patients in a tertiary healthcare setting and to analyze age-, gender- and time-related trends in the prevalence of these intestinal parasites over a seven year period (2006-2012).
Methods:
The presence of various intestinal parasites in a tertiary care setting over a seven year period in different age groups was determined by performing routine stool microscopy. Modified acid-fast staining was performed for stool samples collected from children less than five years of age for the detection of intestinal coccidian parasites. Statistical analysis was carried out to analyze age-related trends in relation to the prevalence of commonly detected intestinal parasites. Seasonal fluctuations in parasite prevalence were evaluated by performing harmonic regression analysis.
Results:
A total of 257,588 stool samples were received over the seven year period for examination. The highest percentage of intestinal parasites was in the 6-10 yr age group. Among the intestinal parasites, Giardia intestinalis had the highest prevalence across most age groups, except in those above 60 yr of age where hookworm became more prevalent. A significant decreasing trend with age was observed for G. intestinalis, whereas for hookworm and Strongyloides stercoralis, an increasing trend with age was seen. Significant linear temporal trends were observed for parasites such as G. intestinalis, Entamoeba histolytica and Ascaris lumbricoides.
Interpretation & conclusions:
While G. intestinalis was more common in the younger age groups, certain soil-transmitted helminths such as hookworm and S. stercoralis showed a higher prevalence in the older populations. Significant temporal trends and seasonality were observed for some of the common intestinal parasites.
Keywords
Prevalence
soil-transmitted helminths
South India
temporal trends
According to the World Health Organization (WHO) estimates, approximately 21 per cent of India's population may be infected with intestinal parasites such as soil-transmitted helminths1 and the global disease burden attributed to intestinal helminths is 39 million disability adjusted life years2. Intestinal parasitic infections are important public health problems in developing countries. For example, hookworm infection is a major cause of enteric blood loss and anaemia in tropical countries, and according to the WHO estimates, 44 million pregnancies face complications because of hookworm infestation3. The prevalence of intestinal helminths and intestinal parasites is estimated by the extrapolation from findings of population surveys carried out in particular communities and settings4. Compared to the prevalence data for helminthic intestinal infections, data regarding the epidemiological distribution and burden of disease due to intestinal protozoan parasites are limited, and global burden of many of these infections is not available.
In India, the estimates of intestinal parasitic infections differ in different parts of the country56. In some studies on soil-transmitted helminths (STHs), the prevalence in the Indian subcontinent is comparable to other countries in Southeast Asia and China7, which in absolute numbers translates to a huge burden of infected cases. According to the WHO estimates, although the disease burden due to intestinal helminths in India is considerable, only 9.97 per cent of the estimated population requiring preventive chemotherapy has received anthelmintics8.
Among the neglected tropical diseases, the STHs such as Ascaris lumbricoides, hookworm, Trichuris trichiura and Strongyloides stercoralis are most prevalent. In 2004, the WHO included intestinal parasites such as Giardia intestinalis and Cryptosporidium species in the WHO expanded list for the ‘Tropical Diseases Initiative’ with a call to classify them as pathogens associated with stunting as has been done with the intestinal helminths9.
Data on prevalence are important to plan effective interventions. Specific population groups at increased risk of intestinal parasite infections should be the target for intervention measures such as chemoprophylaxis10. Although community-based prevalence data are most informative from a public health point of view, hospitals can also act as sentinel facilities because they capture greater morbidity and help provide data on the presence and absence and, to some extent, the relative abundance of specific parasites. Apart from age-related trends, temporal trends and seasonality patterns might also be helpful in developing focussed prevention programmes against intestinal parasites. Seasonal trends also reflect the relationship of intestinal parasites, particularly those with shorter life cycles such as the protozoa, with abiotic factors such as temperature and relative humidity which affect the survival of these parasites in the environment11.
In this study, a retrospective analysis was carried out to determine the prevalence of intestinal parasites and to identify any age-related or temporal trends over a period of seven years (2006-2012) among patients attending a tertiary care hospital in south India.
Material & Methods
The study was carried out at the department of Gastrointestinal Sciences, Christian Medical College, Vellore, India. Microscopy data from all stool samples sent to the parasitology laboratory for routine diagnostic screening from January 2006 to December 2012 were analysed. Stool samples collected from patients known or suspected to be HIV positive were excluded. Samples were collected in laboratory prescribed clean, dry, leak-proof-labelled containers. On receipt, all samples were screened with saline and iodine wet mounts. For samples obtained from children less than five years of age, formalin ether concentration and modified acid-fast staining were performed to detect intestinal coccidian parasites. Demographic details of the patients were obtained from the computerized database of the hospital. Ethical clearance was obtained from the Institutional Review Board of the Christian Medical College, Vellore, for this retrospective study.
Statistical analysis: Prevalence (with 95% confidence intervals) of intestinal parasitic infections was determined and stratified based on gender and different age groups. The Chi-square test was used to determine any association of intestinal parasitic infections with sex and age ranges. The Chi-square test for trend was performed to detect any age-related trends in the prevalence of common intestinal parasites. Proportion distribution of different intestinal parasites in positive samples was analyzed by Chi-square test for trend. Monthly prevalence of intestinal parasites was calculated and plotted for the seven year period for six common intestinal parasites. To assess the seasonal fluctuations in the prevalence of intestinal parasites, a monthly time series of prevalence of each pathogen was compiled and a Poisson harmonic regression model with a single annual hump (accommodating one sine and one cosine function) was fitted. The month of highest prevalence was determined using methods described previously12. The linear trend was assessed by adding the month of the year as a covariate in the model.
All statistical analyses were carried out with GraphPadInstat version 3.0 (GraphPad Software, San Diego, CA, USA), with STATA (version 10, STATA Corp, TX, USA) used for the annual harmonic regression model.
Results
A total of 257,588 stool samples were received over the seven-year period for routine examination. The number of samples received annually ranged from 29,746 in 2007 to 47,640 in 2012. The age of patients ranged from less than one year to a maximum of 98 yr. When stratified as five-year intervals, samples from patients in the age groups 36-40 and 41-45 yr comprised the majority of clinical specimens (12%) while children below the age of five years contributed to 1.8 per cent of the total samples. Number of samples from males (61.3%) was more than females (Table I).
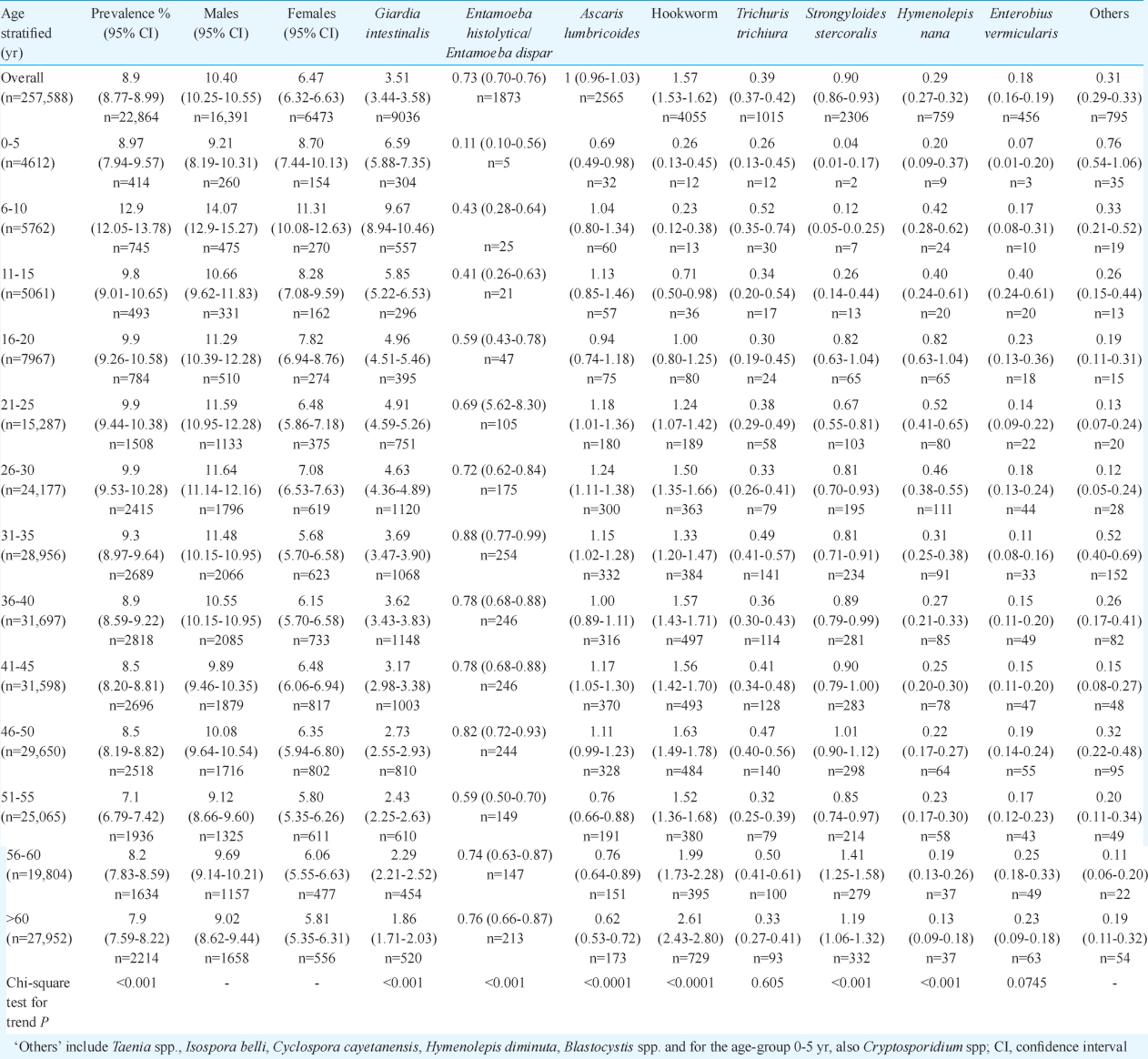
The overall prevalence of intestinal parasitic infections did not vary much over the seven year period, with an overall positivity rate of 8.9 per cent (22,864/257,588), and the highest positivity was observed in 2009 (9.5%). The most common parasite identified was G. intestinalis, found in 3.5 per cent (9036/257,588) of all samples (Table I) and 40 per cent of all positive samples. This was followed by hookworm ova in 1.6 per cent (4055/257,588) of all samples screened and 17.7 per cent (4055/22,864) of all positive samples. A. lumbricoides was found in 11.2 per cent (2565/22,864) followed by S. stercoralis in 10 per cent (2309/22,864), Entamoeba histolytica/Entamoeba dispar in 8.2 per cent (1873/22,864) and T. trichiura in 4.4 per cent (1015/22,864) of all positive samples. The other common parasites seen were Hymenolepis nana found in 3.3 per cent (755/22,864) and Enterobius vermicularis in two per cent (457/22,864) of all positive specimens.
Modified acid-fast staining was performed only for samples from children less than five years of age, and the prevalence of Cryptosporidium spp. was 0.73 per cent overall, comprising 8.2 per cent of all positive specimens in this age group. Blastocystis species, reported from 2009 onwards, was found in 0.22 per cent (335/155,012) of all samples screened for intestinal parasites and 2.9 per cent (389/13,520) of all stool specimens positive for intestinal parasites over the period 2009-2012. In 99 per cent (386/389) of these Blastocystis-positive samples, no other parasite was identified. No significant age associated trend was found for Blastocystis spp. and none of the samples in the age group 0-5 yr was positive for Blastocystis.
Other intestinal parasites which were found rarely in the stool specimens included Taenia spp. seen in only 24 samples over the seven year period and Hymenolepis diminuta in 28 samples. Two patients were positive for ova of Capillaria spp. One was a 31 yr old male patient with Capillaria philippinensis, also confirmed by histopathological examination of intestinal biopsy. The second was a 51 yr old female patient with a ‘spurious’ Capillaria hepatica infection with passage of unembryonated eggs.
Multi-parasitism or presence of more than one species of intestinal parasite in a single specimen was seen in only 0.06 per cent (164/257,588) of all stool specimens screened and comprised 0.7 per cent (164/22,864) of all positive samples. The most common combination observed was G. intestinalis and hookworm (21/164) followed by hookworm and S. stercoralis (19/164), G. intestinalis and A. lumbricoides (16/164) and hookworm and A. lumbricoides (15/164). Multiple parasitic infections were rare in children, with only three of all samples from children less than five years yielding more than one intestinal parasite.
Age-related trends: Across age groups, the highest prevalence of intestinal parasites was found in the age group of 6-10 yr (13%) whereas the lowest prevalence (7.1%) was in the age group 51-55 yr (Table I). When common parasites, with prevalence more than 0.1 per cent, were analyzed, the proportions differed by age group (Fig. 1). G. intestinalis was the dominant parasite across all age groups, except >60 yr, in which hookworm had the highest prevalence (Table I). A significant linear trend was seen for overall prevalence of intestinal parasites across age groups. Among individual parasites, significant age-related trends were observed for G. intestinalis (P<0.001), E. histolytica/E. dispar (P<0.001), hookworm (P<0.0001), A. lumbricoides (P<0.0001) and S. stercoralis (P<0.0001). The prevalence of G. intestinalis showed a decreasing trend with age while hookworm and S. stercoralis showed an increasing trend with age, with the maximum prevalence for both seen in the >60 yr age group (Table I). However, no age-associated trend was observed for E. vermicularis (P=0.0745) or T. trichiura (P=0.605).
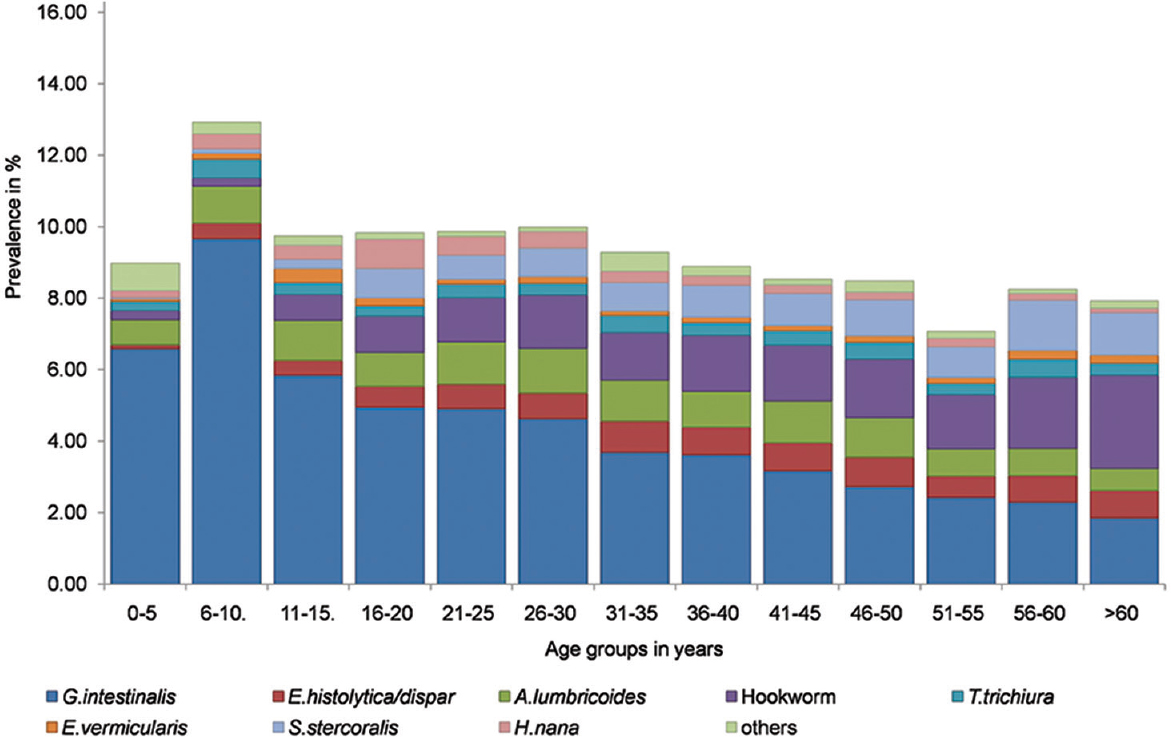
- Prevalence of intestinal parasites in different age groups.
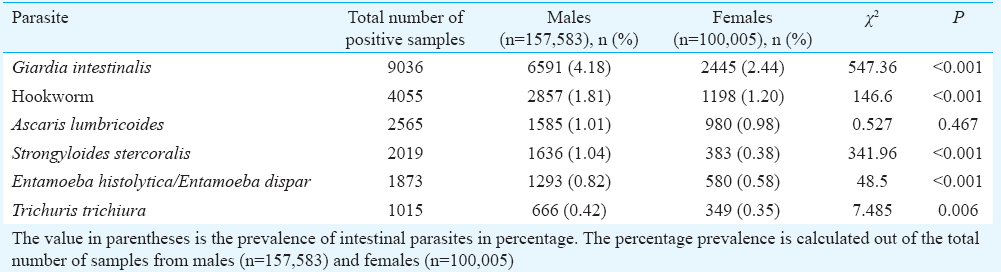
Gender association: Although >60 per cent of samples were from males, there was a significant difference (P<0.001) in the proportion of samples positive for parasites in males (16391/157,583; 10.4%) and females (6473/100,005; 6.5%). The overall prevalence of intestinal parasites in different age groups (Table I) showed significant differences between male and female patients in all age groups (P<0.01), except the 0-5 yr age group (P=0.593). The difference in the prevalence among females and males was significant for all the common parasites, except A. lumbricoides (Table II).
Temporal trends: G. intestinalis was found in a major proportion of positive samples in all years and the proportion contributed by G. intestinalis showed a linear increasing trend over the years (Table III). The proportion contributed by E. histolytica/E. dispar also increased over time, whereas that contributed by A. lumbricoides declined. However, no significant linear trends were seen for the proportion contributed by S. stercoralis or T. trichiura.
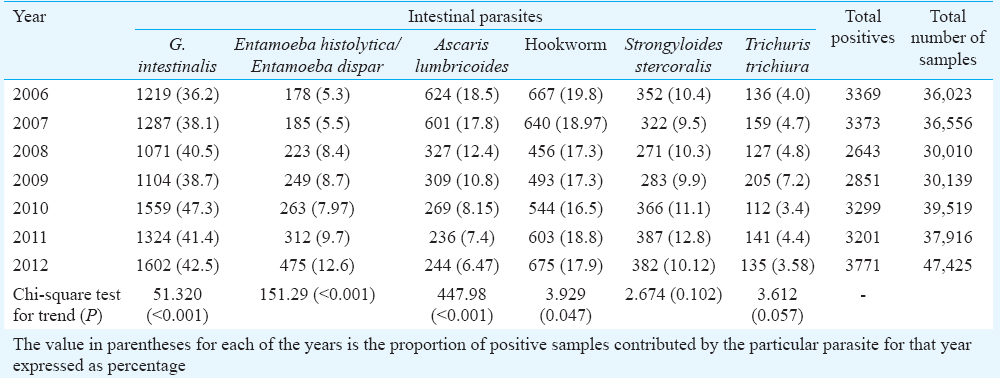
Fig. 2 shows the monthly time series and annual regression analysis models for the six commonest intestinal parasites in relation to the actual monthly time series. Significant seasonality was seen for all the common intestinal parasites, except A. lumbricoides. A significant cosine function (P<0.001) was seen for G. intestinalis with a peak in June. Using the same model, a significant decreasing monthly trend was seen for A. lumbricoides (P<0.001) and hookworm (P=0.004), whereas an increasing trend was observed for E. histolytica/E. dispar (P<0.001).
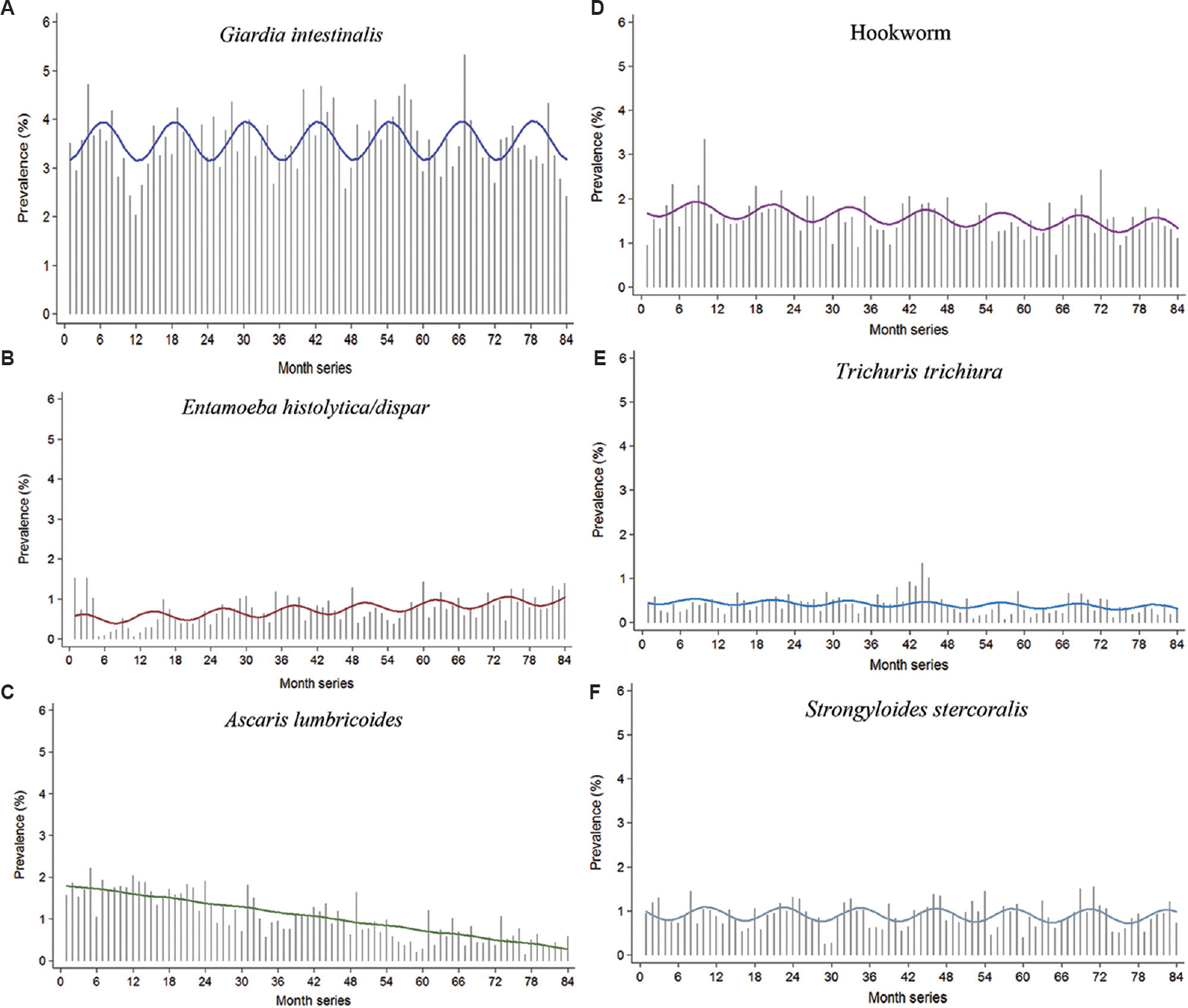
- Annual harmonic regression models for intestinal parasites versus the actual time series. The gray vertical spikes depict observed prevalence of the specific pathogens; the coloured horizontal lines depict the predicted prevalence obtained from the Poisson harmonic regression model.
Discussion
The overall prevalence of intestinal parasitic infections over the seven year period (2006-2012) was 8.9 per cent. This was lower than reports from the Indian subcontinent and elsewhere in the developing world513. This difference could be because of the setting, where many patients might already have been treated in the community before the collection of stool samples. In a similar hospital-based study of intestinal parasites from Rome, Italy, Masucci et al14 reported a total of 11.1 per cent of the total samples screened to be positive for intestinal parasites. Most studies from developing country settings have reported the prevalence of intestinal parasites from community settings and show wide variation. The reasons for such variation, apart from the actual difference in prevalence, could be because of different sampling methods employed, difference in the number of samples examined per patient, inclusion or exclusion of concentration techniques in the workup of samples.
In our study, the prevalence of intestinal parasites varied across age groups with the highest prevalence of 13 per cent seen in the age group 6-10 yr. Pre-school and school-going children have been found to be at the greatest risk for intestinal parasitic infections in some studies7, but others have reported higher prevalence in older age groups15.
The most common intestinal parasite among the patients screened was G. intestinalis. The prevalence of Giardia infection was higher in the younger age groups and it showed a decreasing trend with age. Data from the Global Enteric Multi-Center Study indicate that Giardia occurs commonly in asymptomatic infection and may even play a role in protection from diarrhea16.
In our study, the highest prevalence of hookworm and Strongyloides infection was found in the older population (>60 yr) and a significant correlation was found between older age and infection with these parasites. Similar trends have been reported in other studies16, and the intensity of hookworm infection has also been found to be higher in adults compared to children in some other parts of the world where hookworm disease is endemic17. This association between increased prevalence of hookworm infection and the elderly population in a region or setting might have important implications for the public health policies in countries/regions where such trends are seen, as efforts at control of STHs are usually focussed on the paediatric population17. Unlike hookworm and Strongyloides, there was no particular age-associated trend observed for Trichuris or Ascaris. The maximum intensity of infection with A. lumbricoides and T. trichiura was shown to be at about the age of 5-10 yr3. In our study, the highest prevalence of T. trichiura was in the age group 6-10 yr while that for A. lumbricoides was in the 26-30 yr age group, although the prevalence rates for A. lumbricoides were not significantly higher than that in younger age groups. Most studies in developing country settings have shown higher prevalence of Ascaris infections among children compared to older individuals18. However, some studies have reported higher prevalence in older age group when low-intensity infections were considered19. Similar trends of increasing hookworm infection prevalence with increasing age have been reported by Kaliappan et al20 from a tribal area in southern India. However, these relationships between age and prevalence of particular intestinal parasites, especially helminths, vary in different geographical regions and susceptibility to any helminthic infection, in general, and intestinal helminthic infections, in particular, cannot be considered as a function of age alone.
In our study, a significantly higher proportion of samples from male patients was found to be positive compared to females (10.4 vs. 6.5%). Although males have generally been found to be more predisposed to be positive for intestinal parasites21, the differences observed in the prevalence rates of these infections have not always been significant22. This difference in the prevalence and intensity of parasite infections has been attributed to increased exposure to certain parasites in males because of occupation and activity and also hormonal and immunological mechanisms such as the immunomodulatory effects of testosterone in males which increases their susceptibility to certain parasitic infections23. It has been established that females generally exhibit higher levels of immune responses as compared to males24.
A limitation of this retrospective analysis was the inability to distinguish between cysts of E. histolytica and E. dispar, both of which look similar on microscopy. The WHO has recommended25 the use of methods such as antigen detection and different molecular tools such as nested multiplex polymerase chain reaction (PCR) or real-time PCR for the specific diagnosis of E. histolytica.
The prevailing opinion that Blastocystis spp. are non-pathogenic has undergone a change with studies showing an association with this parasite and clinical disease26. The prevalence of Blastocystis spp. in our study was low, but in the majority of the Blastocystis-positive samples, no other intestinal parasite was concurrently encountered. We were unable to evaluate the association of Blastocystis spp. with clinical disease as enough clinical details were not available to study association.
Multiple intestinal parasitic infections were not common in this study. In a study from Lao People's Democratic Republic, 86.6 per cent of all study participants were found to harbour two or more different intestinal parasites concurrently27. Mehraj et al28 in a study from Karachi reported 10 per cent of all children screened for intestinal parasites had two or more than two intestinal parasites. Application of different diagnostic techniques and screening of multiple samples may be required to estimate the true burden of intestinal multiparasitism5.
Our study also looked at whether there was any seasonality in the prevalence of different intestinal parasites. For STH infections such as hookworm, Strongyloides and T. trichiura, the peak months of prevalence were during September and October, while for Giardia species, the peak was in June. These peaks corresponded to the months of high humidity levels and rainfall in our setting. Although our study was from a hospital setting, this seasonality was likely also a reflection of the picture in the community. For many intestinal parasites, variation in temperature, humidity and rainfall seems to affect the intensity of infections. This has especially been seen for parasites where there are external larval stages which develop in the soil29.
Temporal trend of decreasing STH infection over the seven year period could be a reflection of the same in the community because of the mass administration of albendazole and ivermectin as part of lymphatic filariasis control programmes in India for the past many years. The mass drug administration programme was introduced by the Government of India in 2004 and the population coverage for this programme has improved from 73 per cent in 2004 to 83 per cent in 201330. A study from Tamil Nadu by Kattula et al31 done in community settings among school children also reported much lower rates of STHs compared to previous reports from the area.
The major strength of this study was the long duration coverage and the number of samples analyzed. The limitations of this study included the lack of clinical correlation and the lack of complete testing on all samples, including concentration techniques. Since modified acid fast staining for intestinal coccidian parasites was performed only for samples from children less than five years of age, the actual burden of infection with these parasites could not be correctly estimated. The effect of antiparasitic treatment on the estimation of parasitic infections was also not taken into account as the information was not available for all the patients included in the analysis. Further, since this was a study from a tertiary care centre and a major proportion of patients screened for intestinal parasites were referred from other hospitals and healthcare centres, referral bias could be possible.
In conclusion, our study showed certain age-related trends such as higher prevalence of hookworm and Strongyloides in elderly age groups. Significant seasonality and overall decreasing temporal trends were seen for STHs such as hookworm and A. lumbricoides. Some of these findings may have public health implications.
Conflicts of Interest: None.
References
- Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasites & Vectors. 2014;7:37.
- [Google Scholar]
- Malnutrition and parasitic helminth infections. Parasitology. 2000;121(Suppl S1):S23-38.
- [Google Scholar]
- Hookworm-related anaemia among pregnant women: A systematic review. PLoS Negl Trop Dis. 2008;2:e291.
- [Google Scholar]
- The global burden of intestinal nematode infections - Fifty years on. Parasitol Today. 1997;13:438-43.
- [Google Scholar]
- Prevalence of intestinal parasites in rural Southern Indians. Trop Med Int Health. 1998;3:70-5.
- [Google Scholar]
- The prevalence of intestinal parasitic infections in the urban slums of a city in Western India. J Infect Public Health. 2013;6:142-9.
- [Google Scholar]
- Soil-transmitted helminth infections: Ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521-32.
- [Google Scholar]
- World Health Organization. Soil-Transmitted Helminthiasis: Country Data by Country. 2013. Available from: http://www.who.int/neglected_diseases/preventive_chemotherapy/sth/db
- [Google Scholar]
- “Barriers” to child development and human potential: The case for including the “neglected enteric protozoa” (NEP) and other enteropathy-associated pathogens in the NTDs. PLoS Negl Trop Dis. 2013;7:e2125.
- [Google Scholar]
- Intestinal nematodes: Disease burden, deworming and the potential importance of co-infection. Curr Opin Infect Dis. 2008;21:516-22.
- [Google Scholar]
- The global war against intestinal parasites - Should we use a holistic approach? Int J Infect Dis. 2010;14:e732-8.
- [Google Scholar]
- Seasonal synchronization of influenza in the United States older adult population. PLoS One. 2010;5:e10187.
- [Google Scholar]
- A cross-sectional study of intestinal parasitic infections among schoolchildren in Nan Province, Northern Thailand. Southeast Asian J Trop Med Public Health. 2002;33:218-23.
- [Google Scholar]
- Intestinal parasites isolated in a large teaching hospital, Italy, 1 May 2006 to 31 December 2008. Euro Surveill. 2011;16:pii: 19891.
- [Google Scholar]
- Changing trends in intestinal parasitic infections among long-term-residents and settled immigrants in Qatar. Parasit Vectors. 2010;3:98.
- [Google Scholar]
- Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet. 2013;382:209-22.
- [Google Scholar]
- Emerging patterns of hookworm infection: Influence of aging on the intensity of Necator infection in Hainan Province, People's Republic of China. Clin Infect Dis. 2002;35:1336-44.
- [Google Scholar]
- Age patterns in undernutrition and helminth infection in a rural area of Brazil: Associations with ascariasis and hookworm. Trop Med Int Health. 2008;13:458-67.
- [Google Scholar]
- Prevalence and clustering of soil-transmitted helminth infections in a tribal area in Southern India. Trop Med Int Health. 2013;18:1452-62.
- [Google Scholar]
- Prevalence of tuberculosis, hepatitis B virus, and intestinal parasitic infections among refugees to Minnesota. Public Health Rep. 2002;117:69-77.
- [Google Scholar]
- Prevalence and intensity of infections of Ascaris lumbricoides and Trichuris trichiura and associated socio-demographic variables in four rural Honduran communities. Mem Inst Oswaldo Cruz. 2001;96:303-14.
- [Google Scholar]
- Hormonal and immunological mechanisms mediating sex differences in parasite infection. Parasite Immunol. 2004;26:247-64.
- [Google Scholar]
- Effects of gender and sex steroids on the immune response. J Steroid Biochem. 1990;35:157-72.
- [Google Scholar]
- Helminth and intestinal protozoa infections, multiparasitism and risk factors in Champasack province, Lao People's Democratic Republic. PLoS Negl Trop Dis. 2011;5:e1037.
- [Google Scholar]
- Prevalence and factors associated with intestinal parasitic infection among children in an urban slum of Karachi. PLoS One. 2008;3:e3680.
- [Google Scholar]
- Soil-transmitted helminthiases: Implications of climate change and human behavior. Trends Parasitol. 2010;26:574-81.
- [Google Scholar]
- National Vector Borne Disease Control Programme. Mass drug Administration. New Delhi: Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India; 2015.
- [Google Scholar]
- Prevalence & risk factors for soil transmitted helminth infection among school children in South India. Indian J Med Res. 2014;139:76-82.
- [Google Scholar]






