Translate this page into:
Occurrence, patterns & predictors of hypogonadism in patients with HIV infection in India
Reprint requests: Dr. Deep Dutta, Department of Endocrinology, Post Graduate Institute of Medical Education & Research & Dr. Ram Manohar Lohia Hospital, 1 Baba Kharak Singh Marg, New Delhi 110 001, India e-mail: deepdutta2000@yahoo.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Data on hypogonadism among human immunodeficiency virus (HIV)-infected Indians are not available. This study was aimed to evaluate the occurrence, pattern and predictors of hypogonadism in HIV-infected Indians.
Methods:
Consecutive stable HIV-infected patients, 18-70 yr age, without any severe comorbid state, having at least one year follow up data at the antiretroviral therapy clinic, underwent clinical assessment and hormone assays.
Results:
From initially screened 527 patients, 359 patients (225 males; 134 females), having disease duration of 61.44±39.42 months, 88.58 per cent on highly active antiretroviral therapy (HAART), 40.67 per cent having tuberculosis history and 89.69 per cent with vitamin D insufficiency were analyzed. Testosterone <300 ng/dl was documented in 39.11 per cent males. Primary, hypogonadotropic hypogonadism (HypoH) and compensated hypogonadism were observed in 7.56, 31.56 and 12.44 per cent males, respectively. Males with hypogonadism were significantly older (P=0.009), and had higher opportunistic infections (P<0.001) with longer disease duration (P=0.05). Menstrual abnormalities were observed in 40.3 per cent females, who were significantly older (P<0.001), had lower CD4 count (P=0.038) and higher tuberculosis history (P=0.005). Nearly 46.3, 16.2 and 13 per cent women with menstrual abnormalities were in peri-/post-menopausal state, premature ovarian insufficiency (POI) and HypoH, respectively. Age, CD4 count at diagnosis and 25(OH)D were best predictors of male hypogonadism. Age and CD4 count increment in first 6-12 months following HAART were the best predictors of POI.
Interpretation & conclusions:
Hypogonadism was observed to be a significant problem in HIV-infected men and women in India, affecting 39 and 29 per cent patients, respectively. HypoH was the most common form in males whereas ovarian failure being the most common cause in females.
Keywords
Acquired immunodeficiency syndrome
human immunodeficiency virus
hypogonadism
premature ovarian insufficiency
testosterone
With the increasing burden of human immunodeficiency virus (HIV) epidemic, coupled with better treatment outcomes and longer survival due to highly active antiretroviral therapy (HAART), endocrine abnormalities are increasingly being encountered in HIV-infected patients1. Our group reported thyroid dysfunction to be very common in patients with HIV infection (25.04%) with subclinical hypothyroidism being the predominant form observed in 14.76 per cent patients1. No data are available on the prevalence, pattern and predictors of hypogonadism among HIV-infected patients in India. Early detection and treatment of hypogonadism is important as it is associated with increased frailty, impaired quality of life, poor bone mineral health, metabolic syndrome and adverse cardiovascular outcomes, resulting in increased morbidity and mortality234. The prevalence of hypogonadism among HIV-infected males across the globe has been reported to be variable ranging from 13 to 40 per cent2. Heterogeneity in the definition of hypogonadism, different serum testosterone cut-offs used for diagnosis, variability in demography and clinical characteristics of the cohort evaluated may explain this variability2. In spite of the considerable data on HIV and male hypogonadism from across the globe, data on pattern and predictors of hypogonadism in females with HIV infection are scant. Hence, the aim of this study was to evaluate the presence, patterns and predictors of hypogonadism in males and females with HIV infection attending an antiretroviral therapy (ART) clinic in a tertiary care hospital in north India.
Material & Methods
The study was conducted in the ART clinic at Post Graduate Institute of Medical Education and Research & Dr. Ram Manohar Lohia Hospital, New Delhi, India, during August 2014 to July 2015. Consecutive ambulatory patients, 18-70 yr age, with serologically documented HIV infection, in stable clinical condition without any acute, severe illness, attending the ART clinic were considered. Severely ill patients with multiple comorbid states, who would warrant hospital admission, were excluded. Patients with known primary disorders, afflicting the hypothalamic-pituitary-gonad axis (for example, hypogonadotropic hypogonadism, either isolated or as a part of multiple pituitary hormone deficiency), those with the previous history of glucocorticoid, androgens, anti-androgens or gonadotropin-releasing hormone analogues supplementation, and those with vitamin D and/or calcium supplementation in the last six months were also excluded. Patient records were reviewed and patients having clinical data of at least one year of follow up were further evaluated. Patients with available CD4 cell counts at diagnosis and at first follow up (6-12 months after diagnosis) were included in the study. The study protocol was explained to the patients and only those who gave informed written consent were included. The Institutional Ethics Committee approved the study protocol.
Data were collected from the patients and their records regarding the duration of diagnosis of HIV infection and details of HAART. Data were also collected regarding the past or current evidence of infections including opportunistic infections (bacterial, viral and fungal). History of erectile dysfunction (ED) was taken in males using the International Index of Erectile Function (IIEF-5) Questionnaire3. A detailed history of menstruation was taken in females. All patients underwent detailed clinical assessment including anthropometry. The patients were called the subsequent day in fasting state for blood sampling. Blood samples of 5 ml each were collected in plain and EDTA vacutainers (Becton Dickinson, USA). Serum was separated from blood collected in plain vacutainer and processed immediately for routine biochemical analysis, and one aliquot of serum was stored at −20° C for specific immunological (hormonal) assays. EDTA sample was processed for haematological analysis.
Chemiluminescent microparticle immunoassay (VITROS® ECiQ Immunodiagnostic System, Johnson & Johnson, USA) was used for the estimation of prolactin, testosterone, oestradiol, luteinizing hormone (LH), follicle-stimulating hormone (FSH) and 25-hydroxyvitamin D [25(OH)D]. The analytical sensitivity analytical range and intra- and inter-assay coefficient of variation (CV) respectively, for various assays were as follows: testosterone: 0.16 μg/dl, 4.90-2163 ng/dl, 3.8 and 7.4 per cent; LH: 0.216 mIU/ml, 0.216-200 mIU/ml, 8.8 and 11.3 per cent; FSH: 0.66 mIU/ml, 0.66-200 mIU/ml, 2.8 and 10.1 per cent. CD4 cell count was performed using flow cytometry (Becton Dickinson Immunocytochemistry Systems, USA). Serum calcium, phosphate, alkaline phosphate, electrolytes, lipid profile, liver and renal function tests were done using clinical chemistry autoanalyzer based on dry chemistry micro-slide technology (VITROS® 350 chemistry System, Johnson & Johnson, USA).
Hypogonadism in males was defined as serum testosterone <300 ng/dl45. Patients were subclassified into eugonadism (normal testosterone and normal LH), compensated hypogonadism (normal testosterone and elevated LH), primary hypogonadism/hypergonadotropic hypogonadism (HyperH) (low testosterone and elevated LH) and secondary hypogonadism/hypogonadotropic hypogonadism (HypoH) (low testosterone and low/normal LH)4. The reference range of LH in males in our laboratory was 1.4-9.2 mIU/ml. Females were subclassified into two groups, Group 1: those having regular menstruation and Group 2: those having oligomenorrhoea/amenorrhoea. Patients of Group 2 were further classified as per the aetiology of oligomenorrhoea/amenorrhoea [peri-/post-menopausal, premature ovarian insufficiency (POI), hypogonadotropic hypogonadism and undetermined cause], as per their serum FSH and oestradiol levels. Taking all the phases (early follicular, mid-follicular and luteal phase) of the cycle into consideration, the normal range of oestradiol in our laboratory was 20-350 pg/ml. The reference range of FSH in females was 1.4-21.4 mIU/ml. Oligomenorrhoea was defined as women having menstrual cycle duration of more than 35 days or less than nine cycles in the past year6. Amenorrhoea was defined as absence of menstruation of more than six months duration6. Peri-/post-menopausal state was defined in women having age more than 40 years. POI was defined as women <40 yr of age having oligomenorrhoea/amenorrhoea with serum FSH >40 mIU/ml7. HypoH was defined as women having oligomenorrhoea/amenorrhoea with serum LH lower than the normal reference limit7. Reference range of LH in females was 0.8-12.8 mIU/ml. Serum 25(OH)D ≥30 ng/ml was defined as vitamin D sufficiency, 20-30 ng/ml as vitamin D insufficiency and <20 ng/ml as vitamin D deficiency8.
Statistical analysis: Normality of the distribution of variables was checked using the Kolmogorov–Smirnov test. Independent t test and Wilcoxon rank sum test were done for normally distributed and skewed variables, respectively. Chi-square tests were used for categorical variables. Multiple linear regression analyses were done to determine variables that independently influenced the occurrence of hypogonadism after adjusting for factors in different models. SPSS version 20 (Chicago, IL, USA) was used for analyses.
Results
Five hundred and twenty seven consecutive patients were screened, of whom 359 patients (225 males and 134 females) who fulfilled all inclusion and exclusion criteria and gave informed written consent were included in the study (Fig. 1). The mean duration of HIV infection was 61.44±39.42 months. Eighty eight per cent (198/225) of males and 89.55 per cent (120/134) of females were receiving HAART (Tables I and II). One hundred and forty five of the 359 (40.67%) patients had a history of tuberculosis (Table I), 322 (89.69%) patients had 25(OH)D <30 ng/ml with mean of 20.23±9.13 ng/ml.
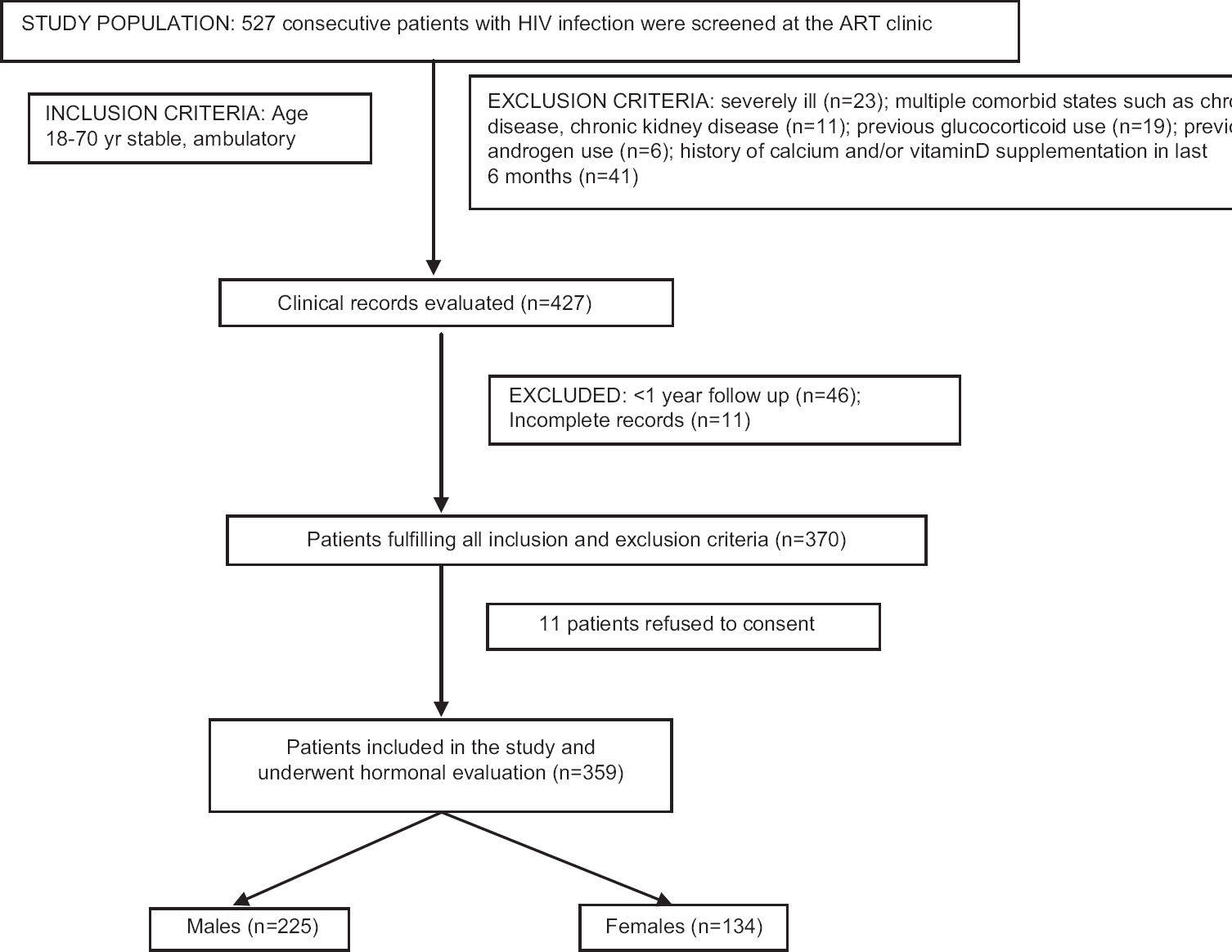
- Flowchart elaborating the study protocol and flow of patients.
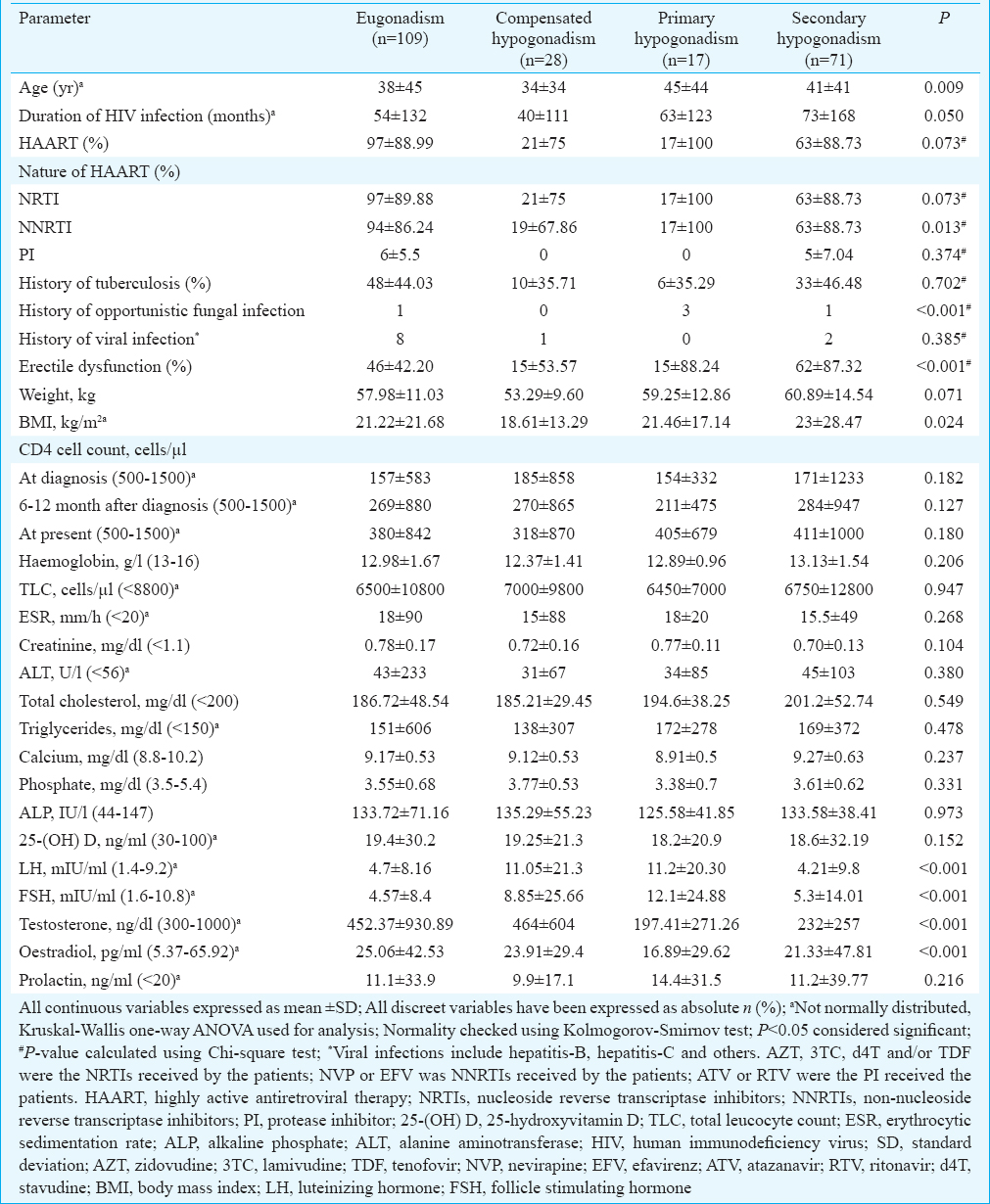
Of the 225 males, 88 (39.11%) with HIV infection had testosterone <300 ng/dl (Fig. 2). Primary, secondary and compensated hypogonadism were observed in 7.56 per cent (17/225), 31.56 (71/225) and 12.44 per cent (28/225) males, respectively (Table I and Fig. 2). One hundred and thirty eight males (61.33%) complained of ED (Table I). ED was significantly more common in men with serum testosterone <300 ng/dl. Patients with testosterone <300 ng/dl (primary and secondary hypogonadism) had significantly lower oestradiol as compared to those with compensated hypogonadism and eugonadism. Male patients with hypogonadism were significantly older (P=0.009) and tended to have a longer duration of HIV infection (P=0.05) as compared to those with eugonadism (Table I). The occurrence of opportunistic infections was significantly higher in males with primary or secondary hypogonadism. CD4 cell counts, use and nature of HAART, haemogram, lipid parameters and 25(OH)D levels were comparable among males with hypogonadism as compared to those without (Table I).
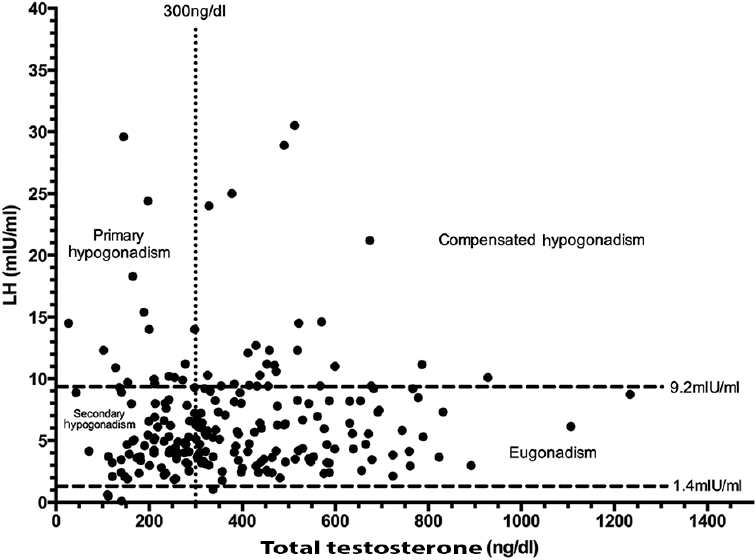
- Scatter plot showing the relationship between serum testosterone and luteinizing hormone (LH) levels in male patients with human immunodeficiency virus infection (n=225). The distribution of patients with eugonadism, compensated hypogonadism, secondary hypogonadism and primary hypogonadism has been elaborated.
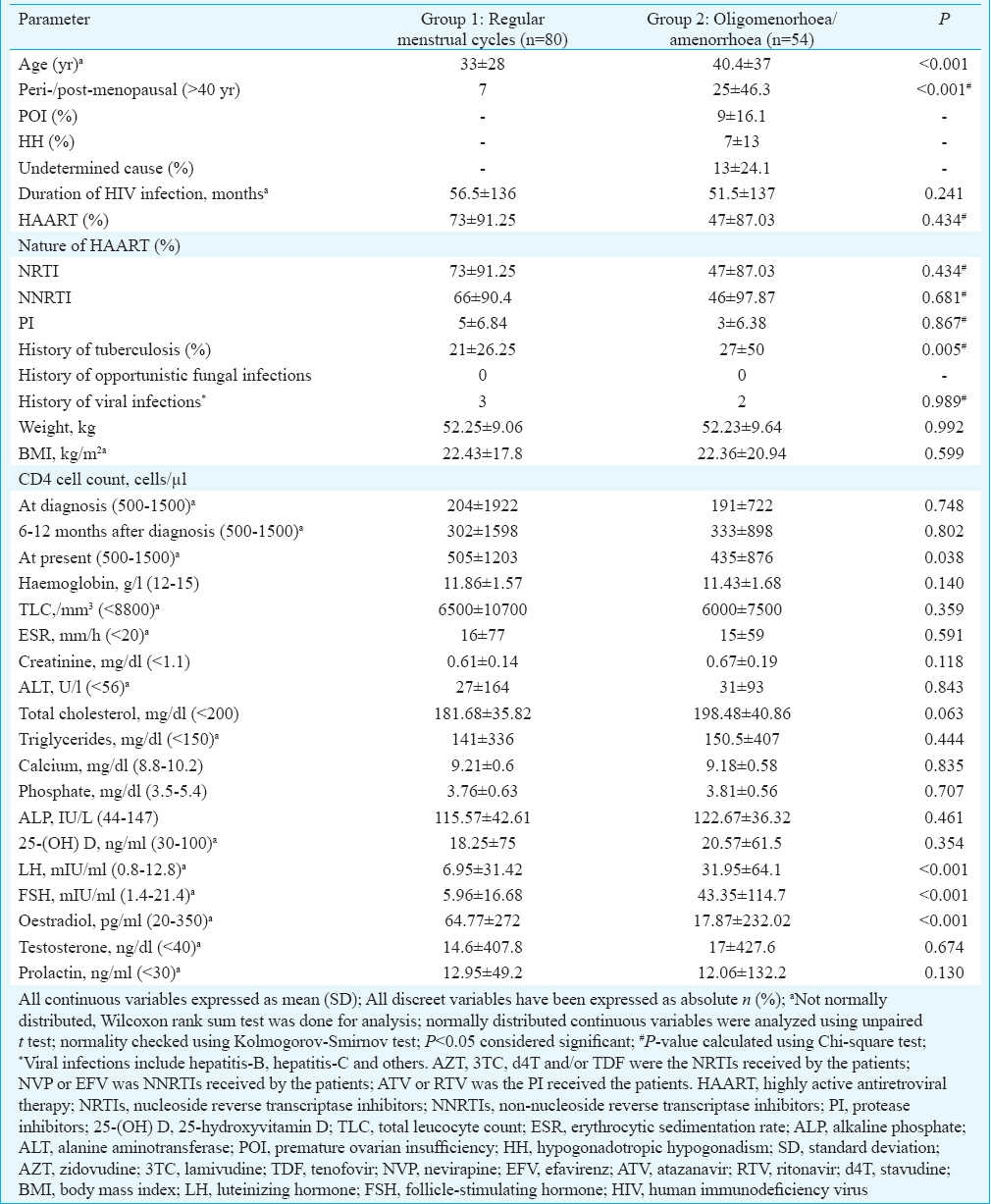
Menstrual abnormalities (oligomenorrhoea/amenorrhoea) were observed in 40.3 per cent (54/134) of HIV-infected females (Table II). Patients with menstrual abnormalities were significantly older (P<0.001), had lower CD4 cell count (P=0.038) and had significantly higher history of tuberculosis (P=0.005) as compared to those with regular cycles (Table II). Twenty five of the 54 women (46.3%) with menstrual abnormalities were in the peri-/post-menopausal state with age >40 yr (Fig. 3A and B). Nine (16.2%) and seven (13%) women had menstrual abnormalities due to POI and HypoH, respectively (Fig. 3A, B and Table II). The cause of menstrual abnormality could not be determined in 13 (24.1%) females (Table II).
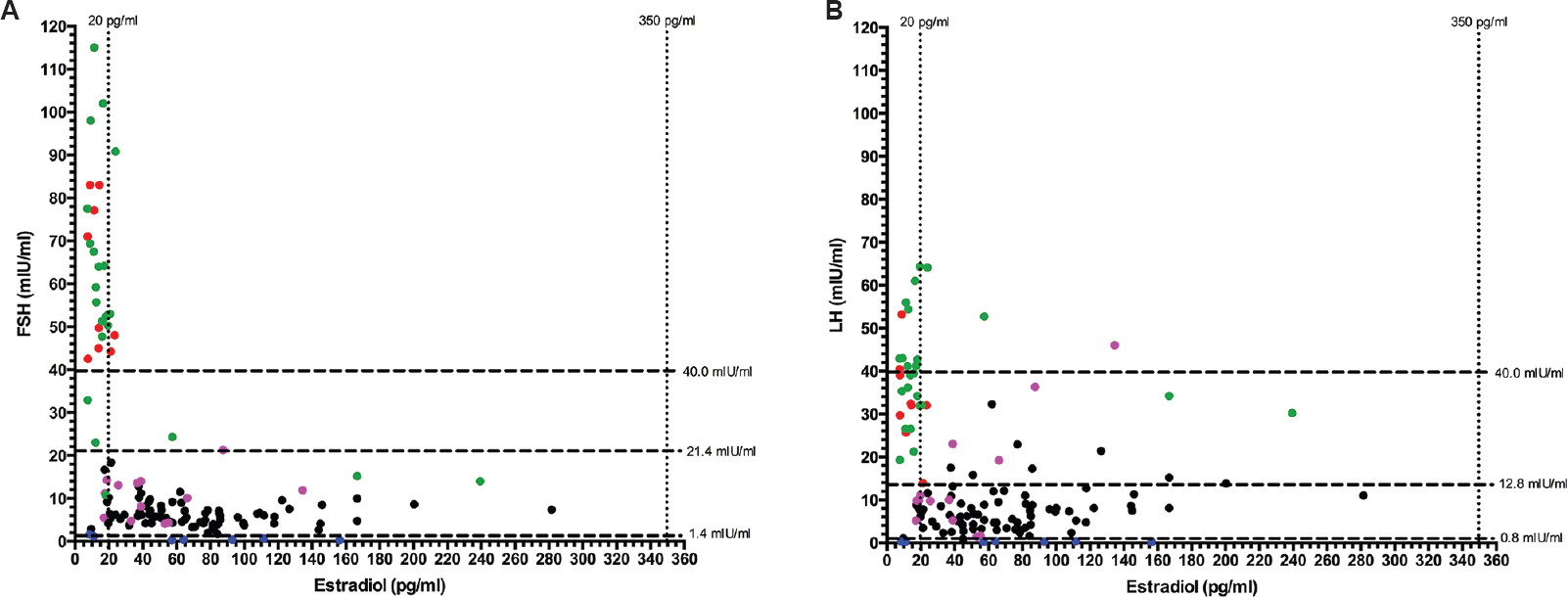
- (A) Scatter plot showing the relationship between follicle-stimulating hormone (FSH) and serum estradiol in females with HIV infection; (B) scatter plot showing relationship between luteinizing hormone (LH) and serum estradiol in females with HIV infection. Black dots: women with regular menses (n=80); Green dots: peri-/post-menopausal women >40 yr age with oligomenorrhea (n=25); Red dots: Women with oligomenorrhoea/amenorrhea due to premature ovarian insufficiency (n=9); Pink dots: Women with oligomenorrhoea/amenorrhea with cause not determined (n=13); Blue dots: Women with oligomenorrhoea/amenorrhoea due to hypogonadotropic/hypogonadism (n=7).
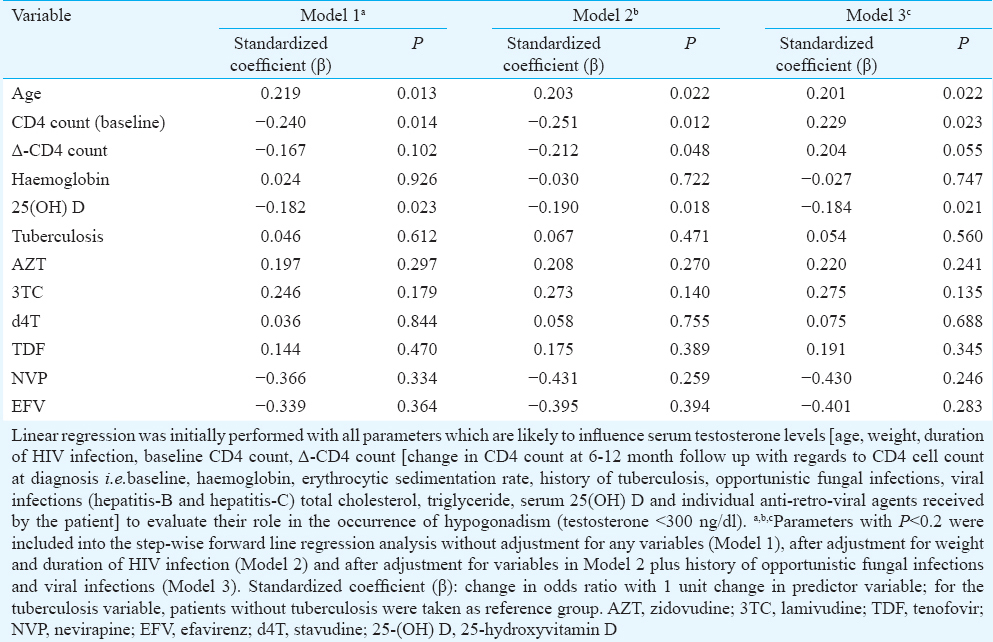
Stepwise linear regression analysis revealed age, CD4 cell count at the diagnosis of HIV infection and 25(OH)D to be persistent predictors of occurrence of low testosterone (<300 ng/dl), at baseline (Model 1), after adjustment for weight and duration of HIV infection (Model 2) and after additional adjustment for opportunistic fungal infections and viral infections (Model 3) (Table III). Increasing age, lower baseline CD4 count and lower 25(OH)D levels were associated with significantly higher occurrence of low serum testosterone (Table III). History of opportunistic infections, tuberculosis and nature of HAART received were not predictors of hypogonadism in males.
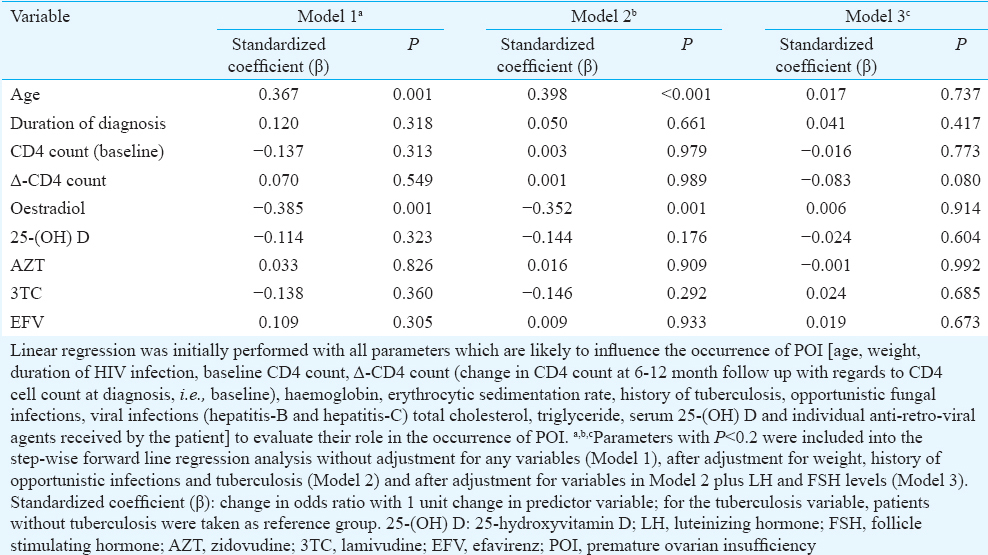
Age was the only consistent predictor of POI in women with HIV infection <40 yr age, with increasing age associated with increased occurrence of POI (Table IV). Serum oestradiol as an independent predictor of POI was lost after adjusting for LH and FSH (Model 3) (Table IV). Δ-CD4 count (change in CD4 count at 6-12 months follow up with regard to baseline) tended to be a good predictor of occurrence of POI, after adjusting for weight, opportunistic infections, tuberculosis, LH and FSH (Model 3). The greater increment in CD4 count was associated with lower risk of POI (P=0.08) (Table IV).
Discussion
Hypogonadism was observed in 39.11 per cent HIV-infected males in our study with HypoH being the predominant form, constituting 80.68 per cent of all patients with testosterone <300 ng/dl, which was in accordance with the previous report9. Rochira et al10 reported HypoH in 86 per cent hypogonadal patients with testosterone <300 ng/dl. Our study showed that males with hypogonadism were significantly older and had HIV infection for longer duration. Increasing age, lower baseline CD4 count and lower 25(OH)D were observed to be predictors of male hypogonadism. Previous studies have demonstrated age, body mass index (BMI) and increased visceral adiposity to be predictors of hypogonadism in males91011. However, literature linking CD4 count and viral load to hypogonadism have been conflicting with some but not all reports documenting a link291011.
Low vitamin D has been reported to be common in patients with HIV infection12. Vitamin D deficiency has been linked with more rapid decline in CD4 cell count, higher occurrence of osteoporosis, cardiovascular disease, diabetes, autoimmune disease and cancer in HIV-infected patients121314. Vitamin D insufficiency among HIV-infected patients in our study was 89.69 per cent, which was higher than that observed in the general Indian population (70-75%)15. Increased prevalence of vitamin D insufficiency in HIV-infected men has been reported from Italy16. Low vitamin D was found to be a predictor of hypogonadism among males in our study. In the European Male Ageing Study17, evaluation of 3369 community-dwelling men aged 40-79 yr in eight European centres, revealed a significant association of vitamin D deficiency with secondary (relative risk ratio 1.16, P=0.05) and compensated (relative risk ratio 1.52, P=0.03) hypogonadism. Similarly, low vitamin D was associated with a higher prevalence of hypogonadism (odds ratio 1.50; 95% confidence interval 1.14-1.97) in 2854 community-dwelling Chinese men evaluated from 16 different centres18. Type-2 diabetes patients with hypogonadism have been reported to have a significantly higher prevalence of vitamin D deficiency as compared to those without hypogonadism19. The underlying mechanism linking vitamin D with hypogonadism has not been well determined. Increased systemic inflammation, inflammatory cytokines and insulin resistance, which are commonly seen with vitamin D insufficiency may have some role182021. It has been demonstrated that supplementation of calcidiol (25-hydroxylated form of vitamin D) and not its precursor cholecalciferol (commonly used for treatment of vitamin D deficiency) is a better way to restore restores 25(OH)D levels in patients with hypogonadism22. Leydig cells in testis express CYP2R1 gene, which encodes the major enzyme involved in 25-hydroxylation of vitamin D. Hypogonadism is associated with decreased expression of CYP2R1, which results in decreased 25-hydroxylation enzyme activity, leading to decreased hydroxylation of cholecalciferol, which may also contribute to low 25(OH)D levels in these patients22.
The mechanism of hypothalamic-pituitary-gonadal axis suppression in HIV-infected patients has not been well studied and is likely to be multifactorial. Low body weight and BMI may be markers of frailty, which is associated with a functional hypothalamic suppression resulting in HypoH. Increased adiposity, especially visceral adiposity, altered body fat distribution secondary to lipodystrophy may lead to increased aromatization of androgens to oestrogen, contributing to LH and FSH suppression210. Body fat distribution and lipodystrophy were not assessed in this study and were limitations of this study. Primary hypogonadism among HIV-infected males in this study was only 7.56 per cent, which was in accordance with previous reports10. However, 12.44 per cent males had compensated hypogonadism (elevated LH with normal testosterone), which might be a precursor to primary hypogonadism later in life. Testicular inflammation, direct testicular damage, reduction in Leydig cell number in patients with more severe disease explains the development of primary hypogonadism223. It has been suggested that the prevalence of primary hypogonadism in HIV-infected males declines with the introduction of HAART23. ED was common among HIV-infected men in this study. The previous studies have also reported a high prevalence of ED among HIV-infected males ranging from 55 to 74 per cent924.
Data on patterns and predictors of hypogonadism in HIV-infected females are scant. A study of 123 HIV-infected women aged >50 yr from England revealed higher rates of premature menopause (6.8%), early menopause (6.8%) and cervical cytological abnormalities (47%)25. A pilot study from Burkina Faso showed higher prevalence of premature menopause in 55 HIV-infected women (5.5%) as compared to normal women (1.4%)26. A study on 78 HIV-infected females from France observed abnormal FSH, inhibin-B and anti-Mullerian hormone in 36, 57 and 23 per cent patients, respectively, suggestive of impaired ovarian reserve and reduced fertility27. Studies from the USA and Latin America have suggested HIV infection to be independently associated with menopause-related symptoms282930. Schoenbaum et al31 reported drug addiction (cocaine and heroin) and CD4 counts to be predictors of early menopause in HIV-infected American women. CD4 counts >200 cells/μl were associated with significantly reduced risk of early menopause31. In a large study of 1139 HIV seropositive women from the USA, in more than half of the women with prolonged amenorrhoea (>1 yr duration), the cause could not be determined and they did not have elevated FSH (ovarian failure)32. In our study, nearly half of the women with menstrual abnormalities were >40 yr age. Sixteen and 13 per cent women had menstrual abnormalities due to POI and HypoH, respectively. The cause could not be determined in nearly a quarter of the patients, even after evaluation of their LH, FSH, oestradiol and testosterone levels, suggesting need for further investigations. Polycystic ovarian syndrome is one of the most common causes of menstrual abnormalities in women in reproductive age group7. Lack of assessment of ovarian morphology in this study was another limitation. Increased age was associated with increased risk of POI in women <40 yr age. Our study showed that a better response to HAART (in terms of increase in CD4 count following initial 6-12 months of therapy) during the early part of the disease was associated with a lower risk of POI later. Lack of detailed assessment of clinical features of hypogonadism along with quality of life (using validated questionnaires) was also a limitation of this study. A few studies have suggested that sex hormone-binding globulin (SHBG) is increased in patients with AIDS333435. Monroe et al35 have suggested the use of free testosterone for the diagnosis of hypogonadism in HIV-infected males, as total testosterone may be falsely elevated secondary to increased SHBG, leading to miss of the diagnosis in many patients. Free testosterone assay was not done in our study. However, free testosterone assay is less reliable, less robust and less easily available as compared to total testosterone assay. Hence it is possible that our study may have slightly underestimated the prevalence of hypogonadism among HIV-infected males.
In conclusion, our findings showed that hypogonadism is a significant problem in HIV-infected men and women in India, affecting 39 and 29 per cent patients, respectively. HypoH was the commonest form in HIV-infected males. Menstrual abnormalities were observed in 40 per cent of HIV-infected women with HyperH being the commonest cause. Increased age was a risk factor of hypogonadism in both sexes. Low baseline CD4 count and increment in CD4 count following initiation of HAART were good predictors of hypogonadism in males and females, respectively. Our study also reported that low 25(OH)D was a good predictor of hypogonadism in HIV-infected males.
Acknowledgment
The authors acknowledge the assistance of the staff of the ART clinic and laboratory technicians of the nursing home laboratory of the department of biochemistry, Dr Ram Manohar Lohia Hospital, New Delhi.
Conflicts of Interest: None.
References
- Prevalence and predictors of thyroid dysfunction in patients with HIV infection and acquired immunodeficiency syndrome: An Indian perspective. J Thyroid Res. 2015;2015:517173.
- [Google Scholar]
- Hypogonadism in the HIV-infected man. Endocrinol Metab Clin North Am. 2014;43:709-30.
- [Google Scholar]
- Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11:319-26.
- [Google Scholar]
- Characteristics of secondary, primary, and compensated hypogonadism in aging men: Evidence from the European Male Ageing Study. J Clin Endocrinol Metab. 2010;95:1810-8.
- [Google Scholar]
- Testosterone therapy in men with androgen deficiency syndromes: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536-59.
- [Google Scholar]
- Hirsutism and oligomenorrhea are appropriate screening criteria for polycystic ovary syndrome in adolescents. Gynecol Endocrinol. 2015;31:625-9.
- [Google Scholar]
- Physiology and pathology of the female reproductive axis. In: Melmed S, Polonsky KS, Larsen PR, Kronenberg HM, eds. Williams textbook of endocrinology. Vol 17. Boston, MA, USA: Saunders Elsevier; 2011. p. :632-3.
- [Google Scholar]
- Vitamin D: Importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79:362-71.
- [Google Scholar]
- Erectile dysfunction and hypogonadism among men with HIV. AIDS Patient Care STDS. 2007;21:9-19.
- [Google Scholar]
- Premature decline of serum total testosterone in HIV-infected men in the HAART-era. PLoS One. 2011;6:e28512.
- [Google Scholar]
- Prevalence of causes of secondary osteoporosis and contribution to lower bone mineral density in HIV-infected patients. Osteoporos Int. 2014;25:1071-9.
- [Google Scholar]
- Vitamin D deficiency may be associated with a more rapid decline in CD4 cell count to <350 cells/μL in untreated HIV-infected adults. Curr HIV Res. 2015;13:517-23.
- [Google Scholar]
- Vitamin D deficiency in HIV infection: Not only a bone disorder. Biomed Res Int. 2015;2015:735615.
- [Google Scholar]
- Vitamin-D supplementation in prediabetes reduced progression to type 2 diabetes and was associated with decreased insulin resistance and systemic inflammation: An open label randomized prospective study from Eastern India. Diabetes Res Clin Pract. 2014;103:e18-23.
- [Google Scholar]
- Serum total estradiol, but not testosterone is associated with reduced bone mineral density (BMD) in HIV-infected men: A cross-sectional, observational study. Osteoporos Int. 2016;27:1103-14.
- [Google Scholar]
- Association of hypogonadism with Vitamin D status: The European Male Ageing Study. Eur J Endocrinol. 2012;166:77-85.
- [Google Scholar]
- Vitamin D is associated with testosterone and hypogonadism in Chinese men: Results from a cross-sectional SPECT-China study. Reprod Biol Endocrinol. 2015;13:74.
- [Google Scholar]
- Vitamin D deficiency in type 2 diabetic patients with hypogonadism. J Sex Med. 2014;11:536-42.
- [Google Scholar]
- Serum Vitamin-D predicts insulin resistance in individuals with prediabetes. Indian J Med Res. 2013;138:853-60.
- [Google Scholar]
- Urinary albumin creatinine ratio predicts prediabetes progression to diabetes and reversal to normoglycemia: Role of associated insulin resistance, inflammatory cytokines and low vitamin D. J Diabetes. 2014;6:316-22.
- [Google Scholar]
- Highly active antiretroviral therapy (HAART) and testicular morphology: Current status and a case for a stereologic approach. J Androl. 2012;33:1130-42.
- [Google Scholar]
- Erectile dysfunction is not a mirror of endothelial dysfunction in HIV-infected patients. J Sex Med. 2012;9:1114-21.
- [Google Scholar]
- Care of HIV-positive women aged 50 and over - Can we do better? Int J STD AIDS. 2014;25:303-5.
- [Google Scholar]
- Is ovarian function impaired in HIV patients? A clinical pilot study in Burkina Faso. Rev Med Brux. 2013;34:397-404.
- [Google Scholar]
- Alterations of ovarian reserve tests in Human Immunodeficiency Virus (HIV)-infected women. Gynecol Obstet Fertil. 2010;38:313-7.
- [Google Scholar]
- Menopause symptoms in HIV-infected and drug-using women. Menopause. 2005;12:348-56.
- [Google Scholar]
- Age at menopause and factors associated with menopause symptoms in HIV-infected women. Menopause. 2006;13:1021.
- [Google Scholar]
- Menopause symptoms in women infected with HIV: Prevalence and associated factors. Gynecol Endocrinol. 2007;23:198-205.
- [Google Scholar]
- HIV infection, drug use, and onset of natural menopause. Clin Infect Dis. 2005;41:1517-24.
- [Google Scholar]
- Effects of human immunodeficiency virus on protracted amenorrhea and ovarian dysfunction. Obstet Gynecol. 2006;108:1423-31.
- [Google Scholar]
- Alterations in the concentrations and binding properties of sex steroid binding protein and corticosteroid-binding globulin in HIV+ patients. J Endocrinol Invest. 1992;15:597-603.
- [Google Scholar]
- Sex hormones, insulin resistance, and diabetes mellitus among men with or at risk for HIV infection. J Acquir Immune Defic Syndr. 2011;58:173-80.
- [Google Scholar]
- Morning free and total testosterone in HIV-infected men: Implications for the assessment of hypogonadism. AIDS Res Ther. 2014;11:6.
- [Google Scholar]






