Translate this page into:
Comparative study of clozapine versus risperidone in treatment-naive, first-episode schizophrenia: A pilot study
Reprint requests: Dr Ajeet Sidana, Department of Psychiatry, Government Medical College & Hospital, Sector 32, Chandigarh 160 030, India e-mail: ajeetsidana@hotmail.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Clozapine may be more useful in treatment-naive patients with first-episode schizophrenia for better symptoms control and improving quality of life. The current study was carried out to compare the efficacy and tolerability of clozapine versus risperidone in treatment-naive, first-episode patients of schizophrenia.
Methods:
This was a comparative, open-label, six months prospective study of treatment-naive, first-episode patients with schizophrenia between the age group of 18 and 40 yr diagnosed as per the International Classification of Diseases-10 (ICD-10) criteria. A total of 63 patients were recruited and randomly assigned to clozapine group or risperidone group using computer-generated random number tables. Eight patients were lost to follow up. The dosages of the respective drugs were kept in therapeutic range of 200-600 mg/day and 4-8 mg/day orally for clozapine and risperidone, respectively.
Results:
On general psychopathology score, after six months of intervention, clozapine led to 60.32 per cent mean reduction in Positive and Negative Syndrome Scale (PANSS) for Schizophrenia total score while risperidone led to 56.35 per cent mean reduction in PANSS total score, which meant more improvement with clozapine. Clozapine group was found to have significant improvement in quality of life (P = 0.04339). On Glasgow Antipsychotic Side-effect Scale, clozapine was superior to risperidone. The most common side effects observed in clozapine group were oversedation (78.96%) and dizziness (55.23%), and in risperidone group, common side effects were rigidity (62.36%), sedation (38.69%), tremors (65.69%) and menstrual irregularities in 80.25 per cent of female patients.
Interpretation & conclusions:
The findings of this preliminary study showed clozapine as a better choice than risperidone in terms of efficacy, tolerability and better quality of life in treatment-naive, first-episode schizophrenia. However, further studies need to be done on a larger group of patients to confirm the findings.
Keywords
Clozapine
first-episode schizophrenia
risperidone
treatment-naive
Schizophrenia is a complex, highly debilitating disorder with profound and disruptive psychopathology that involves cognition, emotion, perception and other aspects of behaviour and accounts for 1.1 per cent of the disability-adjusted life years (DALYs) and 2.8 per cent of years lived in disability (YLDs)1. Van Kanmen and Marder2 stated that clozapine was the first of the atypical antipsychotics to be developed, which was later withdrawn by the manufacturer in 1975 after it was shown to cause agranulocytosis. In 1989, after studies3 demonstrated that clozapine was more effective than any other antipsychotic for treating schizophrenia3, the U.S. Food and Drug Administration (US-FDA) approved clozapine's use only for treatment-resistant schizophrenia (TRS) (www.accessdata.fda.gov/drugsatfda_docs/label/2014/019758s073lbl.pdf). Though clozapine has been found to be the most effective antipsychotic drug (APD) for TRS4, its use is still restricted because of the fear of agranulocytosis. However, the frequency of clozapine-induced agranulocytosis has been shown to be <1 per cent34.
There have been only a few studies available on clozapine as the first-line treatment for schizophrenia567. Girgis et al6 did not find any benefit for clozapine over conventional antipsychotic drug for acute treatment of first episode of schizophrenia or schizoaffective disorder although they mentioned that clozapine led to better adherence and greater tolerability. Lieberman et al8 found that clozapine and chlorpromazine led to approximately 80 per cent reduction in Brief Psychiatric Rating Scale (BPRS) total score. However, in the secondary analysis, clozapine demonstrated an advantage of shorter median time to remission compared with chlorpromazine.
The available data have also suggested that the long-term outcome does not depend on type of antipsychotic used as the first line agent. Sanz-Fuentenebro and colleagues9 compared clozapine with risperidone in patients with first-episode psychosis and suggested that clozapine might have a slightly superior efficacy initially in the treatment of first-episode treatment naive patients with schizophrenia. No published data are available from India. Hence, the current study was planned to compare effectiveness and safety of clozapine and risperidone in a group of treatment-naive, first-episode patients with schizophrenia.
Material & Methods
It was a comparative, open-label, prospective pilot study with intent to treat analysis. The patients were allocated to either of the group on the basis of computer-generated random table number. The patients were followed up for six months after initiation of treatment. Patients were inducted from out- and inpatients both attending the department of Psychiatry, Government Medical College and Hospital, Chandigarh, a tertiary care teaching hospital of north India. Patients with the diagnosis of schizophrenia as per the International Classification of Diseases (ICD-10) criteria10, who gave informed written consent, and fulfilled inclusion and exclusion criteria were enrolled in the study from December 2010 to June 2011, and the study was completed in January 2012. The consecutive patients of first-episode, treatment-naive schizophrenia, between the age group of 18 and 40 years, were included in the study. Patients having history of seizure disorder, heart conduction defects, history of agranulocytosis or total leucocyte counts (TLC) <3500/mm3 and diabetes mellitus, neurological disorders, head injury, movement disorder, lactating or pregnant women, patients with comorbid substance dependence except nicotine and patients with subnormal intelligence were excluded from the study. The patients were allocated to either of the group on the basis of computer-generated random table number. The dosages of the respective drugs were kept in therapeutic range of 200-600 mg/day and 4-8 mg/day for clozapine and risperidone, respectively.
Clozapine was started at the dose of 12.5 mg and risperidone at 2 mg/day and efforts were made to reach an adequate dose in all the patients within one month of starting the treatment, and after reaching this dose, patients were observed for a period of four weeks11. At the end of four weeks after achieving the adequate dose, if the response was not adequate [< 50% reduction in Positive and Negative Syndrome Scale (PANSS)], then in these patients the dose of the respective drug was further increased depending on the serum levels of the concerned drug and attempt was made to bring the serum levels of both the drugs within therapeutic range. The effective levels for clozapine were taken as 200-300 μg/l12 and risperidone as 20-60 μg/l13. However, those who did not respond to clozapine dose could be adjusted to reach a plasma level between 350 and 500 μg/l as per the Maudsley prescribing guidelines13. Patients who showed response to the drugs were continued as per the assessment schedule at one, two, four and at six months. Concomitant medications such as benzodiazepines for restlessness and sleep disturbances, anticholinergic drugs for extrapyramidal side effects and clonidine and amitriptyline for sialorrhoea were permitted wherever required and doses of such medications were documented in both the groups. Sociodemographic and clinical variables of patients were recorded in the prescribed proforma designed for the study.
TLC, differential leucocyte count, platelet count and absolute neutrophil counts were measured on weekly basis for initial 18 wk as per the Maudsley prescribing guidelines13 for patients receiving clozapine and thereafter monthly. Measurements of body mass index, lipid profile, fasting blood sugar and serum drug levels were done at one, two, four and six months. The drug levels were measured by reversed-phase high-pressure liquid chromatography technique14, and blood samples (3-5 ml) were collected into vacuum tubes in the morning, before the administration of morning dose. Besides these assessments, patients were on regular follow up after every two weeks, for six months. Patients were assessed for psychopathology on PANSS15, World Health Organization Quality of Life (WHOQOL)- BREF16, Arizona Sexual Experience Rating Scale (ASEX)17 and side effects on Glasgow Antipsychotic Side-effect Scale (GASS)18 were administered for assessing quality of life, sexual functioning and medication side effects at baseline, one, two, four and six months, respectively. All these scales were used in the morning before administration of morning dose. Patients were asked to bring the empty strips of medicines in both the groups during every follow up visit.
The confidentiality of the information obtained was maintained and the principles enunciated in the ICMR's ethical guidelines for biomedical research on human cases were adhered to19. The study was approved by the Institutional Ethics Committee.
Statistical analysis: The statistical analysis was carried out using Chi-square test for qualitative data and repeated measures ANOVA and MANOVA for quantitative data; t test was applied to compare individual assessments. Data were analyzed using SPSS 16.0 version software (SPSS Inc., Chicago, IL, USA) for heterogeneous data, test was applied after appropriate transformation.
Results
A total of 63 patients were recruited and were randomly assigned to group A (clozapine group, n=28) or group B (risperidone group, n=27) using computer-generated random number table. Eight patients were lost to follow up. The two groups were comparable on sociodemographic variables including mean age, sex, locality, education, occupation, income, family type and marital status. The mean duration of illness was 19.39 ±15.28 months in clozapine group and 18.63 ±14.67 months in risperidone group. The difference in duration of illness between two groups was not significant.
Table I shows comparison between two groups at baseline assessment. No significant differences emerged on PANSS total scores between the two groups at the baseline. In addition, the baseline comparison between the two groups for sexual functioning on ASEX scale (clozapine group: 18.48 ±2.94; risperidone group: 18.17 ±3.03, quality of life on WHOQOL-Bref (clozapine group: 35.07 ±6.74; risperidone group: 33 ±7.95), and body mass indices (clozapine group: body mass index (BMI) 26.13 ±2.756; risperidone group: 25.36 ±2.793 kg/m2 revealed no significance.
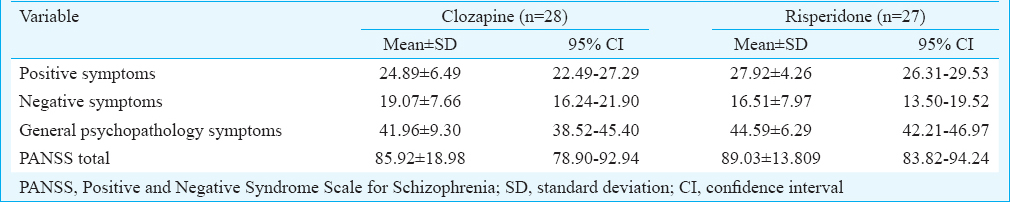
Table II shows the comparison of positive symptoms on PANSS at baseline and across four assessments between the two groups. Both the drugs led to significant reduction in positive symptom score from baseline and two groups were comparable. However, clozapine led to greater recovery in negative symptom [mean = 7.39, 95% confidence interval (CI) = 6.47-8.31, P<0.01] score as well as total PANSS score (mean = 33.46, 95% CI = 31.65-35.27, P< 0.05) compared to risperidone and the difference was significant.
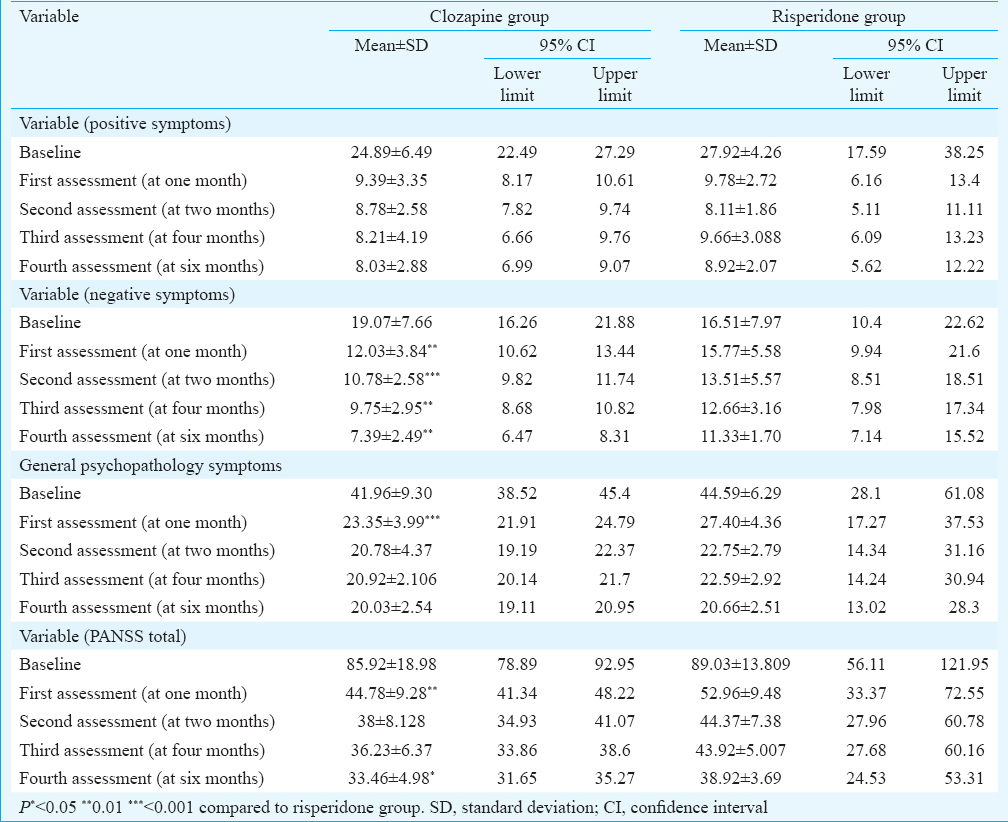
Drug plasma levels were estimated at six months. At this point of time, patients in clozapine group received mean dose of 289.28 mg, with plasma levels of 206.54 μg/l and there was 60.32 per cent reduction in PANSS total score in clozapine group. Similarly, in risperidone at six months, there was 56.35 per cent reduction in total PANSS scores at the mean dose of 6.85 mg, with plasma level of 54.51 μg/l.
The comparison on GASS between the two groups revealed significant difference at each assessment, and clozapine was found to be superior than risperidone. The most common side effects observed in clozapine group were sedation (78.96%), dizziness (55.23%) and drooling of saliva at night (46.32%). The most common side effects noted in risperidone group were rigidity (62.36%), sedation (38.69%), tremors (65.69%) and menstrual irregularities in 80.25 per cent of female patients. However, none of the patients discontinued treatment because of side effects.
Clozapine group was found to have significant improvement in quality of life at all the assessments, and it was found to be significant between the two groups (mean = 49.67, 95% CI = 47.93-51.41, P<0.05) (Table III).
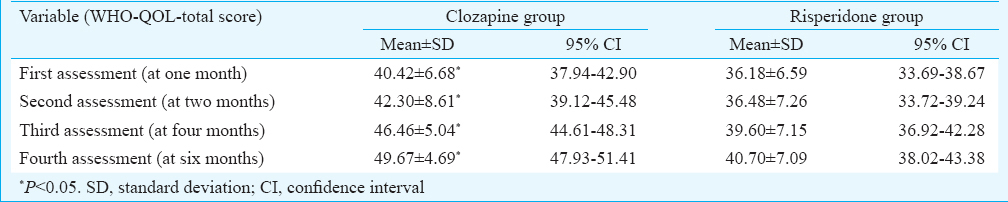
Patients in clozapine group had a significant increase in BMI as compared to the risperidone group from fourth month onward; however, none of the patient in either group developed hypertension, impaired glucose tolerance, dyslipidaemia or any other electrocardiogram abnormalities. Similarly, comparison on ASEX did not reveal any significance.
Discussion
In the present study, both the groups were comparable on sociodemographic variables as well as on severity of psychopathology and quality of life at the baseline. The results showed that both clozapine and risperidone fared equally well in terms of reducing the positive symptoms whereas clozapine was significantly superior to risperidone in reduction of negative symptoms at each assessment.
Earlier studies carried out on clozapine versus typical antipsychotics did not find benefit of clozapine over conventional antipsychotics in patients of first episode of schizophrenia or schizoaffective disorder567. Girgis et al6 studied 160 individuals with treatment-naive, first-episode schizophrenia or schizophreniform disorder who were randomized to clozapine or chlorpromazine treatment for up to two years and reported no significant differences in the effectiveness of two drugs. However, the adherence to clozapine was better than chlorpromazine (26 vs. 10%, P=0.01). Woerner et al5 while studying 34 cases of first episode of schizophrenia or schizoaffective disorder concluded that there was no benefit of clozapine over conventional antipsychotics for acute treatment of the first episode.
The Cochrane schizophrenia group performed a meta-analysis to compare several commonly used second-generation antipsychotics (SGAs, risperidone, olanzapine, clozapine, amisulpride and quetiapine) agents to investigate the efficacy and tolerability of these drugs in patients with schizophrenia or schizophrenia-like psychoses20, and the results showed that among the SGAs, no drug was better than the other. Since many patients included in the meta-analysis were on low or very low doses of clozapine, several had an upper limit of 400 mg/day and five studies used dosages under 210 mg/day; it could be possible that many patients did not receive therapeutic levels of clozapine resulting in poor outcome as compared to the present study. In a study by McEvoy et al21, 91 patients who discontinued treatment with olanzapine, quetiapine, risperidone or ziprasidone because of inadequate efficacy, were randomly assigned to open-label treatment with clozapine (n=49) or blinded treatment with another newer atypical antipsychotic not previously received. The results showed that the Negative Syndrome Scale and total scores decreased more in patients treated with clozapine than in patients treated with quetiapine or risperidone, and treatment discontinuation for any reason was significantly longer for clozapine.
In other studies, clozapine has been found to be effective in resolution of positive and negative symptoms in schizophrenia patients and the average daily dose in these studies was between 523 and 600 mg/day222324. However, in our study, the mean dose of clozapine at six months was 289.28 mg/day.
In another study9, 30 patients of schizophrenia or schizophreniform disorder were randomized to receive clozapine or risperidone and the results showed that patients on clozapine adhered to their original treatment for a longer period than patients on risperidone. In our study also, only 6.6 per cent patients dropped out during six months of the study, and none was due to adverse effects of the drug. The high retention rate (93.4 %), for six months suggested better treatment adherence in clozapine group. The retention rate in case of risperidone during the same follow up period was 82.82 per cent as six patients dropped out within six months. One patient dropped out due to acute dystonia in first two weeks, another patient had akathisia and restlessness, three patients had extrapyramidal side effects and one patient dropped out due to unknown reason and shifted to another hospital.
Robinson et al25 stated that neurological adverse effect of antipsychotic drugs was the main reason of treatment non-adherence and even relapse. Minzenberg et al26 have argued that while the use of adjunctive anticholinergic medications can improve the tolerability of extrapyramidal symptoms, these agents are often associated with adverse cognitive effects and peripheral anticholinergic effects. In our study, on comparison between the two groups on total score on GASS, clozapine was superior to risperidone. On the metabolic parameters, patients in clozapine group had significant weight gain than the risperidone. This is in accordance with the findings reported in other studies2728.
The present study had certain limitations. The sample size was low; being an open-label study, blinding could not be done; placebo arm could not be included due to ethical considerations. In addition, a follow up duration of six months may not be sufficient to interpret metabolic side effects of the drugs. Further, suicidality which is an important variable, could not be measured. The study had several strengths such as randomized sample, first-episode, treatment-naive schizophrenia patients, comparable baseline sociodemographic profile as well as psychopathology score, repeated assessments and follow ups.
In conclusion, the present study showed that clozapine was a better choice than risperidone in terms of efficacy and tolerability in the treatment of treatment-naive, first-episode schizophrenia. In view of these findings and no reports of leucopenia and agranulocytopenia, clozapine may have the potential to be the first-choice antipsychotic which would reduce the failure rates, along with the improvement in quality of life. Further studies on large samples need to be done to confirm these findings.
Conflicts of Interest: None.
References
- Size of burden of schizophrenia and psychotic disorders. Eur Neuropsychopharmacol. 2005;15:399-409.
- [Google Scholar]
- Serotonin-dopamine antagonists. In: Sadock BJ, Sadock VA, eds. Kaplan & Sadock's comprehensive textbook of psychiatry Vol 2. (8th ed). Philadelphia: Lippincot Williams & Wilkins; 2000. p. :2914-38.
- [Google Scholar]
- Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45:789-96.
- [Google Scholar]
- Underuse of clozapine in treatment-resistant schizophrenia. Adv Psychiatr Treat. 2011;17:250-5.
- [Google Scholar]
- Clozapine as a first treatment for schizophrenia. Am J Psychiatry. 2003;160:1514-6.
- [Google Scholar]
- Clozapine v. chlorpromazine in treatment-naive, first-episode schizophrenia: 9-year outcomes of a randomised clinical trial. Br J Psychiatry. 2011;199:281-8.
- [Google Scholar]
- Clozapine's role in the treatment of first-episode schizophrenia. Am J Psychiatry. 2013;170:146-51.
- [Google Scholar]
- Atypical and conventional antipsychotic drugs in treatment-naive first-episode schizophrenia: a 52-week randomized trial of clozapine vs. chlorpromazine. Neuropsychopharmacology. 2003;28:995-1003.
- [Google Scholar]
- Randomized trial of clozapine vs. risperidone in treatment-naïve first-episode schizophrenia: results after one year. Schizophr Res. 2013;149:156-61.
- [Google Scholar]
- International classification of mental and behavioural disorders: Clinical descriptions and diagnostic guidelines (ICD-10). Geneva: World Health Organization; 1992.
- Response of patients with treatment-refractory schizophrenia to clozapine within three serum level ranges. Am J Psychiatry. 1996;153:1579-84.
- [Google Scholar]
- Taylor D, Paton C, Kapur S, eds. The maudsley Prescribing guidelines in psychiatry (11th ed). London: Wiley Blackwell; 2011.
- Determination of risperidone and 9-hydroxyrisperidone in plasma, urine and animal tissues by high-performance liquid chromatography. J Chromatogr. 1992;583:223-30.
- [Google Scholar]
- The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261-76.
- [Google Scholar]
- Development of the World Health Organization WHOQOL-BREF quality of life assessment. The WHOQOL Group. Psychol Med. 1998;28:551-8.
- [Google Scholar]
- The Arizona Sexual Experience Scale (ASEX): reliability and validity. J Sex Marital Ther. 2000;26:25-40.
- [Google Scholar]
- A new self-rating scale for detecting atypical or second-generation antipsychotic side effects. J Psychopharmacol. 2008;22:238-43.
- [Google Scholar]
- Indian Council of Medical Research. In: Ethical guidelines for biomedical research on human participants. Central ethics committee on human research. New Delhi: ICMR; 2006.
- [Google Scholar]
- Clozapine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2010;10:CD006633.
- [Google Scholar]
- Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163:600-10.
- [Google Scholar]
- Refractory schizophrenia and atypical antipsychotics. J Psychopharmacol. 2000;14:409-18.
- [Google Scholar]
- The use of clozapine in the treatment of aggressive schizophrenia. Can J Psychiatry. 1998;43:466-72.
- [Google Scholar]
- Reduction of suicidality during clozapine treatment of neuroleptic-resistant schizophrenia: impact on risk-benefit assessment. Am J Psychiatry. 1995;152:183-90.
- [Google Scholar]
- Predictors of medication discontinuation by patients with first-episode schizophrenia and schizoaffective disorder. Schizophr Res. 2002;57:209-19.
- [Google Scholar]
- Association of anticholinergic load with impairment of complex attention and memory in schizophrenia. Am J Psychiatry. 2004;161:116-24.
- [Google Scholar]
- The distribution of body mass index among individuals with and without schizophrenia. J Clin Psychiatry. 1999;60:215-20.
- [Google Scholar]
- Atypical antipsychotics and weight gain – A systematic review. Acta Psychiatr Scand. 2000;101:416-32.
- [Google Scholar]






