Translate this page into:
Influence of angiotensin converting enzyme (ACE) gene rs4362 polymorphism on the progression of kidney failure in patients with autosomal dominant polycystic kidney disease (ADPKD)
Reprint requests: Dr L.V.K.S. Bhaskar, Sickle Cell Institute, Chattisgarh, Genetic Lab, Department of Biochemistry, Pt. J.N.M. Medical College, Raipur 492 001, Chattisgarh, India e-mail: lvksbhaskar@gmail.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Autosomal dominant polycystic kidney disease (ADPKD) is an inherited systemic disorder, characterized by the fluid filled cysts in the kidneys leading to end stage renal failure in later years of life. Hypertension is one of the major factors independently contributing to the chronic kidney disease (CKD) progression. The renin-angiotensin aldosterone system (RAAS) genes have been extensively studied as hypertension candidate genes. The aim of the present study was to investigate the role of angiotensin converting enzyme tagging - single nucleotide polymorphisms (ACE tag-SNPs) in progression of CKD in patients with ADPKD.
Methods:
In the present study six ACE tagSNPs (angiotensin converting enzyme tag single nucleotide polymorphisms) and insertion/deletion (I/D) in 102 ADPKD patients and 106 control subjects were investigated. The tagSNPs were genotyped using FRET-based KASPar method and ACE ID by polymerase chain reaction (PCR) and electrophoresis. Genotypes and haplotypes were compared between ADPKD patients and controls. Univariate and multivariate logistic regression analyses were performed to assess the effect of genotypes and hypertension on CKD advancement. Mantel-Haenszel (M-H) stratified analysis was performed to study the relationship between different CKD stages and hypertension and their interaction.
Results:
All loci were polymorphic and except rs4293 SNP the remaining loci followed Hardy-Weinberg equilibrium. Distribution of ACE genotypes and haplotypes in controls and ADPKD patients was not significant. A significant linkage disequilibrium (LD) was observed between SNPs forming two LD blocks. The univariate analysis revealed that the age, hypertension, family history of diabetes and ACE rs4362 contributed to the advancement of CKD.
Interpretation & conclusions:
The results suggest that the ACE genotypes are effect modifiers of the relationship between hypertension and CKD advancement among the ADPKD patients.
Keywords
ACE gene
ADPKD
CKD
gene-environment interaction
tagSNP
Autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited systemic disorder, characterized by the fluid filled cysts in the kidneys leading to end stage renal failure in later years of life1. Although the exact genetic factors that favour renal progression have not been identified, studies on mouse and human models suggest that the genetic variations play a significant role in the differential renal progression that is observed in ADPKD23. Intra-familial heterogeneity that is found in the expression of ADPKD, suggests the involvement of environmental factors along with the genetic factors in the progression of ADPKD4. Polycystic kidney disease genes products share sequence homology and are thought to play an important role in cell-cell and cell-matrix interactions and in structure of a receptor-channel complex which regulates renal ion transport5. Patients with ADPKD exhibit non-uniformity of the renal disease progression. This variability was observed from both mouse2 and human6 models, suggesting that disease-modifying loci may play a role in the variable renal progression found in ADPKD.
Hypertension is one of the clinical manifestations in majority of ADPKD cases and correlates with the progressive kidney enlargement with high prognostic values. The renin-angiotensin aldosterone system (RAAS) is known to regulate the blood pressure and fluid balance7. The activity of RAAS system is regulated by the rate of production of angiotensinogen from rennin that is mediated by angiotensin converting enzyme (ACE)8. In view of the role of hypertension on the progression of chronic kidney disease in ADPKD, the gene polymorphisms of ACE are of great interest.
The ACE gene spans over 24 kb with 26 exons and is located on chromosome 17. The presence or absence of a 287-bp repeat sequence [insertion/deletion (I/D) polymorphism] at intron 16 has been used as a common marker in the susceptibility of various disorders9. An initial study showed a deleterious effect of the DD genotype on renal survival in ADPKD10. The direct biological mechanism by which ACE ID polymorphism might influence serum ACE levels remains unclear. In the present study the role of ACE ID and six additional tagging-single nucleotide polymorphisms (tagSNPs) were investigated to unravel the ACE gene modifier effect for renal disease progression in patients with ADPKD.
Material & Methods
A total of 102 consecutive patients affected by ADPKD (55.88% men) and 106 controls (60.38% men) attending the outpatient department of Nephrology, Sri Ramachandra University, Chennai, India, from March 2010 to December 2012 were consecutively selected. The diagnosis of ADPKD was based on previously described Ravine ultrasound criteria11. Patients with other conditions that could influence renal function such as diabetes or pregnancy were excluded. Upon screening, a total of 106 healthy unrelated individuals without family history of polycystic kidney disease or any other kidney related complications (60.38% men) were included as a control. All control subjects were non-diabetic, normotensive and aged between 30-60 yr. The study was approved by the Institutional Ethics Committee of the Sri Ramachandra University, Chennai, India. A written informed consent was obtained from the study participants. The total number of cysts in the ADPKD patients was detected by using ultrasound imaging and the estimated glomerular filtration rate (eGFR) was assessed based on Modification of Diet in Renal Disease (MDRD) formula12. Further, the stage of kidney disease was determined by using eGFR and all the ADPKD patients were divided into two groups: early stages (CKD stages 1-3; eGFR 30 to 90 ml/min/1.73 m2) and advanced (CKD stages 4; eGFR 15-29 ml/min/1.73 m2 and 5; eGFR < 15 ml/min/1.73 m2) stages. Genomic DNA from the peripheral blood samples was extracted by phenol chloroform extraction and ethanol precipitation protocol13.
SNP selection and genotyping: All tagging single nucleotide polymorphisms (tagSNPs) covering ACE gene from the release 2.0 Phase II data of the HapMap Project (www.hapmap.org) were selected using pairwise tagging method14. The tagSNPs were chosen according to the following criterion: r2 ≥0.8 and minor allele frequency of ≥5 per cent in the Gujarati Indians in Houston (GIH) population1415. A common well studied ID polymorphism was also included8. Fluorescence resonance energy transfer (FRET)-based KASPar SNP genotyping assay method (KBioscience, Herts, UK) was used to genotype the tag-SNPs on an Applied Biosystems thermocycler (ABI Prism 9700, Foster City, CA, USA) using the fluorescent probe primers (designed by K Bioscience, UK using Kraken™ software system). The fluorescent endpoint readings were taken using the 7900 SDS software (ABI Prism 7900, Foster City, CA, USA). The genotyping success rate ranged from 99.3 to 100.0 per cent. The polymerase chain reaction (PCR) and electrophoresis were used to detect the ACE gene insertion deletion polymorphism8. All homozygous deletion samples were further subjected to a second PCR amplification with insertion-specific primers to prevent mistyping of heterozygous genotypes16.
Statistical analysis: Allele frequencies were calculated by the gene-counting method17. Genotypes at each SNP were tested for Hardy-Weinberg equilibrium by using χ2 test with one degree of freedom. Chi square test was carried out to check the association between ADPKD and controls. Pairwise linkage disequilibrium (LD) measures (D’ and r2) and haplotype blocks were assessed using the default setting of the Haploview software14. In ADPKD patients univariate and multivariate logistic regression analysis was performed to assess the effect of genotypes and hypertension on CKD progression. The influence of different genotypes on the relationship between different CKD stages and hypertension and their interaction was examined using the Mantel-Haenszel stratified analysis. All statistical analyses were performed using SPSS 16.0 (SPSS, Inc., Chicago, USA).
Results
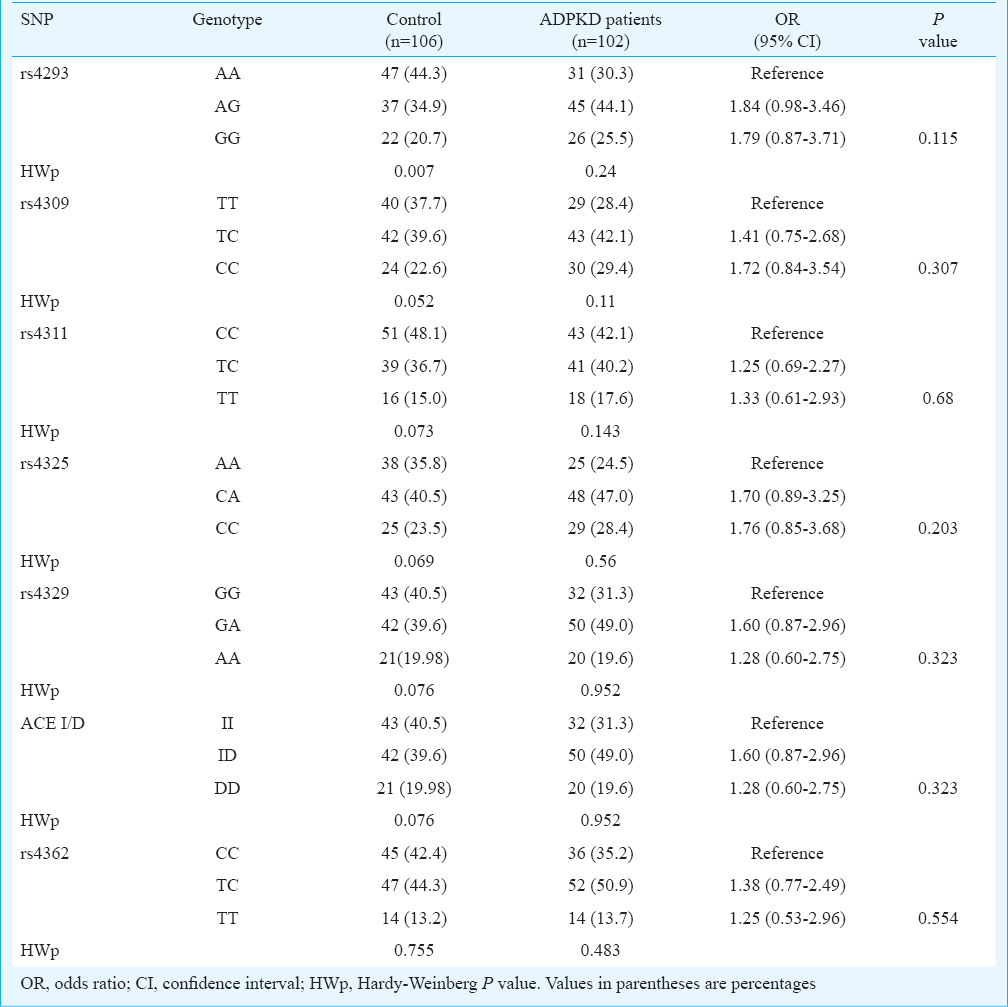
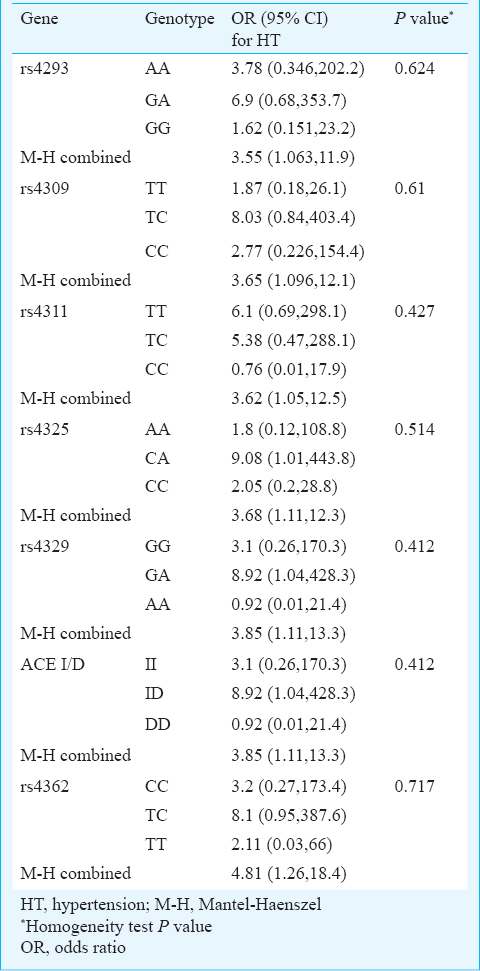
ACE gene polymorphisms and ADPKD: Clinical characteristics of both controls and ADPKD patients are given in Table I. The patients had significantly higher (P<0.001) creatinine and blood urea nitrogen levels and significantly (P<0.001) lower eGFR and calcium levels as compared to controls. For ACE gene GIH population yielded six tagSNPs (rs4293, rs4309, rs4311, rs4325, rs4329 and rs4362)15. The allele and genotype frequencies of the six tagSNPs along with insertion deletion polymorphisms in patients and controls are shown in Table II. All polymorphisms except rs4293 followed Hardy-Weinberg equilibrium. Distribution of ACE genotypes between the control and ADPKD groups was not significant (Table II). Analysis of LD showed a strong and significant LD between the markers by forming two LD blocks (Figure). The first LD block included rs4311 and rs4325, which were separated by 2.4 kb in introns 9 and 13, respectively. The second LD block included rs4329, I/D and rs4362, which encompassed intron 13, intron 16 and exon 21, respectively. The rs4309 SNP located in exon 8 remained outside the LD blocks. Haplotypes constructed using the SNPs located in individual LD blocks are presented in the Figure. Comparison of haplotypes between ADPKD and control groups revealed absence of any significant association with ADPKD.
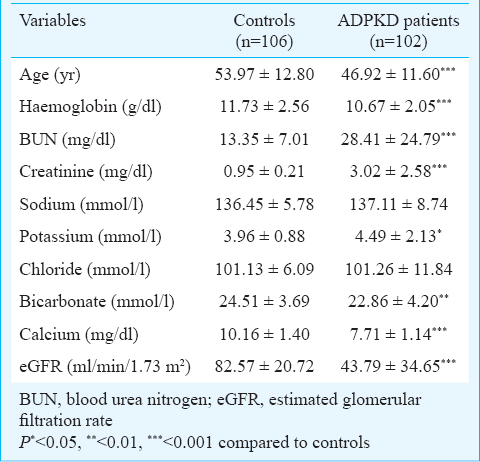
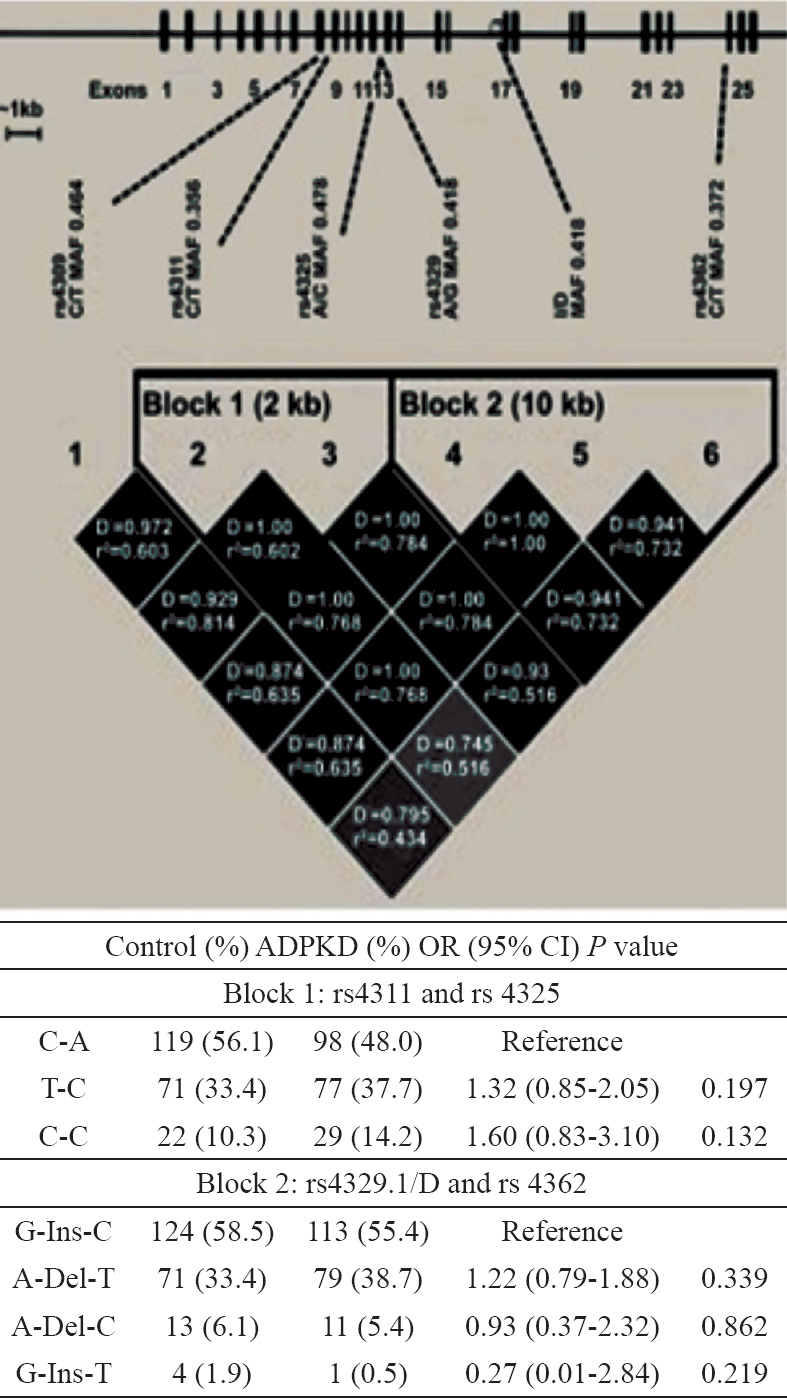
- Linkage disequilibrium (LD) map and haplotype analysis of ACE. Relative positions of the tagSNPs and their MAFs were shown in the upper portion. Pairwise LD measures (D and r2) were shown by the LD map. The ACE gene haplotypes distribution in control and ADPKD patients was shown in the bottom.
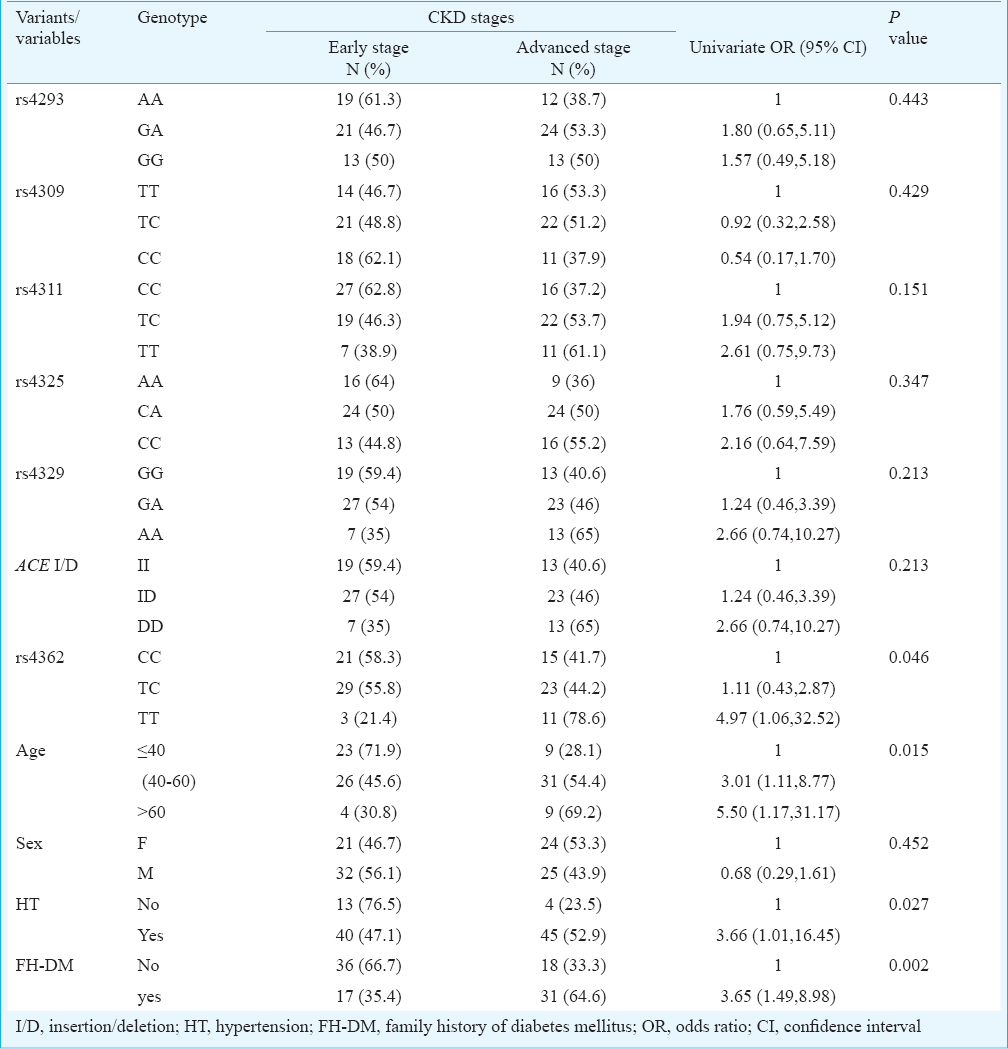
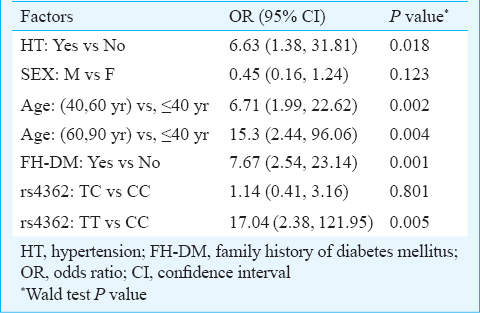
ACE gene polymorphisms CKD advancement: Among ADPKD patients, 49 (48%) showed advanced CKD stage with mean age of 50.73 ± 10.27 yr and 53 (52%) showed moderate progression with 43.4 ± 11.74 yr of age. Distribution of genotypes between these CKD groups revealed that only rs4362 was associated with the development of CKD stage (P=0.046). The TT genotype significantly increased the risk of advanced CKD in ADPKD (OR=4.97; 95% CI: 1.06-32.52) (Table III). The univariate analysis revealed that the age, hypertension and family history of diabetes contributed to the significant advancement of CKD, but the gender had no effect on CKD advancement (Table III). No evidence of heterogeneity of the effect of hypertension on CKD stages was observed among different genotypes of the studied polymorphisms. This indicated the absence of confounding effect of genotype on the relationship between CKD advancement and hypertension. For rs4362 genotypes the Mantel-Haenszel (M-H) combined OR for hypertension was found to be 4.81 and 95 per cent CI is 1.26-18.4 (Table IV). Both univariate (OR=4.97; 95% CI: 1.06-32.52) (Table III) and multivariate analyses (OR=17.04; 95% CI: 2.38-121.95) revealed that the TT genotype of rs4362 increased the risk of CKD advancement in ADPKD after controlling age, sex, hypertension and family history of diabetes (Table V). The adjusted effect of hypertension on CKD advancement was found to be independent of other risk factors (OR=6.63; CI: 1.38-31.81).
Discussion
Analysis of tagSNPs within the ACE gene in ADPKD patients and control subjects did not show any significant association with ADPKD. Strong LD was found among all SNPs studied, covering a region of about 13.8 kb within the ACE gene. Comparison of haplotypes between ADPKD and control groups also revealed the absence of a significant association with ADPKD. The univariate analysis revealed that the age, hypertension, family history of diabetes and ACE rs4362 contributed to the advancement of CKD. The modifier effect of these factors remained even after controlling other variables in multivariate analysis, indicating that the ACE genotypes are effect modifiers of the relationship between hypertension and CKD advancement among the ADPKD patients.
Our study had some limitations. First, it was a nested study, hence there was a possibility of selection bias. Second, was the limited sample size in case group, the estimated 95 per cent CI of the effect of TT genotype on the advancement of CKD stages was found to be wider. Despite these limitations, analysis of tagSNPs of ACE gene revealed that the ACE polymorphism had a significant modifier effect on the progression of renal disease in ADPKD.
In ADPKD, hypertension is an early sign that occurs even before the reduction in glomerular filtration rate. Hence hypertension is one of the most important factors that influences the CKD progression in ADPKD1819. Inappropriate activation of RAAS has been shown to be the major cause in the pathogenesis of hypertension in individuals with ADPKD20. However, the MDRD (Modification of Diet in Renal Disease) study21 demonstrated that the ACE inhibitor therapy or blood pressure control did not reduce the rate of decline in GFR of ADPKD patients. In contrast, pharmacological blockade of the RAAS using ACE inhibitors significantly reduced CKD progression22. ACE is suspected to play a key role and is the first candidate modifier gene that has been investigated in ADPKD23. An initial study of ACE gene found that the DD genotype increased the risk of developing end stage renal failure at an early age in PKD1 patients9. Subsequent studies on the influence of ACE I/D polymorphism on ADPKD progression have yielded conflicting results232425. A comprehensive meta-analysis encompassing all relevant studies did not support the hypothesis that the DD genotype increased risk of developing end stage renal failure in patients with ADPKD26.
The synonymous marker rs4362 (C19329T) located within the 5’end of exon 23 was considered the functional candidate. It has been shown that the rs4362 marks the angiotensin-converting enzyme gene insertion-deletion variant27. A study conducted in Mexican individuals28 revealed that the rs4362 polymorphisms were associated with an increased risk of hypertension and ACE serum levels. These results suggest that the rs4362 polymorphisms can modify the renal function and leads to CKD progression by altering the plasma ACE levels.
A study which examined the detailed renal functions of young ADPKD patients showed abnormal kidney function at younger age group (27 ± 5 yr) and GFR decreased but was not significantly different from that of the normal healthy controls29. Moreover, the measured eGFR in relation to age in the hypertensive children with ADPKD was an observed risk for increased renal volume and decreased renal function as compared children with normal blood pressure30. These results suggest that the renal compensation mechanism might terminate as the age increases and with the acceleration of associated risk factors.
In conclusion, our study showed that rs4362 polymorphism in ACE gene had a significant modifier effect on the progression of renal disease in patients with ADPKD.
Acknowledgment
This research was partially supported by “GATE-Young Faculty Research Grant” of the Sri Ramachandra University, Porur, Chennai.
Conflicts of Interest: None.
References
- Trans-heterozygous Pkd1 and Pkd2 mutations modify expression of polycystic kidney disease. Hum Mol Genet. 2002;11:1845-54.
- [Google Scholar]
- Novel PKD1 and PKD2 mutations in autosomal dominant polycystic kidney disease (ADPKD) Nephrol Dial Transplant. 2011;26:2181-8.
- [Google Scholar]
- Variable progression of autosomal dominant polycystic kidney disease: genetic and molecular counterparts. Nephrol Ther. 2006;2(Suppl 2):S104-8.
- [Google Scholar]
- Autosomal dominant polycystic kidney disease: molecular genetics and molecular pathogenesis. Hum Genet. 2000;107:115-26.
- [Google Scholar]
- Modifier genes play a significant role in the phenotypic expression of PKD1. Kidney Int. 2005;67:1256-67.
- [Google Scholar]
- The brain renin-angiotensin system controls divergent efferent mechanisms to regulate fluid and energy balance. Cell Metab. 2010;12:431-42.
- [Google Scholar]
- PCR detection of the insertion/deletion polymorphism of the human angiotensin converting enzyme gene (DCP1) (dipeptidyl carboxypeptidase 1) Nucleic Acids Res. 1992;20:1433.
- [Google Scholar]
- Angiotensin-converting enzyme (ACE) I/D genotype and renal ACE gene expression. Kidney Int. 2001;60:1124-30.
- [Google Scholar]
- Association of the angiotensin I converting enzyme gene deletion polymorphism with early onset of ESRF in PKD1 adult polycystic kidney disease. Kidney Int. 1997;52:607-13.
- [Google Scholar]
- Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet. 1994;343:824-7.
- [Google Scholar]
- A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461-70.
- [Google Scholar]
- Molecular cloning : a laboratory manual (3rd ed). Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 2001.
- Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263-5.
- [Google Scholar]
- The estimation of gene frequencies in a random-mating population. Ann Hum Genet. 1955;20:97-115.
- [Google Scholar]
- Potentially modifiable factors affecting the progression of autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2011;6:640-7.
- [Google Scholar]
- The role of parental hypertension in the frequency and age of diagnosis of hypertension in offspring with autosomal-dominant polycystic kidney disease. Kidney Int. 2003;64:1792-9.
- [Google Scholar]
- Role of renin-angiotensin-aldosterone system gene polymorphisms and hypertension-induced end-stage renal disease in autosomal dominant polycystic kidney disease. Iran J Kidney Dis. 2014;8:265-77.
- [Google Scholar]
- Dietary protein restriction and the progression of chronic renal disease: what have all of the results of the MDRD study shown? Modification of diet in renal disease study group. J Am Soc Nephrol. 1999;10:2426-39.
- [Google Scholar]
- Does blockade of the renin-angiotensin-aldosterone system slow progression of all forms of kidney disease? Curr Hypertens Rep. 2010;12:369-77.
- [Google Scholar]
- Influence of the ACE gene polymorphism in the progression of renal failure in autosomal dominant polycystic kidney disease. Am J Kidney Dis. 1999;34:273-8.
- [Google Scholar]
- Association of the ACE gene polymorphism with the progression of autosomal dominant polycystic kidney disease. J Korean Med Sci. 2000;15:431-5.
- [Google Scholar]
- ACE gene I/D polymorphism and the presence of renal failure or hypertension in autosomal dominant polycystic kidney disease (ADPKD) Nephrol Dial Transplant. 2007;22:1483.
- [Google Scholar]
- Influence of ACE I/D gene polymorphism in the progression of renal failure in autosomal dominant polycystic kidney disease: a meta-analysis. Nephrol Dial Transplant. 2006;21:3155-63.
- [Google Scholar]
- Renin-angiotensin system haplotypes and the risk of myocardial infarction and stroke in pharmacologically treated hypertensive patients. Am J Epidemiol. 2007;166:19-27.
- [Google Scholar]
- Single nucleotide polymorphisms of the angiotensin-converting enzyme (ACE) gene are associated with essential hypertension and increased ACE enzyme levels in Mexican individuals. PLoS One. 2013;8:e65700.
- [Google Scholar]
- Early renal abnormalities in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2010;5:1091-8.
- [Google Scholar]
- Prospective change in renal volume and function in children with ADPKD. Clin J Am Soc Nephrol. 2009;4:820-9.
- [Google Scholar]






