Translate this page into:
Serum levels of phosphorylated heat shock protein 27 (pHSP27) are associated with bone mineral density in pre- & postmenopausal women: A pilot study
Reprint requests: Dr M. Ikram Khatkhatay, Division of Molecular Immunodiagnostics, National Institute for Research in Reproductive Health (ICMR), J.M. Street, Parel, Mumbai 400 012, Maharashtra, India e-mail: khatkhatayi@nirrh.res.in
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution NonCommercial ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Phosphorylated heat shock protein 27 (pHSP27) has been implicated in the pathogenesis of osteoporosis. Oxidative stress and proinflammatory cytokines, which are known to be involved in aetiology of osteoporosis, can trigger HSP27 phosphorylation. Since pHSP27 is present in circulation, it was hypothesized that serum pHSP27 would be elevated in low bone mineral density (BMD) condition and might serve as an indicator of osteoporosis/osteopenia. Hence, the aim of this study was to examine serum levels of pHSP27 in relation with BMD in pre- and postmenopausal women.
Methods:
Premenopausal (30 to 40 yr) and postmenopausal (50 to 60 yr) women having either low BMD (osteopenia/osteoporosis) or high BMD were selected (n=80) from a prospective cohort (n=200). Serum levels of pHSP27; along with levels of oestradiol, malondialdehyde, total antioxidant capacity, interleukin (IL)-1, IL-6, tumour necrosis factor - alpha, (TNF-α), c-telopeptide fragments of collagen type I (CTX-1) and osteocalcin were estimated.
Results:
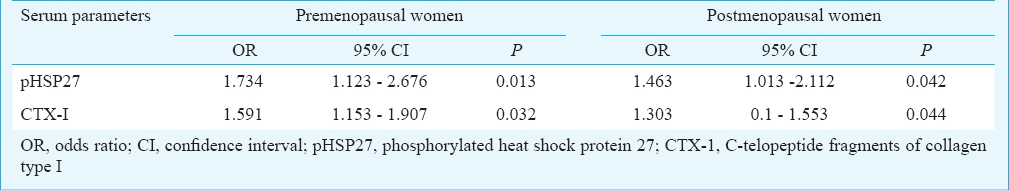
The serum levels of pHSP27 were significantly elevated in low BMD groups in premenopausal and postmenopausal categories (P<0.05). It also exhibited a significant odds ratio (OR) to differentiate between low and high BMD in both premenopausal (OR=1.734, P=0.013) and postmenopausal (OR=1.463, P=0.042) categories. Additionally, area under the curve to predict low BMD was non-significantly higher for pHSP27 than CTX-1 in premenopausal and postmenopausal categories.
Interpretation & conclusions:
This study highlights a novel relation between serum pHSP27 and BMD in Indian women however, these findings need to be confirmed in larger studies.
Keywords
Bone mineral density (BMD)
osteoporosis
phosphorylated HSP27
postmenopause
premenopause
Osteoporosis is a systemic skeletal disease characterized by low bone mineral density (BMD) and increased susceptibility for fragility fracture. Recently it has been reported that 50 million people have osteoporosis or low bone mass in India1. It is more prevalent in females than in males, and more so, in postmenopausal women due to decline in levels of estradiol. Besides its direct protective actions on the skeleton, estradiol also has antioxidant and anti-inflammatory properties23. Hence, menopause is usually accompanied with a rise in oxidative stress and proinflammatory cytokines45. This leads to the enhanced formation and activity of osteoclasts (bone resorbing cells) compared with that of osteoblasts (bone forming cells), leading to a net decrease in BMD6. It is well recognized that oxidative stress and proinflammatory cytokines exert adverse effects on skeletal health. Even so, the mechanisms underlying the complex aetiology of postmenopausal osteoporosis are not completely understood.
Oxidative stress and proinflammatory cytokines can directly induce phosphorylation of heat shock protein 27 (HSP27)78910. HSP27 is a molecular chaperone belonging to the small heat shock protein group. Phosphorylated HSP27 (pHSP27) level has been found to be significantly elevated in serum of patients with advanced lung cancer compared with early stages11. In our recent proteomics study, we found pHSP27 to be upregulated in osteoclast precursors in low BMD condition and exogenous pHSP27 could increase the migration of osteoclast precursors towards RANKL representing the bone milieu12. In addition, it has been previously shown that number of osteoclasts formed is inversely related to BMD13. Thus, it can be deduced that elevated pHSP27 in osteoclast precursors may lead to their increased migration towards the bone milieu and thereby increased osteoclastogenesis which can contribute to the pathogenesis of osteoporosis. As oxidative stress and proinflammatory cytokines can drive phosphorylation of HSP27, it would be imperative to know whether elevation of pHSP27 in low BMD condition can also be seen in serum and used as an indicator of bone health.
Hence, to examine the association of serum pHSP27 with BMD, in unison with its inducers, we sought to determine serum levels of pHSP27 in pre- and postmenopausal Indian women with low (osteopenia/osteoporosis) versus high BMD, along with oxidative stress indicators (malondialdehyde and total antioxidant capacity), proinflammatory cytokines [interleukin (IL)-1, IL-6 and tumour necrosis factor-α) (TNFα)] and also oestradiol, in a nested case control study. We also compared the predictive ability of pHSP27 with bone turnover markers, osteocalcin (bone formation marker) and C-telopeptide fragments of collagen type I or CTX-1 (bone resorption marker).
Material & Methods
This study was carried out at the National Institute for Research in Reproductive Health (NIRRH), Mumbai, India, from March 2011 to April 2013. The protocol was approved by the Institutional Ethics Committee for clinical studies. Premenopausal women (n=100) aged 30 to 40 yr with regular menstrual cycles and postmenopausal women (n=100) aged 50 to 60 yr having achieved menopause at least one year prior attending the health clinic of NIRRH, were consecutively enrolled. All these women gave their written informed consents to participate in the study. All the participants were apparently healthy, physically active, and none had nutritional deficiencies as per their dietary recall. Exclusion criteria comprised smoking habits, alcohol consumption, obesity, diabetes, hypertension, chronic disorders or malignancies, arthritis or any other bone disease, regular intake of any drugs or medication. Dual energy X-ray absorptiometry (DXA, QRR 1000, Hologic Inc., USA) was used for measurement of BMD (g/cm2) at hip (femoral neck or FN) and at spine (lumbar spine 1-4 or LS) for all 200 enrolled participants.
Participants were categorized as osteoporotic (T score ≤ - 2.5 at either of the two sites); osteopenic (T score between -1 and -2.5 at either of the two sites) and normal (T score ≥ -1 at both the sites) in accordance with World Health Organization criteria14.
The classification of low and high BMD subjects was essentially based on centile distribution of BMD values only. Accordingly, BMD values of the women belonging to pre- and postmenopausal categories were arranged according to centile, of whom, women with either low BMD (osteoporosis/osteopenia) i.e. the women having the lowest BMD (≤20th centile) or high BMD i.e. those having the highest BMD (≥ 80th centile) were selected. The selected women (total n = 80) included 20 women in each of the four categories (i) premenopause low BMD, (ii) premenopause high BMD, (iii) postmenopause low BMD, and (iv) postmenopause high BMD.
Fasting blood was collected from the selected participants (n = 80) within a period of three months from DXA measurement and serum was separated immediately and stored at -80°C. Blood was collected from 0900 to 1000 h. For premenopausal women blood collection was done on menstrual day 5 to day 8 and for postmenopausal women on any convenient day.
HSP27 phosphorylation occurs at serine 15, 78 and 82, however, serine 78 and 82 are the major sites of phosphorylation15. Hence, serum levels of HSP27 phosphorylated at serine 78/82 and also total HSP27 were estimated using commercial sandwich ELISA kits (R & D systems, USA). Phosphorylated HSP27 was expressed as percentage of phosphorylation calculated by estimating ratio of phosphorylated fraction to total HSP27 multiplied by 100 for each individual sample. This was done to exclude the possibility that the rise in its phosphorylated form was simply due to rise in total expression, hence, to assess an actual change in phosphorylation status. Further, 17 - β estradiol was measured in serum using a competitive ELISA kit (ImmunoDiagnostic Systems, Canada). The thiobarbituric acid reactive substances (TBARS) assay kit (Cayman Chemical, USA) was used for estimation of malondialdehyde (MDA), a byproduct of lipid peroxidation which is used as an indicator of oxidative stress. Total Antioxidant Capacity kit (Cayman Chemical, USA) was used for estimating antioxidant status. That proinflammatory cytokines IL - 1β, IL-6 and TNF-α were measured using sandwich ELISA kits (BD Biosciences, USA). Intact human osteocalcin was measured using a commercially available solid phase Enzyme Amplified Sensitivity Immunoassay (DIASource Immunoassays, Belgium) and C-telopeptide fragments of collagen type I or CTX-I was measured using Serum CrossLaps® ELISA (Nordic Bioscience Diagnostics, Denmark). The intra- and inter-assay coefficients of variations were less than 10 and 15 per cent, respectively for all assays.
Statistical analysis: Data were represented as median and interquartile range (IQR). Graphpad Prism version 6 (GraphPad Software, Inc., USA) was used to compare parameters between two independent groups using Mann Whitney U tests and also for Spearman rank correlation. Multivariate logistic regression was performed using Statistical Package for Social Sciences (SPSS) version 16 for windows (SPSS, IBM, USA) Receiver operating characteristic (ROC) curve was computed to calculate area under the curve (AUC) using MedCalc software version 13 (Med Calc Software, Ostend, Belgium). For correlation, data of all samples were considered (n=80). DeLong test was applied to compare AUCs using MedCalc software version 13. For logistic regression and ROC curve, data were categorized as (i) premenopause low BMD (n=20) and premenopause high BMD (n=20), and (ii) postmenopause low BMD (n=20) and postmenopause high BMD (n=20).
Results
Baseline characteristics of the selected participants are shown in Table I. Age and body mass index (BMI) were not significantly different, whereas, BMD (z and T scores) at FN and at LS were significantly different (P<0.0001) in cases and controls. All women in the low BMD group were previously undiagnosed and untreated cases of osteopenia/osteoporosis at FN and/or LS; while participants in the high BMD group were normal at both FN and LS.
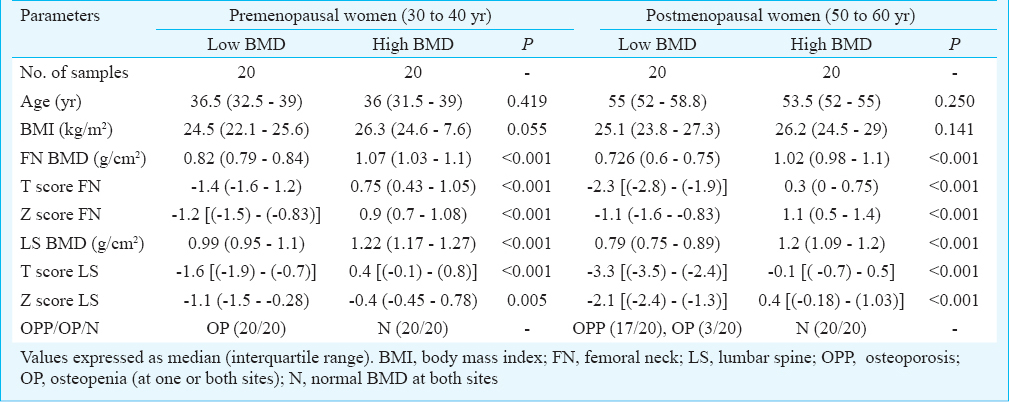
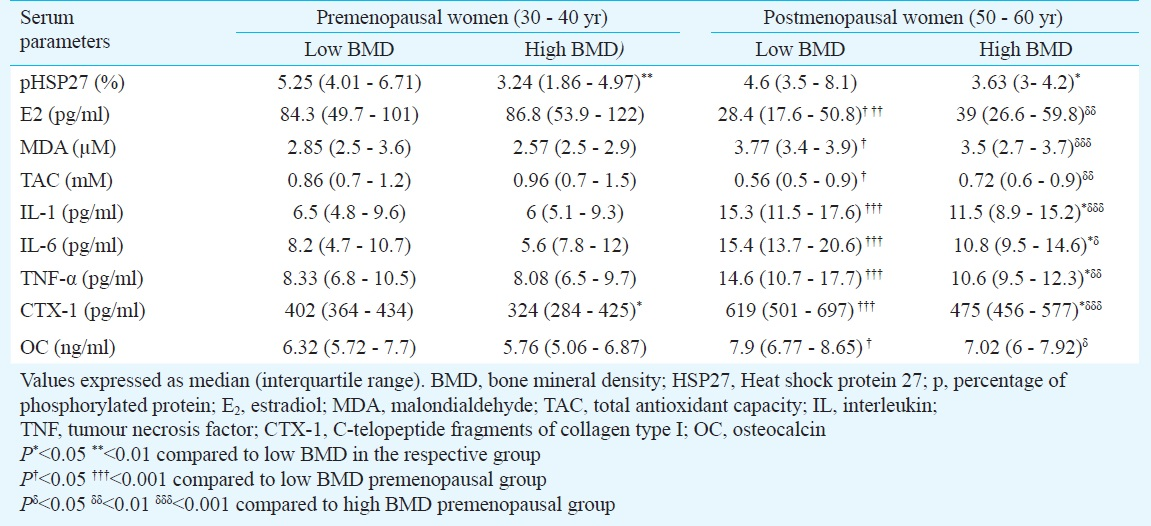
Comparison of serum parameters based on BMD and menopause: Table II shows the comparison of serum parameters based on BMD and menopause. The pHSP27 was significantly elevated in low BMD groups in both pre- and postmenopausal categories. Also, C-telopeptide fragments of collagen type I (CTX-1) was significantly higher in low BMD in both categories. Median levels of oestradiol (E2), total antioxidant capacity (TAC) were marginally raised in high BMD condition, whereas, MDA and OC were marginally raised in low BMD condition, in both pre- and postmenopausal categories, however, not significantly different. Further, IL-1, IL-6 and TNF-α were significantly higher in low BMD condition in post- but not in premenopausal category.
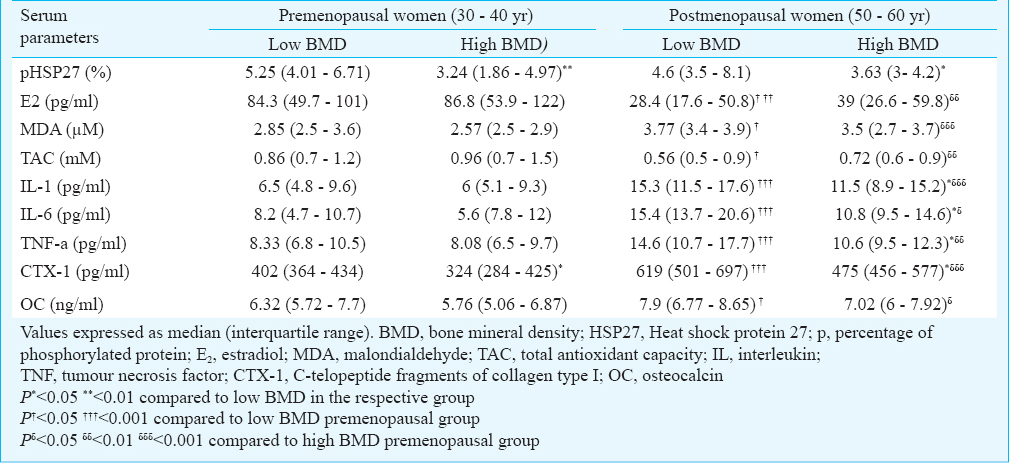
On comparing premenopausal versus postmenopausal women within similar BMD conditions, E2 and TAC were significantly higher in pre, while, MDA, IL-1, IL-6, TNF-α, CTX-1 and OC were significantly higher in post menopausal category. Besides, pHSP27 was not significantly different in pre versus post categories within similar BMD conditions.
Relation of serum parameters with BMD: pHSP27 showed a significant inverse correlation with BMD at FN (r = -0.320, P = 0.004) and at LS (r = -0.315, P = 0.004) (Fig. 1). Further, E2 positively correlated with BMD at FN (r = 0.268, P= 0.016) and at LS (r = 0.321, P= 0.004). Also, TAC positively correlated with BMD at FN (r = 0.358, P = 0.001) and at LS (r = 0.354, P = 0.001). Conversely, MDA inversely correlated with BMD at FN (r = -0.276, P = 0.01) and at LS (r = -0.367, P = 0.001). IL-1, IL-6 and TNF-α exhibited inverse correlation with BMD at FN (IL-1 r = -0.321, P = 0.004; IL-6 r = -0.275, P = 0.01; TNF -α r = -0.304, P = 0.006) and at LS (IL-1 r = -0.368, P = 0.001; IL-6 r = -0.228, P = 0.04; TNF-α r = -0.327, P = 0.003). Besides, OC was inversely correlated with BMD at FN (r = -0.248, P = 0.027) and at LS (r = -0.221, P = 0.049). Also, CTX-1 was inversely correlated with BMD at FN (r = -0.404, P<0.0001) and at LS (r = -0.330, P= 0.003).
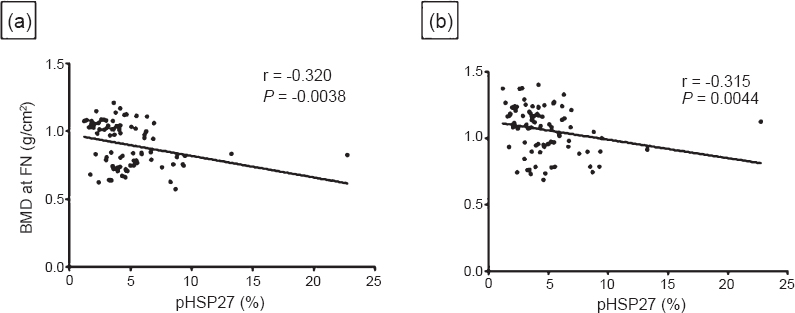
- Correlation of phosphorylated HSP27 with BMD at (a) Femur neck (FN) and (b) Lumbar spine (LS) in women (n=80).
Relation of serum parameters with pHSP27: pHSP27 showed significant positive correlation with MDA (r = 0.456, P< 0.0001), IL-1 (r = 0.318, P = 0.004) and TNF-α (r = 0.369, P = 0.0008) and an inverse correlation with TAC (r = -0.363, P = 0.0009) (Fig. 2). However, pHSP27 did not correlate with IL-6 and E2, and there was no correlation between pHSP27 and OC or between pHSP27 and CTX-1.
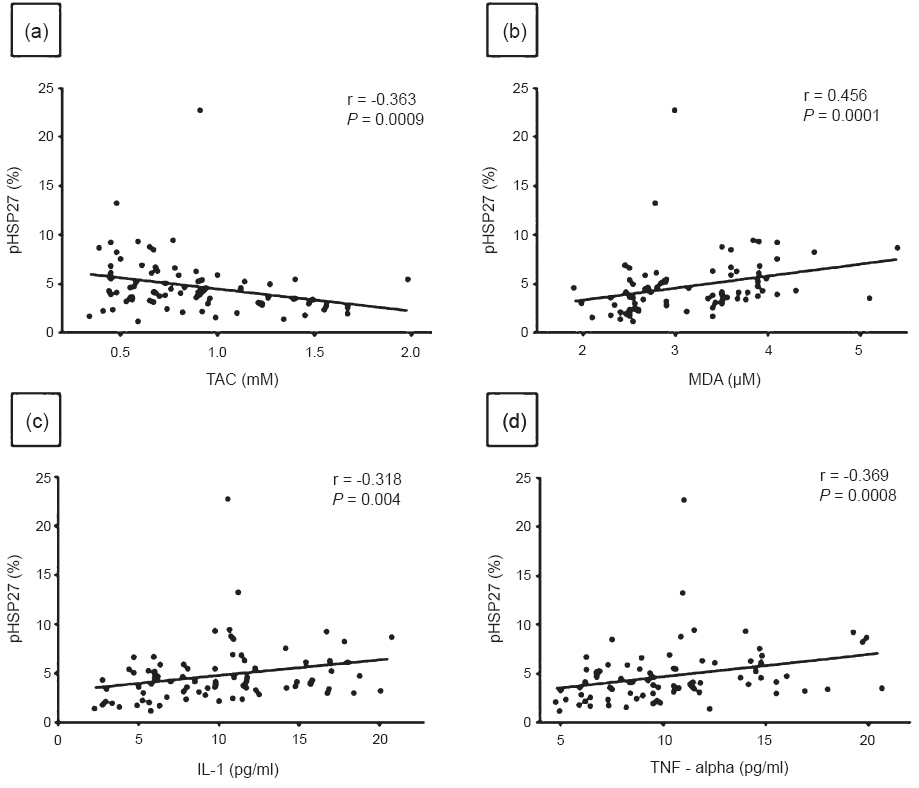
- Correlation between phosphorylated HSP27 (pHSP27) and (a) Total antioxidant capacity (TAC), (b) Malondialdehyde (MDA), (c) Interleukin -1 (IL-1) and (d) Tumour necrosis factor - alpha (TNF-α), in Indian women (n=80).
Predictive ability of pHSP27: Using multivariate logistic regression, it was found that pHSP27 and CTX-1 exhibited significant odds ratios to predict low BMD condition (Table III). The odds ratio exhibited by pHSP27 was higher than CTX-1 for both pre and post categories. Further, since pHSP27 and CTX-1 showed significant odds ratio in both categories, ROC curves were computed to assess their predictive ability. The pHSP27 showed significant AUC for predicting low BMD in both pre (AUC = 0.765, P = 0.0004) and post (AUC = 0.688, P = 0.0308) categories (Fig. 3). Likewise, CTX-1 showed significant AUC for predicting low BMD in pre (AUC = 0.695, P = 0.0271) and post (AUC = 0.686, P= 0.0001) categories. In both categories, the AUC exhibited by pHSP27 was higher than CTX-1, although the difference in AUCs was not significant.
- Receiver operating characteristic (ROC) curves showing area under the curve (AUC) exhibited by phosphorylated HSP27 and CTX-1 to distinguish between low BMD (n=20) and high BMD (n=20) in pre- and postmenopausal Indian women.
Discussion
In this study, pHSP27 was found to be significantly elevated in low BMD condition compared with high BMD condition. Oxidative stress and proinflammatory cytokines can trigger phosphorylation of HSP277-10. In agreement with this, the MDA, TAC, IL-1 and TNF - α showed direct correlation with pHSP27 in our study. Although IL-6 is considered a proinflammatory cytokine, it also has anti-inflammatory properties, which may be a possible reason for lack of association with pHSP2716. Bone turnover markers (CTX-1 and OC) showed no correlation with pHSP27 but serum levels of pHSP27 were associated with oxidative stress and proinflammatory cytokines.
It is well known that oxidative stress and proinflammatory cytokines have adverse effects on bone density. Concordantly, we observed that MDA, IL-1, IL-6 and TNF - α were inversely correlated with BMD, while, TAC was positively correlated with BMD. Yet, median levels of MDA and TAC were not significantly higher and lower in low BMD condition, respectively, for both pre- and postmenopausal categories. This implies a marginally altered oxidative balance in low BMD and is consistent with a previous study addressing the link between oxidative stress and BMD, although, significant alterations have also been reported in another study1718. In our study, IL-1, IL-6, TNF-α were significantly raised in women with low BMD compared to women with high BMD in post menopausal women. However, this was not the case in pre category, probably since low BMD group in precategory comprised women with osteopenia (20/20), whereas, low BMD group in post category comprised women mostly with osteoporosis (17/20). Studies in the past have also shown a significant rise in proinflammatory cytokines in postmenopausal osteoporosis and have postulated a synergistic mechanism resulting in their simultaneous elevation192021.
The median levels of E2 marginally declined in low BMD condition, in both pre and post categories. This is plausible since significant decline in E2 may not be the only basis for postmenopausal osteoporosis22. E2 and BMD showed a direct correlation with each other, consistent with another study, though a few studies have refuted an association232425.
Bone turnover markers, OC (bone formation marker) and CTX-1 (bone resorption marker) had an inverse correlation with BMD as shown in earlier studies2627. In both pre- and postmenopausal categories, CTX-1 was significantly higher in low BMD condition, which was concordant with earlier reports2728.
On comparing pre- versus postmenopausal categories within similar BMD conditions, E2 and TAC were significantly elevated in pre, whereas, MDA, IL-1, IL-6, TNF-α, CTX-1 and OC were significantly elevated in postmenopausal group. These results were expected as menopause results in decline in levels of E2 with a concomitant rise in oxidative stress, proinflammatory cytokines and bone turnover markers452930. However, pHSP27 was not significantly different in pre versus post categories within similar BMD conditions. This may be attributed to the fact that E2 can induce HSP27 expression and secretion, and in turn, HSP27 can modulate E2 signaling3132. Hence, E2 being significantly elevated in pre, can induce HSP27 expression and secretion, whereas, oxidative stress and proinflammatory cytokines, being significantly elevated in post category, can trigger HSP27 phosphorylation, perhaps leading to a net uniformity in serum levels of pHSP27. Therefore, pHSP27 may serve as an early indicator of bone health, irrespective of menopausal status.
In our study, serum pHSP27 was able to distinguish between low and high BMD in both pre and post categories. Apart from pHSP27, CTX-1 also showed significant ability to predict low BMD in both pre- and post categories, although with a lower odds ratio than pHSP27. Further, ROC curves revealed that both pHSP27 and CTX-1 exhibited a significant area under the curve to predict low BMD in pre and post categories. Taken together, pHSP27 may be a candidate serum marker of low BMD and may potentially aid in the diagnosis of osteoporosis. A study on non-small cell lung cancer reported significantly raised pHSP27 in serum of patients with advanced stage of cancer compared with early stage11.
Our preliminary data show that pHSP27 may be a potential marker for diagnosis of osteoporosis/osteopenia in premenopausal as well as postmenopausal women; however, its usefulness needs to be addressed through larger studies. Although BMD measurement is gold standard for diagnosis of osteoporosis/osteopenia, the translational utility of pHSP27 may be important especially in conditions wherein DXA may not be a suitable option like pregnancy, etc. The case control nature of this study limited our ability to study a linear trend between pHSP27 and BMD; hence, prospective studies with large sample size including co-morbid diseases are needed.
Acknowledgment
Authors thank Dr S.D. Kholkute, former Director, and Dr S.D. Mahale, Director, NIRRH, for their support. This work was funded by Board of Research in Nuclear Sciences - Department of Atomic Energy (BRNS-DAE) and Indian Council of Medical Research (ICMR). The first author (BD) acknowledges BRNS - DAE for granting Senior Research Fellowship. Authors thank Shrimati Pratibha Kokate, Neha Minde, Anamika Gomle and Dipika Belekar, for help during enrollment of participants, and Dr Deepa Mhatre for suggestions.
Conflicts of Interest: None.
References
- The Asia-Pacific Regional Audit-Epidemiology, Costs, and Burden of Osteoporosis in India 2013: A report of International Osteoporosis Foundation. Indian J Endocrinol Metab. 2014;18:449-54.
- [Google Scholar]
- A crucial role for thiol antioxidants in estrogen-deficiency bone loss. J Clin Invest. 2003;112:915-23.
- [Google Scholar]
- Anti-inflammatory properties of estrogen. I. In vivo suppression of leukocyte production in bone marrow and redistribution of peripheral blood neutrophils. Cell Immunol. 1992;142:67-78.
- [Google Scholar]
- Changes in proinflammatory cytokine activity after menopause. Endocr Rev. 2002;23:90-119.
- [Google Scholar]
- Direct and indirect estrogen actions on osteoblasts and osteoclasts. Ann N Y Acad Sci. 2006;1068:173-9.
- [Google Scholar]
- HSP27 phosphorylation-mediated resistance against actin fragmentation and cell death induced by oxidative stress. Cancer Res. 1996;56:273-9.
- [Google Scholar]
- Interleukin-1-induced intracellular signaling pathways converge in the activation of mitogen-activated protein kinase and mitogen-activated protein kinase-activated protein kinase 2 and the subsequent phosphorylation of the 27-kilodalton heat shock protein in monocytic cells. Mol Pharmacol. 1994;46:1077-83.
- [Google Scholar]
- Interleukin (IL)-6 signaling leads to phosphorylation of the small heat shock protein (Hsp)27 through activation of the MAP kinase and MAPKAP kinase 2 pathway in monocytes and monocytic leukemia cells. Leukemia. 1995;9:288-94.
- [Google Scholar]
- Tumor necrosis factor-alpha induces changes in the phosphorylation, cellular localization, and oligomerization of human hsp27, a stress protein that confers cellular resistance to this cytokine. J Cell Biochem. 1995;58:248-59.
- [Google Scholar]
- Discrimination of clinical stages in non-small cell lung cancer patients by serum HSP27 and HSP70: A multi-institutional case–control study. Clin Chim Acta. 2012;413:1115-20.
- [Google Scholar]
- Menopause and osteoporosis. In: Annual report. Mumbai: National Institute for Research in Reproductive Health (ICMR); 2013. p. :97-9.
- [Google Scholar]
- Spontaneous osteoclast formation from peripheral blood mononuclear cells in postmenopausal osteoporosis. FASEB J. 2005;19:410-2.
- [Google Scholar]
- Consensus development conference: diagnosis, prophylaxis and treatment of osteoporosis. Am J Med. 1993;94:646-50.
- [Google Scholar]
- Human HSP27 is phosphorylated at serines 78 and 82 by heat shock and mitogen-activated kinases that recognize the same amino acid motif as S6 kinase II. J Biol Chem. 1992;267:794-803.
- [Google Scholar]
- The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophy Acta. 2011;1813:878-88.
- [Google Scholar]
- Oxidative stress and bone resorption interplay as a possible trigger for postmenopausal osteoporosis. Bio Med Res Int 2014 2014:569563.
- [Google Scholar]
- Antioxidant status in patients with osteoporosis: A controlled study. Joint Bone Spine. 2009;76:514-8.
- [Google Scholar]
- Increase in cytokine production (IL-1 beta, IL-6, TNF-alpha but not IFN-gamma, GM-CSF or LIF) by stimulated whole blood cells in postmenopausal osteoporosis. Maturitas. 1997;26:63-71.
- [Google Scholar]
- Estrogen, cytokines, and pathogenesis of postmenopausal osteoporosis. J Bone Mineral Res. 1996;11:1043-51.
- [Google Scholar]
- The mechanisms of estrogen regulation of bone resorption. J Clin Invest. 2000;106:1203-4.
- [Google Scholar]
- From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr Rev. 2010;31:266-300.
- [Google Scholar]
- Analysis of correlation between blood biochemical indicators and bone mineral density of post-menopausal women. Mol Biol Rep. 2011;38:939-48.
- [Google Scholar]
- Bone mineral density and 17 beta-estradiol correlation in postmenopausal women. Ginecol Obstet Mex. 2004;72:95-102.
- [Google Scholar]
- Dehydroepiandrosterone (DHEA) as a possible source for estrogen formation in bone cells: correlation between bone mineral density and serum DHEA-sulfate concentration in postmenopausal women, and the presence of aromatase to be enhanced by 1,25-dihydroxyvitamin D3 in human osteoblasts. Mech Ageing Dev. 2002;123:1107-14.
- [Google Scholar]
- The association of serum osteocalcin with the bone mineral density in post menopausal women. J Clin Diagn Res. 2013;7:814-6.
- [Google Scholar]
- Hormonal profiles and biochemical indices of bone turnover in Indian women. Osteoporos Int. 2007;18:923-9.
- [Google Scholar]
- Biochemical markers of bone turnover and response of bone mineral density to intervention in early postmenopausal women: an experience in a clinical laboratory. Clin Chem. 2001;47:1083-8.
- [Google Scholar]
- Changes in cytokines, biomarkers of bone turnover and hormones are associated with bone loss in postmenopausal Indian women. Int J Endocrinol Metab. 2012;10:399-403.
- [Google Scholar]
- Age-related changes in bone turnover markers and ovarian hormones in premenopausal and postmenopausal Indian women. J Clin Lab Anal. 2007;21:55-60.
- [Google Scholar]
- Transcriptional activation of heat shock protein 27 gene expression by 17beta-estradiol and modulation by antiestrogens and aryl hydrocarbon receptor agonists. J Mol Endocrinol. 2001;26:31-42.
- [Google Scholar]
- Heat shock protein 27 protects against atherogenesis via an estrogen-dependent mechanism: Role of selective estrogen receptor beta modulation. Arterioscler Thromb Vasc Biol. 2009;29:1751-6.
- [Google Scholar]






