Translate this page into:
Relation of serum 25 hydroxyvitamin D levels to bone mineral density in southern Chinese postmenopausal women: A preliminary study
The first two authors contributed equally to the study.
Reprint requests: Dr Qiu-Shi Wei, Department of Rehabilitation, General Hospital of Guangzhou Military Command of PLA, NO. 111, Liu-Hua Road, Guangzhou 510010, P.R. China e-mail: weiqshi@126.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution NonCommercial ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Vitamin D insufficiency is prevalent in postmenopausal women and has been related to low bone mineral density (BMD). However, controversial results have been reported for the relationship between serum 25-hydroxyvitamin D [25(OH)D] levels and BMD. This study was done to investigate whether serum 25(OH)D levels were associated with BMD in postmenopausal women living in Guangzhou in southern China.
Methods:
This cross-sectional study involved 119 asymptomatic postmenopausal women, aged 48-85 yr, who were consecutively selected from Guangzhou city. BMD was measured at the lumbar spine and femoral neck. The correlation between serum 25(OH)D levels and BMD wes investigated.
Results:
With increasing serum 25(OH)D levels categorized as <20, 20-30, and ≥30ng/ml, the PTH levels decreased gradually (P=0.031). Bivariate correlation analyses showed an inverse relationship between serum 25(OH)D and PTH levels after controlling for age and BMI (r=-0.209, P=0.023). Although subjects with vitamin D<30 ng/ml had significantly lower BMD, age- and BMI-adjusted serum 25(OH)D was weakly correlated with BMD at femoral neck (r=0.185, P=0.045), and not at lumbar spine (r=0.172, P=0 0.063). In multiple regression analyses, serum 25(OH)D was a predictor for BMD at femoral neck (R2=0.424). However, serum β-CTX was a determinant for BMD at lumbar spine (R2=0.361).
Interpretation & conclusions:
Serum 25(OH)D levels showed a positive correlation with BMD at femoral neck and serum β-CTX levels were inversely correlated with BMD at lumbar spine in postmenopausal women. Further studies are needed to elucidate the clinical impact of these findings.
Keywords
Bone mineral density
cohort studies
25 hydroxyvitamin D
osteoporosis
postmenopausal women
Vitamin D is considered essential for bone health in postmenopausal women1. In some studies, vitamin D insufficiency has been reported to be associated with low bone mineral density (BMD) and increased bone loss23. However, the results reported so far have been controversial4567. Sun exposure is considered as the most important source of vitamin D8. Previous studies in Caucasians910, Africans11, and Asians412131415 indicated that the participants though had abundant sunlight exposure, but vitamin D insufficiency was prevalent among populations around the world.
Circulating 25-hydroxyvitamin D [25(OH)D] level is a biomarker of the vitamin D status of an individual. Unfortunately, the cut-off levels of serum 25(OH)D that represent vitamin D insufficiency are not clearly defined16.A circulating level of < 20 ng/ml of 25(OH)D is widely used as deficiency, while a circulating level of < 30 ng/ml as insufficiency117. Garnero et al7 showed that 73.0 per cent of French healthy postmenopausal women had 25(OH)D levels less than 30 ng/ml, and serum 25(OH)D levels were not related to BMD at hip. Bhattoa et al18 in their study on Hungarian postmenopausal women indicated high prevalence (56.7%) of vitamin D deficiency in association with BMD at femoral neck. Another study on Moroccan postmenopausal women showed high prevalence (85.3%) of vitamin D insufficiency in relation to asymptomatic osteoporotic vertebral fractures19. In Asia, available evidences from Shanghai12, Hongkong4, and India14 indicated that participants had abundant sunlight exposure, but vitamin D insufficiency was widespread. We undertook this study to investigate the relationship between serum 25(OH)D levels and BMD in postmenopausal women in Guangzhou, a city in the southern of China.
Material & Methods
In this cross-sectional study 165 healthy women (mean age 63.82 ± 10.61 yr; range 48-85 yr) who participated in the annual health examination at the General Hospital of Guangzhou Military Command of PLA were evaluated from July to August 2012. Only those women were selected who confirmed to be living for at least five years in Guangzhou. Informed written consent was obtained from all subjects. The study protocol was approved by the Ethical Committee of General Hospital of Guangzhou Military Command of PLA. Age, height, weight, and age of menopause were recorded and body mass index (BMI) was calculated.
Exclusion criteria included diabetes mellitus, hyperparathyroidism, thyroid disorders, liver and kidney diseases, cancer, rheumatoid arthritis and medical treatment with bisphosphonate, diuretics, adrenal and thyroid-related drugs, corticosteroids, vitamine D and/or calcium supplements within the preceding three months. Forty six women were excluded.
Serum collection and analysis: Blood samples (4 ml) were collected from each subject in the morning after an overnight fast. The blood was centrifuged for 10 min at 1000 x g to obtain serum. The serum was placed in Eppendorf tubes and stored at -80°C until used.
The levels of serum 25(OH)D, parathyroid hormone (PTH), type 1 procollagen amino-terminal propeptide (P1NP), and beta-carboxy-terminal cross-linking telopeptide of type I collagen (β-CTX) were measured by electrochemiluminescence immunoassay (ECLIA) (Cobase 411, Roche Diagnostics, Mannheim, Germany). The intra- and inter-assay coefficients of variation (CVs) for 25(OH)D were 3.0 to 7.5 per cent and 5.5 to 13.6 per cent, respectively. The normal reference range for serum 25(OH)D was ≥30 ng/ml (≥75 nmol/l). The lower limit of detection of 25(OH)D was 3 ng/ml (7.5 nmol/l), and the higher limit was 70 ng/ml (175 nmol/l). The intra- and inter-assay CVs for PTH were 4.5 and 6.4 per cent, respectively. The normal reference range for serum PTH was 15-65 pg/ml (1.6-6.9 pmol/l). The lower limit of detection of PTH was 1.20 pg/ml (0.127 pmol/l), and the higher limit was 5000 pg/ml (530 pmol/l). The intra- and inter-assay CVs for P1NP were 6.5 and 6.1 per cent, respectively. The lower limit of detection of P1NP was 5 μg/l, and the higher limit was 1200 μg/l. The intra- and inter-assay CVs for β-CTX were 4.3 and 5.8 per cent, respectively. The lower limit of detection of β-CTX was 0.010 μg/l (10 pg/ml), and the higher limit was 6.00 μg/l(6000 pg/ml).
Serum calcium and phosphorus (Cobase501, Roche Diagnostics, Mannheim, Germany) were measured by colorimetric method (Hitachi, Automated Biochemistry Analyzer). The normal laboratory range for serum calcium was 2.15-2.55 mmol/l and for serum phosphorus was 0.97-1.62 mmol/l, according to the kit manufacturers.
Bone mineral density examination: BMD was determined using Dual Energy X-ray Absorptiometry (DEXA; Lunar Prodigy, GE Medical Systems, Madison, USA). Both spine region including lumbar vertebrae 1-4 and femoral neck area l BMD were obtained. To eliminate operator differences, all women were tested by the same operator during the study. Duplicate measurements were obtained from thirty subjects who underwent a repeat assessment on the same day, and the precision errors were calculated using the root mean square method20. The coefficients of variation for the lumbar spine and femoral neck BMD were 0.60 and 0.89 per cent, respectively.
Statistical analysis: Normally distributed data were expressed as mean ± SD, and abnormally distributed data were shown as medians and interquartile ranges. All analyses were performed using SAS version 8.0 (SAS Institute, Cary, NC, USA). Normal distribution of data was ascertained by the Shapiro-Wilk test21. Results that were not normally distributed, were log transformed before analysis. For normal distribution data, one-way ANOVA and Dunnett's post-hoc test were performed to compare the results of multiple groups. Because serum PTH and β-CTX levels were still abnormally distributed after log transformation, the groups were compared using Kruskal-Wallis test. The frequency comparison between each group was tested by chi-square. Bivariate correlation analyses with Spearman's rank correlation test was performed to determine the relationship between pairs of bone related variables which did not follow a normal distribution. Partial correlation analyses were used to adjust for age and BMI. Unadjusted and age-, BMI-adjusted Pearson correlation analyses were used when bone related parameters followed a normal distribution. Independent relationships between BMD and other variables were determined by multiple regression22. The selected independent variables had significant association with BMD on bivariate correlation analyses. The multiple-regression analysis was carried out by the step-wise method. The probability of F was applied to select the variables to be included in the model, the variables with P less than 0.10 were entered and variables with P larger than 0.11 were removed from the model.
Results
The mean age of women (n=119) was 62.76 ± 9.82 yr (range 48-85 yr), and mean BMI was 23.50 ± 3.50 kg/m2 (range 15.82-34.63). The mean serum concentrations of calcium and 25(OH)D were 2.34 ± 0.14 mmol/l (range 1.97-2.83) and 25.98 ± 8.19 ng/ml (range 7.93-49.69), respectively. The median (interquartile range) serum concentrations of phosphorus, PTH, P1NP and β-CTX were 1.26 (1.14-1.39) mmol/l, 7.66 (3.35-18.30) pmol/l, 42.88 (33.07-62.79) μg/l, and 0.42 (0.25-0.60) μg/l, respectively. The mean BMD values of lumbar spine and femoral neck were 0.95 ± 0.16 (range 0.54-1.36) and 0.77 ± 0.12 (range 0.46-1.00)g/cm2, respectively. When the study group was analyzed according to the presence/absence of the fragility fracture, 10 women (8.40%) with spine fracture and three (2.52%) with hip fracture were identified; 29 (24.37%) reported at least one fall in the past 12 months.
Serum 25(OH)D concentrations were divided into three subgroups17: deficiency (≥10 and <20 ng/ml), insufficiency (≥20 and <30 ng/ml), and sufficiency (≥30 ng/ml). Thirty (25.2%) postmenopausal women had 25(OH)D levels below 20 ng/ml; 77 (64.7%) had below 30 ng/ml. Women who spent less than 30 min a day time for sun exposure had lower serum 25(OH)D levels. (P<0.001) among the three subgroups. Serum 25(OH)D concentrations were higher in women who exercised at least three times per week (P<0.001) among the three subgroups. In subgroups with decreasing mean 25(OH)D levels, the incidence of the vertebral fragility fracture increased (P<0.01), and a similar trend was observed for fall in the past 12 months (P<0.001) (Table I). In addition, serum 25(OH)D concentrations increased as the PTH levels decreased (P<0.05). The BMD at lumbar spine and femoral neck increased with increasing mean 25(OH)D levels (P<0.05). Women with 25(OH)D less than 30 ng/ml had significantly lower BMD T scores at both the spine and femoral neck (Table I).
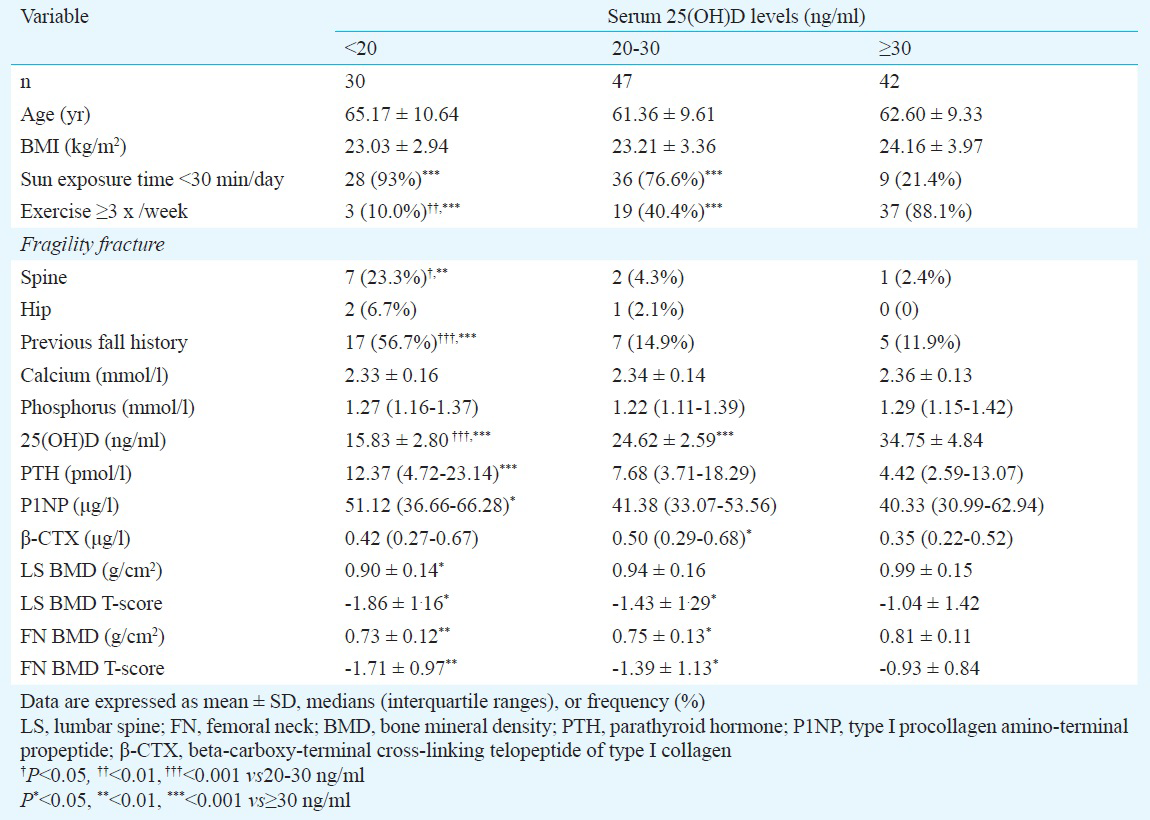
Serum 25(OH)D, PTH levels in relation to anthropometric and biochemical markers: No significant correlations were observed between serum 25(OH)D levels and age, BMI, serum calcium, phosphorus, PTH, P1NP and β-CTX levels. However, correlations between the serum 25(OH)D and PTH levels persisted after adjustment for age and BMI. No correlation between age-after and BMI-adjusted 25(OH)D and the remaining variables was found (Table II).
Serum PTH levels were negatively correlated with age, and positively correlated with calcium and P1NP levels. No significant correlations were found between serum PTH levels and BMI, serum phosphorus and β-CTX levels. However, age- and BMI-adjusted PTH levels were positively associated with calcium levels. No association between age- and BMI- adjusted PTH and the remaining variables was found (Table II).
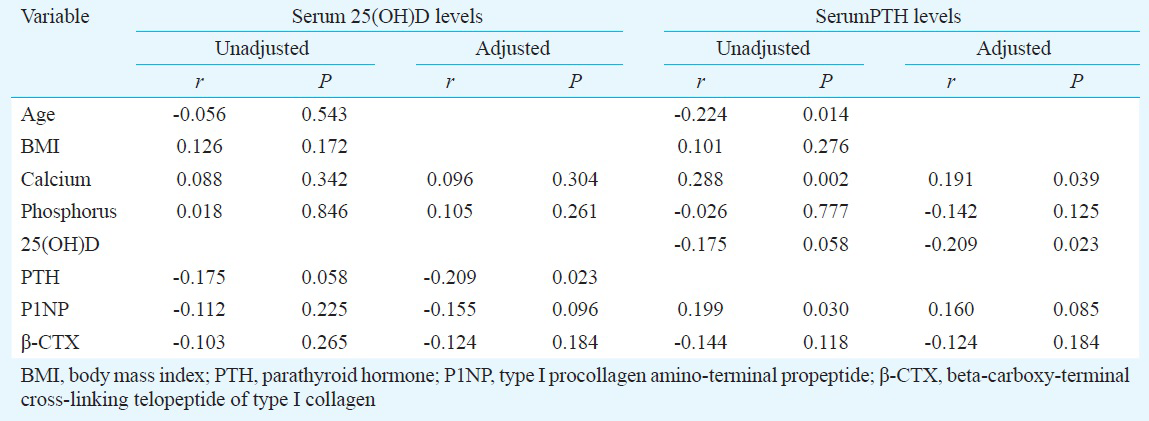
BMD in relation to anthropometric and biochemical markers: BMD at lumbar spine was positively correlated with BMI and negatively correlated with age, P1NP, and β-CTX levels. BMD at femoral neck was negatively correlated with age and positively correlated with BMI and serum 25(OH)D levels (Table III). After controlling for age and BMI, BMD at lumbar spine showed significant inverse correlation with serum P1NP and β-CTX levels. However, age- and BMI- adjusted BMD at femoral neck was positively correlated with serum phosphorus and 25(OH)D levels (Table III).
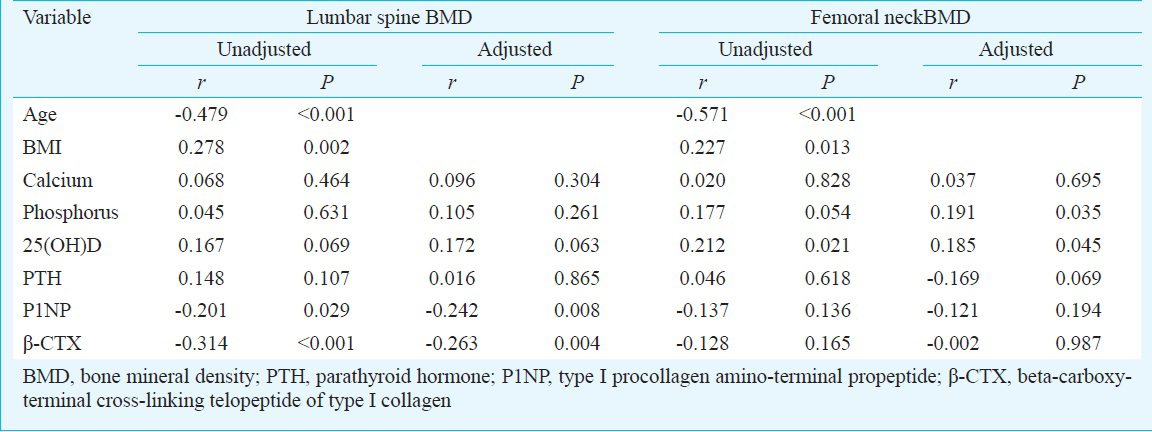
Multiple regression analysis for BMD as a dependent variable: Multiple regression analysis was performed to determine the predictor of BMD after adjustment for age and BMI. When age, BMI, serum 25(OH)D, P1NP and β-CTX levels were included as the independent variables, only age, BMI and serum β-CTX were the significant predictors for BMD at lumbar spine (Table IV). When age, BMI, serum phosphorus, 25(OH)D, P1NP and PTH levels were included as the independent variables, age, BMI, serum phosphorus and 25(OH)D levels were the significant determinants for BMD at lumbar spine (Table IV).
Discussion
In our study 64.7 per cent postmenopausal women had vitamin D insufficiency, in line with the previous studies of French postmenopausal women (73.0%)7, Moroccan healthy postmenopausal women (85.3%)19 and Indian postmenopausal women (86.0%)2324. Guangzhou is a flourishing international commercial city with a population of over 12 million. The main reason of vitamin D insufficiency may be attributed to the lifestyle change of people with less outdoor activities and air pollution related haze from vehicular sources that decreases sunlight exposure, thereby reducing the ultraviolet-B (UVB)-induced vitamin D synthesis in the skin825. Our results indicated that exercising at least three times a week and sun exposure less than 30 min a day were needed to obtain optimum serum 25(OH)D concentrations.
Serum 25(OH)D level is the best clinical indicator of the vitamin D status in blood. Wat et al4 reported that an optimal level of 25(OH)D to suppress serum PTH concentrations was 30 ng/ml. Because PTH began to rise when 25(OH)D levels were below 30 ng/ml, whereas PTH levels did not change when 25(OH)D levels were above 30 ng/ml4. Lu et al12 showed that the levels of procollagen 1 N-terminal peptide (P1NP) and beta C-telopeptide of collagen (β-CTX) started to increase when serum 25(OH)D levels were less than 30 ng/ml. In our study, with increasing serum 25(OH)D levels categorized as <20, 20-30, and ≥30ng/ml, the PTH levels gradually decreased and BMD at lumbar spine and femoral neck increased. Our results also indicated that serum 25(OH)D level of at least 20 ng/ml was needed to maintain high BMD at lumbar spine and suppress circulating PTH and P1NP levels, and that 30 ng/ml of 25(OH)D was needed to obtain high BMD at femoral neck and suppress circulating β-CTX levels.
Age and BMI, known risk factors for vitamin D insufficiency26, did not show any correlation with 25(OH)D levels in our study. Our results also showed that serum 25(OH)D was inversely correlated with PTH levels after adjustment for age and BMI. This result was in agreement with previous studies on Asian and European women6712. However, studies from USA and Israel have shown no association between 25(OH)D and PTH levels after controlling age and BMI527. Allali et al6 showed that participants with low 25(OH)D levels had higher bone turnover. We found that age- and BMI- adjusted 25(OH)D levels did not correlate with bone turnover markers. This suggests that it will be unsuitable to use increased bone turnover levels as a surrogate indicator for vitamin D insufficiency in subjects under investigation.
Vitamin D status is considered as an important determinant of bone health. However, there is a controversy regarding the association between 25(OH)D levels and BMD. Some groups have reported a positive association between serum 25(OH)D levels and BMD at the hip and spine in men and women2518 while others found no association467. Our data demonstrated a marginal association between serum 25(OH)D levels and lumbar BMD in postmenopausal women. However, in multiple regression analysis, serum 25(OH)D was not a predictor for lumbar BMD. We also found that femoral neck BMD was significantly and positively related to 25(OH)D after controlling age and BMI. Multiple regression analysis confirmed that serum 25(OH)D was a predictor of BMD at femoral neck. Our findings were similar to those reported earlier251828. von Mühlen et al5 showed that age-adjusted 25(OH)D levels were related to BMD at hip in postmenopausal women (aged 50-97 yr). Bhattoa et al18 showed that 25(OH)D levels were significantly associated with BMD at femoral neck in postmenopausal women. Nakamura et al28 showed that higher serum 25(OH)D levels were associated with increased BMD of the femoral neck, and that a serum 25(OH)D level of at least 70 nmol/l was needed to obtain high BMD of the femoral neck in home-dwelling postmenopausal Japanese women. Garnero et al7 indicated that 25(OH)D levels were not related to BMD at hip in postmenopausal women (mean age: 62.2 yr). The results of the current study demonstrated that vitamin D status was an important determinant of hip bone health. Based on our results, it may be recommended to take vitamin D supplements when the circulating 25(OH)D level was below 30 ng/ml, especially, in postmenopausal women. It was also found that with decreasing mean 25(OH)D levels, the incidence of fall and the vertebral fragility fractures increased. Our results suggested that vitamin D status was also linked with vertebral fragility fracture risk and normal levels of circulating 25(OH)D could reduce falls leading to lower fracture rate.
The analysis of our data showed that serum β-CTX was a determinant of BMD at lumbar spine. This result was consistent with a previous study, which also showed that there was a negative correlation between serum β-CTX and lumbar BMD29. Yoshimura et al30 indicated that serum β-CTX in women could predict the occurrence of spinal osteoporosis. We also found that femoral neck BMD was significantly and inversely related to phosphorus after adjustment of age and BMI and there was a marginal association between serum PTH levels and BMD at femoral neck. This suggested that serum β-CTX was a determinant of BMD at lumbar spine, and it would be unsuitable to use serum PTH and bone turnover levels as an indicator for BMD at femoral neck in women under investigation.
This study had some limitations. First, the study was a population-based research were participants were sampled from annual health examination at a general hospital. There could be a selection bias as healthy adults are more likely to participate in the study. Second, this was a cross-sectional design, hence, no sufficient causality could be demonstrated for the relationship between serum 25(OH)D and other variables.
In conclusion, vitamin D insufficiency was common but largely ignored health problem in healthy postmenopausal women living in Guangzhou. Low serum 25(OH)D levels were associated with high serum PTH levels and low BMD at femoral neck. Serum 25(OH)D levels were not associated with lumbar spine BMD and bone turnover markers. However, serum β-CTX levels were inversely correlated with lumbar spine BMD. Further studies are needed to elucidate the clinical impact and precise mechanisms of the above findings.
Acknowledgment
This study was supported in part by grants from the project of the National Natural Science Foundation of China (81273778). Authors acknowledge colleagues at the departments of Rehabilitation, Nuclear Medicine and Physical Examination Center, General Hospital of Guangzhou Military Command of PLA for their invaluable assistance during the execution of the present study, and J.L. Wang and L. Huang for improving language of the paper.
Conflicts of Interest: None.
References
- Vitamin D deficiency among postmenopausal women with osteoporosis. J Clin Diagn Res. 2013;7:336-8.
- [Google Scholar]
- Vitamin D status and bone mineral density in women of reproductive and postmenopausal age groups: a cross-sectional study from south India. J Assoc Physicians India. 2011;59:698-704.
- [Google Scholar]
- Influence of vitamin D levels on bone mineral density and osteoporosis. Ann Saudi Med. 2011;31:602-8.
- [Google Scholar]
- Prevalence and impact of vitamin D insufficiency in southern Chinese adults. Ann Nutr Metab. 2007;51:59-64.
- [Google Scholar]
- Vitamin D, parathyroid hormone levels and bone mineral density in community-dwelling older women: the Rancho Bernardo Study. Osteoporos Int. 2005;16:1721-6.
- [Google Scholar]
- High prevalence of hypovitaminosis D in Morocco: relationship to lifestyle, physical performance, bone markers, and bone mineral density. Semin Arthritis Rheum. 2009;38:444-51.
- [Google Scholar]
- Associations of vitamin D status with bone mineral density, bone turnover, bone loss and fracture risk in healthy postmenopausal women. The OFELY study. Bone. 2007;40:716-22.
- [Google Scholar]
- Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31:48-54.
- [Google Scholar]
- High prevalence of vitamin D insufficiency in healthy Irish adults. Ir J Med Sci. 2008;177:131-4.
- [Google Scholar]
- Prevalence and correlates of vitamin D status in African American men. BMC Public Health. 2009;9:191.
- [Google Scholar]
- High prevalence of vitamin D insufficiency in China: relationship with the levels of parathyroid hormone and markers of bone turnover. PLoS One. 2012;7:e47264.
- [Google Scholar]
- High prevalence of vitamin D deficiency among children aged 1 month to 16 years in Hangzhou, China. BMC Public Health. 2012;12:126.
- [Google Scholar]
- Regional variation and determinants of vitamin D status in sunshine-abundant Thailand. BMC Public Health. 2011;11:853.
- [Google Scholar]
- Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18-28.
- [Google Scholar]
- Prevalence and seasonal variation of hypovitaminosis D and its relationship to bone metabolism in community dwelling postmenopausal Hungarian women. Osteoporos Int. 2004;15:447-51.
- [Google Scholar]
- Hypovitaminosis D and prevalent asymptomatic vertebral fractures in Moroccan postmenopausal women. BMC Womens Health. 2012;12:11.
- [Google Scholar]
- Omentin-1, visfatin and adiponectin levels in relation to bone mineral density in Iranian postmenopausal women. Bone. 2012;51:876-81.
- [Google Scholar]
- The relationship between serum resistin, leptin, adiponectin, ghrelin levels and bone mineral density in middle-aged men. Clin Endocrinol (Oxf). 2005;63:131-8.
- [Google Scholar]
- The analysis and selection of variables in linear regression. Biometrics. 1976;32:1-49.
- [Google Scholar]
- Postmenopausal osteoporosis: Our experience. Indian J Endocrinol Metab. 2012;16(Suppl 2):S421-2.
- [Google Scholar]
- Prevalence of vitamin D insufficiency in postmenopausal south Indian women. Osteoporos Int. 2005;16:397-402.
- [Google Scholar]
- The impact of atmospheric pollution on vitamin D status of infants and toddlers in Delhi, India. Arch Dis Child. 2002;87:111-3.
- [Google Scholar]
- Vitamin D status in relation to obesity, bone mineral density, bone turnover markers and vitamin D receptor genotypes in healthy Saudi pre- and postmenopausal women. Osteoporos Int. 2011;22:463-75.
- [Google Scholar]
- The relationship between serum 25(OH)D and parathyroid hormone levels. Am J Med. 2011;124:1165-70.
- [Google Scholar]
- Vitamin D status, bone mass, and bone metabolism in home-dwelling postmenopausal Japanese women: Yokogoshi Study. Bone. 2008;42:271-7.
- [Google Scholar]
- Prevalence of vitamin D insufficiency and low bone mineral density in elderly Thai nursing home residents. BMC Geriatr. 2012;12:49.
- [Google Scholar]
- Biochemical markers of bone turnover as predictors of osteoporosis and osteoporotic fractures in men and women: 10-year follow-up of the Taiji cohort. Mod Rheumatol. 2011;21:608-20.
- [Google Scholar]






