Translate this page into:
Evaluation of profertility effect of probiotic Lactobacillus plantarum 2621 in a murine model
Reprint requests: Dr Vijay Prabha, Department of Microbiology, Panjab University, Chandigarh 160 014, India e-mail: satishvijay11@yahoo.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Urogenital infections of bacterial origin have a high incidence among the female population at reproductive age, affecting the fertility. Strains of Escherichia coli can colonize the vagina and replace natural microflora. Lactobacillus the predominant vaginal microorganism in healthy women, maintains the acidic vaginal pH which inhibits pathogenic microorganisms. Studies on Lactobacillus have shown that these can inhibit E. coli growth and vaginal colonization. An alternative therapeutic approach to antimicrobial therapy is to re-establish Lactobacillus in this microbiome through probiotic administration to resurge fertility. Therefore, the aim of the present study was to determine the capability of L. plantarum 2621 strain with probiotic properties, to prevent the vaginal colonization of E. coli causing agglutination of sperms and to evaluate its profertility effect in a murine model.
Methods:
Screened mice were divided into five groups i.e. control group, E. coli group, Lactobacillus group, prophylactic and therapeutic groups. The control group was infused with 20 µl PBS, E.coli group was administered with 106 cfu/20 µl E. coli, and probiotic group was administered with Lactobacillus (108 cfu/20 µl) for 10 consecutive days. In prophylactic group, the vagina was colonized with 10 consecutive doses of Lactobacillus (108 cfu/20 µl). After 24 h, it was followed by 10 day intravaginal infection with E. coli (106 cfu/20 µl) whereas for the therapeutic group vagina was colonized with (106 cfu/20 µl) E. coli for 10 consecutive days, followed by 10 day intravaginal administration with Lactobacillus after 24 h.
Results:
Upon mating and completion of gestation period, control, probiotic and the therapeutic groups had litters in contrast to the prophylactic group and the group administered with E. coli.
Interpretation & conclusions:
Results indicated that Lactobacillus intermitted colonization of pathogenic strains that resulted in reinforcement of natural microflora and resurge fertility.
Keywords
Escherichia coli
infertility
intravaginal administration
Lactobacillus
spermagglutination
vaginal lavages
The prevalence of male factor infertility has been reported to be 30 per cent while female factor infertility is found to be 40-55 per cent12. Infections may lead to male infertility with a prevalence of 15 per cent3 while in females it accounts for 30-40 per cent4. Bacterial interactions with spermatozoa lead to morphological defects as well as changes in functional parameters of spermatozoa in vitro5. These may in turn, lead to a decrease in the fertilization potential of the sperm. Escherichia coli is one of the dominant bacteria isolated from the semen samples of males with infertility. E. coli inhibits spermatozoa motility in vitro and readily adheres to and agglutinates sperm6. Antibiotics have been extensively used as effective therapy for the treatment of bacterial infections but due to increasing drug resistance of urogenital pathogens, development of alternative therapeutics is vital. Lactobacillus has been shown as an alternative to antibiotics in bacterial infections which on intravaginal administration colonized by eradicating the pathogens7.
Lactobacilli are predominant microorganisms balancing the normal vaginal milieu. Lactobacilli comprise 70 per cent of the total microorganisms isolated (i.e at the level of 107-108 cfu/g) from the vaginal fluid influencing the vaginal ecosystem89. Recolonizing the vagina with live lactobacilli may offer a choice of treatment. However, the use of lactobacilli in improving compromised fertility induced by bacteria has not been evaluated. Carefully designed trials using well characterized probiotic strains and treatment regimens are required to evaluate the effect of probiotics on fertility outcome. L. plantarum 2621 has been shown to display in vitro properties relevant to colonization as assessed by adherence to vaginal epithelial cells10. The present study was undertaken to determine the capability of L. plantarum 2621 to overcome vaginal colonization and infertility induced by spermagglutinating E. coli, in a mouse model.
Material & Methods
This study was conducted in the Microbiology department of Panjab University, Chandigarh, India. The strain of E. coli used in the present study was isolated earlier in our laboratory from the semen sample of a patient with complains of infertility. A standard strain of L. plantarum MTCC 2621 was procured from Microbial Type Culture Collection (MTCC), Institute of Microbial Technology (IMTECH), Chandigarh, India. These strains were maintained on Luria agar (LA) and de Mann Rogosa Sharpe (MRS) agar (5% CO2 ). Strains were stored as glycerol stocks at -60°C.
Animals: Sexually mature, 5-6 wk old male (25±2 g) and 4-5 wk old female (22±2 g) BALB/c mice were used in the present study. Animals were maintained in laboratory conditions (12:12, dark:light cycle), housed in plastic cages and fed with standard pellet diet and water ad libitum. Experimental protocols were approved by Institutional Animal Ethics Committee.
Extraction of mouse spermatozoa: Male mice (6-7 wk old) were used for isolation of the spermatozoa. Mice were sacrificed by cervical dislocation and sperms from the cauda epididymis and vas deferens were collected by teasing in different media kept at 37°C to maintain sperm motility. Motility of mouse spermatozoa was maintained in phosphate buffer saline (PBS, pH 7.2). Isolated sperms were utilized to screen mouse vagina harbouring spermagglutinating strains.
Sperm bacteria interaction: Sperm bacteria interaction was studied in vitro by incubating equal volumes (50 µl) of sperm sample and (50 µl) cell culture to evaluate spermagglutinating property at 37°C for 30 min and 1h. The adherence of immotile spermatozoa to each other or of motile spermatozoa to mucus strands, non-sperm cells or debris was considered to be non-specific aggregation. Agglutination specifically refers to motile spermatozoa sticking to each other, head-to-head, tail-to-tail or in a mixed way.
Screening of animals: The animals were first screened for micro-organisms that naturally inhabit the BALB/c mouse vagina. The microbiota of the mice induced in estrous cycle by whitten effect was studied from vaginal samples taken with sterile cotton swabs moistened with physiological saline. Swabs were cultured at 37°C on brain heart infusion (BHI) agar, eosin methylene blue (EMB) agar for 24 h and MRS agar in 5 per cent CO2 for 48 h. Strains growing on BHI plates were further checked for spermagglutinating/immobilizing properties. Mice harbouring Lactobacillus (growth on MRS agar) / E. coli (growth on EMB agar with green sheen) or any other bacteria (growth on BHI) with spermagglutinating/immobilizing property were excluded from the study. Screened mice were divided into five groups, i.e. control group, E. coli group, Lactobacillus group, prophylactic and therapeutic group with three mice per group and the experiments were done twice amounting to total number of animals used in this study 30. In the first group as control six mice were inoculated intravaginally with PBS, the second group was used to induce the vaginal infection with spermagglutinating E. coli, the third group was used to study colonization by L. plantarum 2621 and the remaining two groups were used to evaluate the preventive and therapeutic effects.
Preparation of inoculum: E. coli was cultivated in Luria broth at 37°C for 24 h. Cell culture was centrifuged at 7267 g for 20 min and washed twice with PBS (50 mM, pH 7.2). Cells were suspended in the same buffer and adjusted to a concentration of 106 colony forming units (cfu)/20 µl. Similarly, L. plantarum 2621 was grown in MRS broth and was adjusted to a concentration of 108 cfu/20 µl.
Effect of spermagglutinating strain of E. coli on fertility outcome: To demonstrate the colonization and induction of infertility, a group of female mice were administered intravaginally with 106 cfu of E. coli per mouse in 20 µl PBS for 10 consecutive days at 24 h interval. As control, mice were inoculated intravaginally with 20 µl of PBS for 10 consecutive days. Vaginal lavages were taken every third day upto 35 days, so as to monitor vaginal colonization. The re-isolated bacteria from the vaginal swabs were further confirmed as E. coli by culture characteristics and biochemical tests (Hi-Motility biochemical identification kit, Hi-Media Laboratories, Mumbai, India) and were also checked for spermagglutinating activity in vitro.
On day 12, these female mice were allowed to mate with breeder male mice in the ratio 2:1, to check the effect on fertility outcome. Upon mating, next day the females were separated and mating was confirmed by observing the presence of vaginal plug in all the female mice.
Effect of probiotic L. plantarum 2621 on fertility outcome: The mice of Lactobacillus group were administered by intravaginal inoculation with 20 µl of the suspension of Lactobacillus in PBS adjusted to 108 cfu/20 µl. Vaginal lavages were taken every third day upto 35 days and cultured on MRS agar (5% CO2 for 48 h) so as to monitor vaginal colonization. On day 12, these female mice were allowed to mate with breeder male mice in the ratio 2:1, to check the effect on fertility outcome. Upon mating, next day the females were separated and mating was confirmed by observing the presence of vaginal plug in all the female mice.
Prophylactic effect of L. plantarum 2621 against spermagglutinating E. coli induced infertility: The preventive effect of Lactobacillus on the infertility induced by spermagglutinating organisms was studied by administering Lactobacillus (108 cfu/20 µl) for 10 consecutive days into the vagina of mice, 24 h later followed by a challenge of spermagglutinating E. coli (106 cfu/20 µl ) for 10 consecutive days intravaginally. Lavages were taken at day 3 interval upto 35 days to monitor the vaginal colonization and viability of Lactobacillus and spermagglutinating E. coli checked on MRS (5% CO2 for 48 h), LA and EMB agar. Mating was allowed 24 h after the last inoculation as described before.
Therapeutic effect of L. plantarum 2621 against spermagglutinating E. coli induced infertility: Screened mice were administered intravaginally with the spermagglutinating E. coli (106 cfu/20 µl) for 10 consecutive days to establish an infection, 24 h later followed by infusion with Lactobacillus (108 cfu/20 µl) for 10 consecutive days to ameliorate the impairment caused by E. coli. Lavages were taken on every 3rd day upto 35 days to assess the establishment of infection and evaluation of colonization of Lactobacillus and viability of the organisms on MRS (5% CO2 for 48 h), LA and EMB agar. Mating was allowed 24 h after the last inoculation.
Statistical analysis: All bacterial counts were expressed as mean ± SD. An analysis of variance was used for differences in numbers of viable microorganisms from various groups.
Results
Microbiological analysis of normal flora in mice from vaginal samples was performed by light microscopy and culture characteristics. Gram staining of sample slides showed that higher percentage (48%) of mice harboured Gram +ve cocci followed by Gram -ve rods (23%), very small percentage (8.3%) of mice were found to harbour Lactobacillus. These observations correlated with the white colonies on BHI, transparent colonies on Luria agar plates under aerobic conditions. There was growth on very few MRS agar plates. This indicated absence of lactobacilli strains in a large percentage of mice.
Effect of spermagglutinating strain of E. coli on fertility outcome: Lavages taken at regular intervals demonstrated persistence of E. coli in the mouse vagina at high counts of 4.5 log10 cfu/ml for more than 17 days (7 days after last inoculation), thereafter started decreasing gradually by day 24 to 1.5 log10 cfu/ml and was cleared by day 31 and the vaginal cultures were thereafter negative for the respective organism (Figure A). Upon mating on day 12 with the proven male breeder mice in 2:1 ratio in the presence of organisms all the mice failed to conceive and no pregnancy related changes were observed. These results were in contrast to the control mice inoculated with PBS which showed consistent pregnancy related changes such as weight gain, strings of pearl by palpation by the day 14, abdominal distension and the delivery of pups at the end of gestation period by day 21.
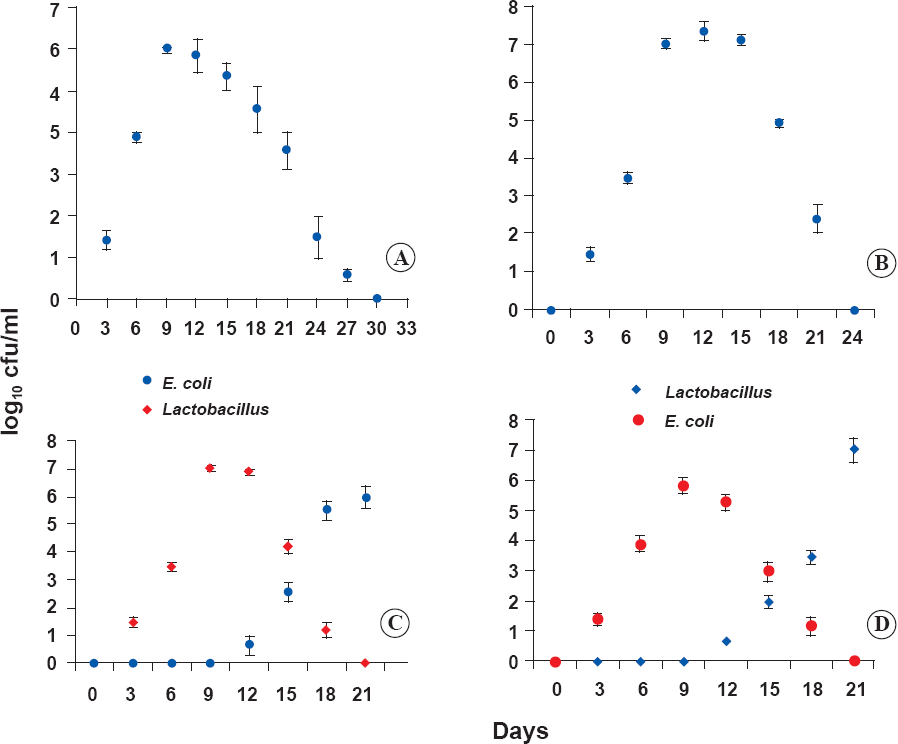
-
A. Vaginal establishment of E. coli in BALB/c mice. Results are shown as mean ± SD (n=3 observations). Figure B. Vaginal colonization of L. plantarum 2621 in female mice. Results are shown as mean ± SD (n=3 observations). Figure C. Prophylactic effect of L. plantarum 2621 against E. coli in BALB/c mice. Results are shown as mean ± SD (n=3 observations). Figure D. Therapeutic effect of L. plantarum 2621 on E. coli. Results are shown as mean ± SD (n=3 observations). Significant differences found between the untreated control (Figure A) and the treated group (P<0.05).
Effect of probiotic L. plantarum 2621 on fertility outcome: Lavages taken at regular intervals verified the colonization of Lactobacillus in the vaginal samples. Lactobacillus was observed to persist in the vagina in high counts of 7.12 log10 cfu/ml for 15 days, thereafter started decreasing to the count of 2.4 log10 cfu/ml on day 21 and was cleared by day 24 and the vaginal cultures were negative thereafter for respective organism (Figure B). Mice allowed to mate on day 12 with the proven breeder male mice in 2:1 ratio in the presence of Lactobacillus in the vagina were all fertile. These results were analogous to the control group inoculated with PBS and showed pregnancy related changes.
Prophylactic effect of L. plantarum 2621 against spermagglutinating E. coli: It was observed Lactobacillus colonized in high counts of 7.12 log10 cfu/ml on day 10, however, its number decreased progressively after the challenge with each dose of E. coli. The count decreased to 4.1 log10 cfu/ml on day 15 and was cleared by day 21 and cultures were thereafter negative for Lactobacillus. With increase in number of doses of E. coli challenge the colonization of vagina with E. coli tends to increase to 5.52 log10 cfu/ml on day 18 and by the end of 10 inoculations E. coli replaces Lactobacillus completely (Figure C). These mice after mating on day 22 in the absence of Lactobacillus and presence of E. coli failed to conceive, no pregnancy related changes were observed.
Therapeutic effect of L. plantarum 2621 against spermagglutinating E. coli: To evaluate the therapeutic effect, vaginal lavages collected on regular time intervals were cultured on EMB, Luria agar and MRS (5% CO2, 48 h) agar at 37°C. High counts of E. coli (5.8 log10 cfu/ml) were found to colonize the vagina by the last inoculation on day 10. Thereafter its number decreased to 3.01 log10 cfu/ml on day 15 once the Lactobacillus started establishing after the day four of Lactobacillus inoculation and E. coli was cleared by day 21. The cultures were found negative for E. coli as Lactobacillus completely removed the E. coli (Figure D). A significant difference between the untreated control (Figure A) and the treated group (Figure D) was found (P<0.05). These mice when allowed to mate in the absence of E. coli and presence of Lactobacillus were fertile.
Discussion
This study was carried out to evaluate the effect of lactobacilli on infertility due to the presence of spermagglutinating E. coli. Many studies have related the absence of lactobacilli, with genital infections such as bacterial vaginosis1112. Lactobacillus is the prominent organism present in the healthy human vagina that comprises 70 per cent of the total organisms isolated1314. Lactobacilli play an important role in gastrointestinal tract, urinary tract and in the vagina815. Substances such as H2 O2, lactic acid and bacteriocin are produced by lactobacilli to protect the human vagina131617. To ascertain the important factors in pathogenesis of infertility and the effect of lactobacilli on fertility, mouse model was used for intravaginal inoculations that can simulate ascending genital tract infections in female1819. L. plantarum 2621 was able to colonize the mouse vagina efficiently. This showed association between the colonization observed in vivo with our in vitro studies of Lactobacillus attachment to the vaginal epithelium10. Other investigators2021 have used a large number of doses to ensure the colonization of Lactobacillus. Mice inoculated with 108 cfu/20 µl of Lactobacillus for 10 consecutive days upon mating on day 12 were all fertile delivering litters on completion of gestation period. Same was not true with the spermagglutinating E. coli, as all mice administered with 106 cfu/20 µl of E. coli were rendered infertile. These results were in parallel to the persistence of this strain in high numbers for more than 18 days and were similar to our earlier findings22. Bacterial interactions with spermatozoa lead to morphological defects as well as changes in functional parameters of spermatozoa in vitro. These may in turn lead to a decrease in the fertilization potential of sperm. Villegas et al23 demonstrated that a single incubation with Enterococcus fecalis, E. coli and Staphylococcus aureus induced apoptosis in human sperm.
The prophylactic and therapeutic studies were carried out to study the effect of lactobacilli on spermagglutinating E. coli strain in vivo. In the prophylactic group 10 inoculations of spermagglutinating E. coli could successfully eliminate the Lactobacillus on day 21. Moreover, upon mating in the presence of E. coli and absence of lactobacilli female mice were rendered infertile. The infertility could be due to the high dose of spermagglutinating organisms causing infection that rendered the mice infertile. However, Lactobacillus administration in the mouse vagina after E. coli establishment resulted in complete elimination of the pathogen resulting in fertility and exhibiting a significant antimicrobial effect in post-infection treatment. Results obtained were parallel to the results of group administered with LactobacillusLactobacillus. These findings were similar to studies by Pascual and his group7 reaffirming antimicrobial property in vitro and in vivo.
From the above results it could be concluded that the presence of probiotic strain L. plantarum 2621 in vagina had no effects on fertility whereas spermagglutinating organism induced infertility in mice. The therapeutic study indicated that establishment of spermagglutinating organism and its displacement by probiotic strain restored the fertility.
Acknowledgment
This work was supported by the financial support from the University Grants Commission (UGC), New Delhi, India
References
- Sexually transmitted infections and sexual function in relation to male fertility. Korean J Urol. 2013;54:149-56.
- [Google Scholar]
- Ureaplasma urealyticum, Ureaplasma parvum, Mycoplasma hominis and Mycoplasma genitalium infections and semen quality of infertile men. BMC Infect Dis. 2007;7:129-37.
- [Google Scholar]
- Impact of genital infections on fertility. J Turkish German Gynecol Assoc. 2005;6:197-203.
- [Google Scholar]
- Genital tract infection in asymptomatic infertile men and its effect on semen quality. Iran J Public Health. 2006;35:81-4.
- [Google Scholar]
- The presence of bacterial species in semen and sperm quality. J Assist Reprod Genet. 2009;26:47-56.
- [Google Scholar]
- Vaginal colonization and activity of the probiotic bacterium Lactobacillus fermentum L23 in a murine model of vaginal tract infection. J Med Microbiol. 2010;59:360-4.
- [Google Scholar]
- The identification of vaginal Lactobacillus species and the demographic and microbiologic charactristics of women colonized by these species. J Infect Dis. 1999;180:1950-6.
- [Google Scholar]
- Genetic diversity of vaginal lactobacilli from women in different countries bassed on 16sRNA gene sequence. J Appl Microbiol. 2002;92:451-9.
- [Google Scholar]
- Potential of probiotic Lactobacillus plantarum 2621 for the management of Infertility. Br Microbiol Res J. 2014;4:1585-96.
- [Google Scholar]
- Lactobacillus rhamnosus L60, a potential probiotic isolated from the human vagina. J Gen Appl Microbiol. 2008;54:141-8.
- [Google Scholar]
- Hydrogen peroxide producing lactobacilli and acqusition of vaginal infections. J infect Dis. 1996;4:1058-63.
- [Google Scholar]
- Emerging role of lactobacilli in the control and maintenance of the vaginal bacterial microflora. Rev Infect Dis. 1990;12:856-72.
- [Google Scholar]
- Pathogenic relevance of Lactobacillus: a retrospective review of over 200 cases. Eur J Clin Microbiol Infect Dis. 2005;24:31-40.
- [Google Scholar]
- Adherence of human vaginal lactobacilli to vaginal epithelial cells and interaction with uropathogens. Infect Immun. 1998;66:1985-9.
- [Google Scholar]
- Interference in initial adhesion of uropathogenic bacteria and yeasts to silicone rubber by a Lactobacillus acidophilus biosufactant. J Med Microbiol. 1998;47:1081-5.
- [Google Scholar]
- A new computer model for estimating the impact of vaccination protocols and its application to the study of Chlamydia trachomatis genital infections. Vaccine. 1995;13:119-27.
- [Google Scholar]
- Factors influencing the induction of infertility in a mouse model of Chlamydia trachomatis ascendiing genital tract infection. J Med Microbiol. 1998;47:599-605.
- [Google Scholar]
- Structural and ultrastructural studies of the urinary tract of mice inoculated with Lactobacillus fermentum. BJU Int. 2003;91:878-82.
- [Google Scholar]
- Phase I trial of a Lactobacillus crispatus vaginal suppository for prevention of recurrent urinary tract infection in women. Infect Dis Obstet Gynecol. 2007;2007:35387.
- [Google Scholar]
- Infertility as a consequence of spermagglutinating Staphylococcus aureus colonization in genital tract of female mice. PLoS One. 2012;7:e52325.
- [Google Scholar]
- Bacteria induce expression of apoptosis in human spermatozoa. Apoptosis. 2005;10:105-10.
- [Google Scholar]






