Translate this page into:
Nucleic acid amplification tests (NAATs) for gonorrhoea diagnosis in women: Experience of a tertiary care hospital in north India
Reprint requests: Dr Seema Sood, Department of Microbiology, All India Institute of Medical Sciences, Ansari Nagar, New Delhi 110 029, India e-mail: seemalsood@rediffmail.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Gonorrhoea is among the most frequent of the estimated bacterial sexually transmitted infections (STIs) and has significant health implications in women. The use of nucleic acid amplification tests (NAATs) has been shown to provide enhanced diagnosis of gonorrhoea in female patients. However, it is recommended that an on-going assessment of the test assays should be performed to check for any probable sequence variation occurring in the targeted region. In this study, an in-house PCR targeting opa-gene of Neisseria gonorrhoeae was used in conjunction with 16S ribosomal PCR to determine the presence of gonorrhoea in female patients attending the tertiary care hospitals.
Methods:
Endocervical samples collected from 250 female patients with complaints of vaginal or cervical discharge or pain in lower abdomen were tested using opa and 16S ribosomal assay. The samples were also processed by conventional methods.
Results:
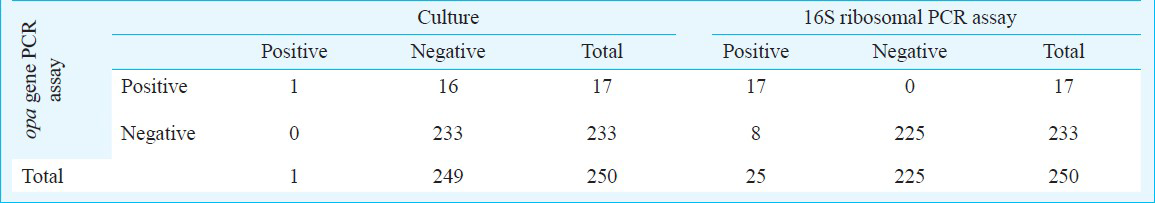
Of the 250 female patients included in the study, only one was positive by conventional methods (microscopy and culture) whereas 17 patients were found to be positive based on PCR results.
Interpretation & conclusions:
The clinical sensitivity of conventional methods for the detection of N. gonorrhoeae in female patients was low. The gonococcal detection rates increased when molecular method was used giving 16 additional positives. Studies should be done to find out other gene targets that may be used in the screening assays to detect the presence of gonorrhoea.
Keywords
Gonorrhoea
NAATs
Neisseria
opa gene
PCR
16S ribosomal gene
A significant proportion of gonococcal infections in women are asymptomatic which if left untreated, may spread to sexual partners and may lead to long term complications12. In symptomatic women also due to overlapping clinical presentation, syndrome based approach may lead to over-diagnosis and over-treatment thereby facilitating emergence and spread of antimicrobial resistance in Neisseria gonorrhoeae. Therefore, timely and accurate identification of these infections is crucial and reliance is being placed on nucleic acid amplification tests (NAATs) which are considered as the gold standard for diagnosis34. However, in light of sequence-related problems that are unique to N. gonorrhoeae, the Centers for Disease Control and Prevention (CDC), Australian Public Health Laboratory Network (PHLN) and also the UK Health Protection Agency (HPA) recommend use of a supplementary PCR targeting a different gene for confirming the results of a screening test on urogenital specimens5678. It is further recommended that any N. gonorrhoeae NAAT should be validated prior to being introduced as a routine diagnostic test for any prospective patient population. Also, the performance of the selected test requires an ongoing assessment to confirm its continued utility, given the high frequency of sequence variation reported for the organism. In our earlier study9, we described the use of 16S ribosomal screening assay along with an in-house opa-gene based supplemental PCR assay for detection of N. gonorrhoeae. We also observed that the use of NAATs in male patients did not lead to an increase in yield in terms of number of positives detected as compared to conventional methods (microscopy/culture). Therefore, the present study was designed to use an in-house opa gene based PCR and 165 ribosomal PCR to determine the presence of gonorrhoea in women attending a tertiary care hospital in north India.
Material & Methods
Sample collection and processing: A total of consecutive 250 female patients in the age group of 15-45 yr with cervical/vaginal discharge or lower abdominal pain attending the Dermatology and Gynaecology OPDs of All India Institute of Medical Sciences (AIIMS) and Dermatology OPD of Dr R.M.L. Hospital, New Delhi, from February 2010 to May 2012 were included in the study. Endocervical swabs were collected from these patients in triplicate. One swab was used for smear preparation for microscopic examination by Gram's staining. Culture was done on modified Thayer Martin medium containing vancomycin, colistin, nystatin trimethoprin (VCNT) (A) inhibitors and chocolate agar (Columbia agar base plus sheep blood) using the second swab. The isolates were confirmed positive by oxidase superoxol test and rapid carbohydrate utilization test (RCUT)10. The third swab sample was used for DNA extraction using commercially available QIAamp DNA mini kit (Qiagen Sciences Inc., US) according to the manufacturer's instructions. The study protocol was approved by the Ethics Committee, AIIMS, New Delhi, India.
PCR of DNA extracted from patients’ samples: opa-gene and 16S ribosomal gene based PCRs were performed as described earlier9. All samples were checked for inhibition using β-globin gene based PCR9.
Statistical analysis: Sensitivity, specificity, positive predictive (PPV) and negative predictive values (NPV) of tests were calculated using online available tool [http://faculty.vassar.edu/lowry/VassarStats.html]. True positives were defined as ones which were positive by culture and/or positive by both 16S ribosomal gene and opa-gene based PCR.
Results & Discussion
Identification of gonococcal infections in women by conventional methods is interfered with by many factors, and the presence of complicated mixed microflora of the vagina and cervix plays a key role4. Introduction of NAATs has achieved significant progress in diagnosis of gonorrhoea in women not only because a low detection limit is of crucial importance for diagnosis of asymptomatic infections which is of the order of 50 to 80 per cent in women6. To date, these tests have provided high specificity (95-100%), and the best sensitivity of all diagnostic methods (95%)11. Because of their accuracy these are considered as the gold standard for diagnosis and good for different samples, such as cervical swabs or non-invasive samples such as urine12.
In the present study, the 16S ribosomal PCR was used as the screening assay followed by opa based PCR as the supplemental assay. The use of two assays is important especially when used in low prevalence populations like ours, especially considering the social impact of a positive result. Other studies have also reported opa gene-based assay to be sensitive, specific and reliable for detection of N. gonorrhoeae in clinical samples and/or for confirmation of less specific tests611. Unlike the real-time PCR formats used by others613 our opa assay was a conventional PCR feasible for laboratories not having access to real-time PCR machines.
Based on PCR results, opa-gene based PCR was positive in 17 patients (Fig. 1). These 17 were also positive by 16S ribosomal PCR (Fig. 2) and hence were considered true positives. However, only one patient was positive by conventional methods (microscopy and culture) (Table). 16S ribosomal PCR revealed eight extra positives that were negative by opa based PCR. The extra positives had very faint bands which could not be analysed further by sequencing. Further, as the sensitivity of the opa assay is equivalent to the 16S ribosomal assay, it was considered unlikely that these samples were false negative by the opa PCR. In our earlier study9 all discrepant results during the course of validation were resolved by sequencing and it was observed that opa gene as a target was highly specific for detection of N. gonorrhoeae. The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for the opa based PCR assay were found to be 100 per cent (77.1-100%, 95% CI), 100 per cent (98-100%, 95% CI), 100 per cent (77-100%, 95% CI) and 100 per cent (98-100%, 95% CI), respectively. The higher number of positives in 16S ribosomal gene assay may perhaps be due to cross-reaction with commensal Neisseria, Lactobacillus or other flora in the female genital tract and the frequent horizontal genetic exchange within the Neisseria genus. Cross-reaction with commensal Neisseria sp. was evident with the 16S ribosomal assay during the standardization studies in our laboratory and has been reported by others also1415. Further, N. gonorrhoeae species comprises a broad range of subtypes which exhibit considerable genetic variation. The changing prevalence of these sub-types in a patient population can have a significant impact on the success of any N. gonorrhoeae NAAT6. Therefore, assays need to be routinely monitored to identify any new variation in the target sequence used. The cross-reactivity and non-specificity observed with the 16S ribosomal PCR prompts the need for evaluation of PCRs targeting alternative regions in the 16S ribosomal gene or alternative gene targets to determine another assay with a better performance, to be used in conjunction with the opa based assay.
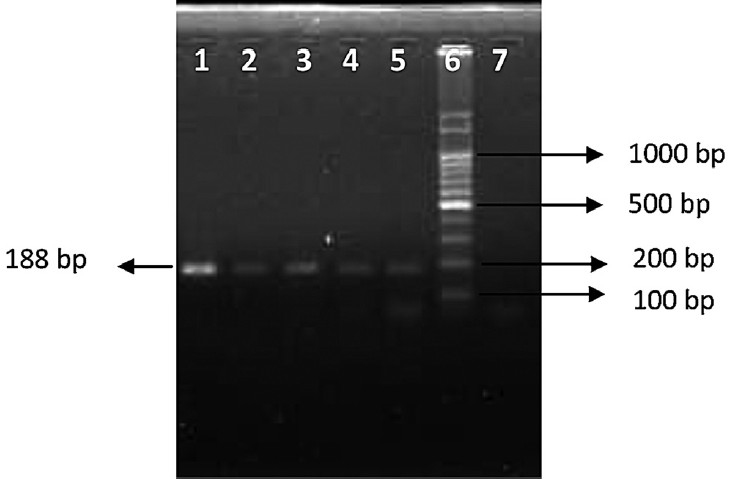
- PCR results with patient samples (opa). Lane 1: N. gonorrhoeae (ATCC 49226), Lanes 2-5: Patients samples, Lane 6: 100 bps ladder, Lane 7: Negative control. Lanes 1-5 were positive.

- PCR results with patient samples (16S). Lane 1: Negative control, Lane 2: N. gonorrhoeae (ATCC 49226), Lane 3-8: Patient samples, Lane 9: 100 bps ladder. Lanes 2, 3, 6, 7, 8 were positive.
The clinical sensitivity of conventional methods for the detection of N. gonorrhoeae in female patients was low (16 false negatives). It is also speculated that a good percentage of women attending the STI clinics are routed through the Gynaecology OPD and have thus been exposed to antibiotics. This coupled with the presence of very diverse flora of the female genital tract reduces the chances of getting positive results by conventional methods. The gonococcal detection rate increased significantly when molecular method was used (6.8%). World over, the estimated prevalence of gonococcal infection in general population has been reported to be 0.7 -7 per cent1617, and an increase of about 15-35 per cent has been reported in specific high risk groups such as sex workers1819. All positive cases in the present study were from the STI clinics.
The extra positives obtained by 16S ribosomal assay could not be analysed as the bands obtained were very faint and hence not sufficient for sequencing. A further study for determining the reason for cross-reaction needs to be conducted.
The present study was carried out in compliance with the recommendation that an ongoing assessment of gene targets for diagnostic purposes must be performed. The use of molecular tests (16S ribosomal assay as screening and opa gene based assay as supplemental assay) increased the yield of positives in female patients. However, the cross-reactivity and non-specificity observed with 16S ribosomal assay suggest the need for appraisal of other gene targets that may be used as a screening assay in diagnosis of gonorrhoea.
Acknowledgment
The work was supported by grant no. BT/PR11930/MED/29/118/2009 from the Department of Biotechnology, Ministry of Science and Technology, Government of India, New Delhi. The authors acknowledge Shri Rajinder Singh, Lab Technician, STD Laboratory, Department of Microbiology, AIIMS, New Delhi, for his assistance.
References
- Antimicrobial resistance in Neisseria gonorrhoeae. 2001. Document no. WHO/CDS/CSR/DRS/2001.3. Geneva: World Health Organization; Available from: http://www.who.int/csr/resources/publications/drugresist/WHO_CDS_CSR_DRS_2001_3/en/
- [Google Scholar]
- Global prevalence and incidence of selected curable sexually transmitted infections: overview and estimates. Geneva: World Health Organization; 2001.
- [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59:1-110.
- [Google Scholar]
- Are we satisfied with tools for the diagnosis of gonococcal infection in females? J Chin Med Assoc. 2011;74:430-4.
- [Google Scholar]
- Guidelines for the use and interpretation of nucleic acid detection tests for Neisseria gonorrhoeae in Australia: a position paper on behalf of the Public Health Laboratory Network. Commun Dis Intell Q Rep. 2005;29:358-65.
- [Google Scholar]
- Nucleic acid amplification testing for Neisseria gonorrhoeae: an ongoing challenge. J Mol Diagn. 2006;8:3-15.
- [Google Scholar]
- Screening tests to detect Chlamydia trachomatis and Neisseria gonorrhoeae infections-2002. MMWR Recomm Rep. 2002;51:1-38.
- [Google Scholar]
- Detection of Neisseria gonorrhoeae using molecular methods. 2010. National Standard Method QSOP 62. Available from: http://www.hpa-standardmethods.org.uk/pdf_sops.asp
- [Google Scholar]
- Evaluation of an opa gene-based nucleic acid amplification test for detection of Neisseria gonorrhoeae in urogenital samples in North India. Epidemiol Infect. 2012;140:2110-6.
- [Google Scholar]
- Laboratory diagnosis of gonorrhoea. WHO Regional Publication. In: South East Asia series No 33. Geneva: WHO; 1999.
- [Google Scholar]
- Detection of Chlamydia trachomatis and Neisseria gonorrhoeae by enzyme immunoassay, culture, and three nucleic acid amplification tests. J Clin Microbiol. 2001;39:1751-6.
- [Google Scholar]
- Evaluation of a rapid antigen detection test for Neisseria gonorrhoeae in urine sediment for diagnosis of gonococcal urethritis in males. J Infect Chemother. 2004;10:208-11.
- [Google Scholar]
- Evaluation of opa-based real-time PCR for detection of Neisseria gonorrhoeae. Sex Transm Dis. 2005;32:199-202.
- [Google Scholar]
- Diagnostic implications of 16S ribosomal assay for gonorrhoea. Sex Transm Infect. 2010;86:461-4.
- [Google Scholar]
- Evaluation of conventional and real-time PCR assays using two targets for confirmation of results of the COBAS AMPLICOR Chlamydia trachomatis/Neisseria gonorrhoeae test for detection of Neisseria gonorrhoeae in clinical samples. J Clin Microbiol. 2005;43:2231-5.
- [Google Scholar]
- Prevalence of Neisseria gonorrhoeae infection in young subjects attending community clinics in South London. Sex Transm Infect. 2008;84:117-21.
- [Google Scholar]
- Reproductive tract infections in primary healthcare, family planning, and dermatovenereology clinics: evaluation of syndromic management in Morocco. Sex Transm Infect. 1998;74:S95-105.
- [Google Scholar]
- Diagnosis of gonococcal infection in high risk women using a rapid test. Sex Transm Infect. 2006;82(Suppl):v26-8.
- [Google Scholar]
- Evaluation of simple diagnostic algorithms for Neisseria gonorrhoeae and Chlamydia trachomatis cervical infectiosn in female sex workers in Abidjan, Côte d’Ivoire. Sex Trans Infect. 1998;74:S106-11.
- [Google Scholar]






