Translate this page into:
Emergence of tigecycline & colistin resistant Acinetobacter baumanii in patients with complicated urinary tract infections in north India
*For correspondence: drneelampgi@yahoo.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications and was migrated to Scientific Scholar after the change of Publisher.
Sir,
Acinetobacter spp. were considered to be quiet bystanders till their role in hospital acquired infections was described1. Of the many species of Acinetobacter which have been described, Acinetobacter calcoaceticus - Acinetobacter baumanii complex (Acb complex) is clinically the most important. Usually a beta lactam drug in combination with aminoglycosides is used to treat such infections. Other effective treatment regimens include fluoroquinolones and carbapenems. Worldwide, treatment of multidrug resistant Acinetobacter infections poses a great therapeutic challenge to clinicians. The wide array of antimicrobial resistance mechanisms that have been described for Acinetobacter spp. are impressive and rival those of other nonfermentative Gram-negative pathogens. To set guidelines for treatment of infections due to Acb complex and other nonfermenters, it is imperative that the isolates be classified depending upon their degree of drug resistance.
Multiple citations have appeared in literature making an attempt to label multi drug resistant (MDR) Acinetobacter strains, but these studies vary in even laying down the basic criteria for labelling a strain as MDR or non-MDR2. These include ones with no definitions at all3, to some which include a single class, usually carbapenems or cephalosporins4. Then there are others which involve all classes of drugs except the polymixin group of drugs5. Resistance to all four classes in addition to polymixins is then labelled as pan drug resistant (PDR) Acb complex5.
A prospective study was conducted to report emergence of PDR Acb complex which were also resistant to tigecycline and colistin. All consecutive isolates of Acb complex obtained between February 2007 to June 2008 from urine culture of patients with complicated urinary tract infection (UTI) attending the Postgraduate Institute of Medical Education and Research, Chandigarh, India, were included. Only first isolate from the patient was included and repeat isolates were excluded. Complicated UTI was defined as infection developing in a patient with anatomically, physiologically or functionally compromised urinary tract. Clinical profiles of the patients were noted on a proforma. The isolates were identified by standard laboratory methods6. Pseudomonas aeruginosa ATCC 27853 was used as a quality control in antibiotic susceptibility determinations.
Antimicrobial susceptibility was done by disc diffusion method as per the c0 linical and l0 aboratory s0 tandards i0 nstitute (CLSI) guidelines7 using the Muller-Hinton agar (Difco, USA) and antimicrobial discs (Oxoid, UK and Hi-Media, Mumbai). The following antimicrobial agents (μg) were used - cefotaxime (30), cefoperazone (75), gentamicin (10), amikacin (30), ciprofloxacin (5), nalidixic acid (30), norfloxacin (10), co-trimoxazole (25), nitrofurantoin (30), imipenem (10), piperacillin and tazobactam (75+10) and cefoperazone and sulbactam (75+30), tigecycline (15) and colistin (10). The isolates were labelled as susceptible, intermediate sensitive and resistant based on inhibition zones as per standard guidelines. In addition, the MIC of tigecycline and colistin was also determined of all the isolates that were resistant to the first and second line drugs by using the E-test (AB Biodisk, Solna, Sweden). In our study, isolates were tested for four basic classes of drugs, namely beta-lactam/beta-lactamase inhibitor combinations, aminoglycosides, fluoroquinolones and carbapenems. Isolates which were resistant to beta lactams/beta lactamase inhibitor combination, aminoglycosides and fluoroquinolones classes were termed as MDR while those resistant to all four classes including carbapenems were termed as carbapenem resistant MDR. Isolates which were resistant to all classes including the glycicyclins and polymixins were labelled as PDR.
During the study period, 32,416 samples of urine were submitted, of which 6218 (19.1%) had significant bacteriuria. Of these, a total of 224 isolates of Acb complex were obtained. Complete clinical details were available for only 205 patients. The male to female ratio was 55.1 to 44.9 (113 males, 92 females). The age of the patients ranged from 2-80 yr with an average of 32.3 yr. Thirty seven patients were children under the age of 14 yr (24 admitted and 13 outpatients). Overall admitted (166 patients) to outpatient (39 patients) ratio was 4.2: 1. Of the admitted patients, maximum patients were from Obstetrics and Gynecology unit (58 patients, 34.9%), followed by ICU patients (38, 22.8%), Urology (21, 12.6%), Medical Wards (15 patients, 9%) and Surgical Wards (10 patients, 6%). Majority of outpatients (26 patients, 66%) were attending Urology OPD. One hundred and three patients (50.3%) underwent urinary catheterization, either admitted to or attending the outpatient clinic of the Urology department (56 patients, 27.6%) or post-operative cases from gynaecology department (47 patients 22.7%). Obstructive uropathy was present in 31 (15.1%), urosepsis in 12 (5.8%), underlying renal disease in 14 (6.8%), pyelonephirits in 4 (1.9%), blunt trauma in 6 (2.9%) and malignancy in 16 (7.8%) cases. Empiric treatment consisted of a third generation cephalosporin alone in 63 cases, a third generation cephalosporin in combination with amikacin in 36 patients, fluoroquinolones alone / in combination with amikacin in 76 patients, piperacillin-tazobactum in 10, cefoperazone-sulbactum in 11 and carbapenems alone or in combination with amikacin in 9 cases. Antibiotics were changed in 63 patients according to the susceptibility report if patient was not responding clinically or was in impending sepsis. Such patients were treated with piperacillin-tazobactum (22 cases), cefoperazone-sulbactum (20 cases) and carbapenems (13 cases). Intravenous colistin was administered only in 8 cases. None of the patients died. Follow up of the patients beyond one week was not carried out. The Table shows the resistance (%) of the Acb complex to the commonly used antimicrobials.
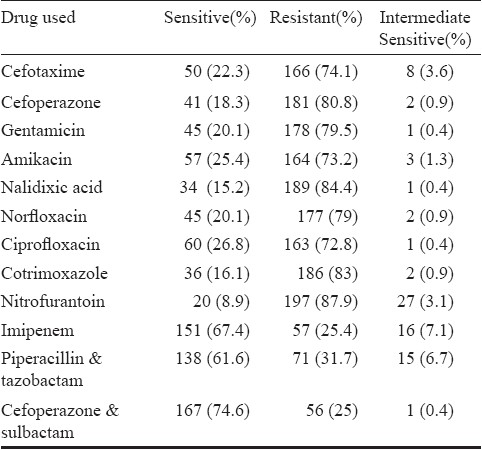
Of the total 224 isolates studied, 93 (41.5%) were labelled as MDR while 50 (22.3%) were also resistant to carbapenems. The most significant finding was the occurrence of PDR isolates, eight isolates (3.5% of total and 16% of carbapenem resistant MDR) were resistant to both tigecycline and colistin. Considered alone 32 (14.2%) of the total and 32 (64%) of the carbapenem resistant MDR isolates were resistant to tigecycline, while 8 of total (3.5%) and 8 of 50 carbapenem resistant (16%) were resistant to colistin alone. The MIC ranged from 0.016 to 128 μg/ml for tigecycline and 0.016 to 256 μg/ml for colistin. The MIC50 and MIC90 for tigecycline was found to be 6 and 32 μg/ml respectively while that for colistin was 0.125 and 4 μg/ml respectively.
In the present study, 3.5 per cent of the total and 16 per cent of the carbapenem resistant MDR strains were found to be PDR i.e., resistant to both tigecycline and colistin. This finding is significant as it heralds the beginning of the post-antibiotic era where only a few therapeutic options would be available for treatment8. Comparison with reports in literature is difficult as tigecycline is a relatively new drug and studies conducted before 2005 did not include it. Various authors have reported a resistance rate to colistin between 1.8 to 2 per cent89, while resistance to tigecycline varies from being nonexistent to 66 per cent1011. In our study, 14.2 per cent of the total and 64 per cent of the carbapenem resistant MDR isolates were resistant to tigecycline.
In India, infections caused by Acb complex pose a therapeutic challenge owing to their multi-drug resistance1213. Karthika et al14 showed that most of the Acinetobacter isolates showed complete or high resistance to imipenem (100%), meropenem (89%), amikacin (80%), cefotaxime (89%) and ciprofloxacin (72%). In another study of Acinetobacter blood stream infections from a tertiary care hospital, susceptibility to ceftazidime, cefoperazone- sulbactum, piperacillin- tazobactum, amikacin and ciprofloxacin was 44.6, 48.2, 32.1, 46.4 and 48.2 per cent respectively15. In a study by Behra et al16 only 42 per cent of metallo beta lactamase (MBL) producing Acb complex were susceptible to tigecycline. These results are similar to our study.
Mezzatesta et al10 from Italy had reported 90 per cent of the isolates from their hospital to be resistant to first line drugs, with imipenem resistance being 50 per cent. Kuo et al17 reported MDR rates as 21.4 and 8.9 per cent in catheterized patients and respiratory samples respectively. However, they did not report any presence of carbapenem resistant MDR or pan drug resistant organisms. In contrast, reports from India have shown MDR rates to vary from 9 to 90 per cent1819. In the present study, we report 3.5 per cent of Acb complex to be pan drug resistant.
As carbapenems are the cornerstones of therapy for treatment of Acb complex infections, it is essential that resistance to this class of drugs be delineated properly. For this reason in the present study, isolates which were resistant to beta-lactam/beta-lactamase inhibitor combinations, aminoglycosides and fluoroquinolones were labelled as MDR. In addition, if resistance was also seen to carbapenems those were then labelled as carbapenem resistant MDR strains.
With the ever increasing spectrum of infections that Acb complex can cause, the emergence of pan drug resistance signifies the need to introduce strict preventive measures in hospitals and to use novel agents or combination therapy.
References
- Attributable mortality of Acinetobacter baumannii infections in critically ill patients: a systematic review of matched cohort and case-control studies. Crit Care. 2006;10:R48.
- [Google Scholar]
- The diversity of definitions of multidrug-resistant (MDR) and pandrug-resistant (PDR) Acinetobacter baumannii and Pseudomonas aeruginosa. J Med Microbiol. 2006;55:1619-29.
- [Google Scholar]
- Multidrug resistant Acinetobacter baumannii isolates from a teaching hospital. J Infect Chemother. 2003;9:187-90.
- [Google Scholar]
- Considerations in control and treatment of nosocomial infections due to multidrug-resistant Acinetobacter baumannii. Clin Infect Dis. 2003;36:1268-74.
- [Google Scholar]
- Treatment of multidrug-resistant Acinetobacter baumannii ventilator-associated pneumonia (VAP) with intravenous colistin: a comparison with imipenemsusceptible VAP. Clin Infect Dis. 2003;36:1111-8.
- [Google Scholar]
- Acinetobacter, Achromobacter, Chryseobacterium, Moraxella and other nonfermentative gram-negative rods. In: Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA, eds. Manual of clinical microbiology (9th ed). Washington DC: ASM Press; 2007. p. :770-802.
- [Google Scholar]
- CLSI. Performance standards for antimicrobial susceptibility testing. 17th Informational Supplement. M100-S17. Wayne, PA: Clinical and Laboratory Standards Institute; 2007.
- [Google Scholar]
- Susceptibility of the Acinetobacter calcoaceticus-A.baumannii complex to imipenem, meropenem, sulbactam and colistin. Int J Antimicrob Agents. 2004;23:487-93.
- [Google Scholar]
- Antibiotic resistance among clinical isolates of Acinetobacter in the UK, and in vitro evaluation of tigecycline (GAR-936) J Antimicrob Chemother. 2002;49:479-87.
- [Google Scholar]
- In vitro activity of tigecycline and comparators against carbapenem-susceptible and resistant Acinetobacter baumannii clinical isolates in Italy. Ann Clin Microbiol Antimicrob. 2008;7:4.
- [Google Scholar]
- High tigecycline resistance in multidrug-resistant Acinetobacter baumannii. J Antimicrob Chemother. 2007;59:772-4.
- [Google Scholar]
- Correlation between biofilm production and multiple drug resistance in imipenem resistant clinical isolates of Acinetobacter baumannii. Indian J Med Microbiol. 2008;26:333-7.
- [Google Scholar]
- Epidemiology of bacterial colonization at intensive care unit admission with emphasis on extended-spectrum beta-lactamases and metallo-beta-lactamase producing g0 ram-negative bacteria - an Indian experience. J Med Microbiol. 2010;59:955-60.
- [Google Scholar]
- Phenotypic and genotypic assays for detecting the prevalence of metallo-beta-lactamases in clinical isolates of Acinetobacter baumannii from a South Indian tertiary care hospital. J Med Microbiol. 2009;58:430-5.
- [Google Scholar]
- Blood stream infections in cancer patients: a single center experience of isolates and sensitivity pattern. Indian J Cancer. 2010;47:184-8.
- [Google Scholar]
- Tigecycline susceptibility report from an Indian tertiary care hospital. Indian J Med Res. 2009;129:446-50.
- [Google Scholar]
- Multidrug-resistant Acinetobacter baumannii bacteraemia: clinical features, antimicrobial therapy and outcome. Clin Microbiol Infect. 2007;13:196-8.
- [Google Scholar]
- Changing trends in bacteriology of burns in the burns unit, Delhi, India. Burns. 2003;29:129-32.
- [Google Scholar]
- Acinetobacter baumannii-an emerging nosocomial pathogen in the burns unit Manipal, India. Burns. 2001;27:140-4.
- [Google Scholar]





