Translate this page into:
Covalent immobilization of lipase, glycerol kinase, glycerol-3-phosphate oxidase & horseradish peroxidase onto plasticized polyvinyl chloride (PVC) strip & its application in serum triglyceride determination
Reprint requests: Dr Chandra Shekhar Pundir, Department of Biochemistry, M. D. University, Rohtak, 124 001, Haryana, India. e-mail: pundircs@rediffmail.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Reusable biostrip consisting enzymes immobilized onto alkylamine glass beads affixed on plasticized PVC strip for determination of triglyceride (TG) suffers from high cost of beads and their detachments during washings for reuse, leading to loss of activity. The purpose of this study was to develop a cheaper and stable biostrip for investigation of TG levels in serum.
Methods:
A reusable enzyme-strip was prepared for TG determination by co-immobilizing lipase, glycerol kinase (GK), glycerol-3-phosphate oxidase (GPO) and peroxidase (HRP) directly onto plasticized polyvinyl chloride (PVC) strip through glutaraldehyde coupling. The method was evaluated by studying its recovery, precision and reusability.
Results:
The enzyme-strip showed optimum activity at pH 7.0, 35°C and a linear relationship between its activity and triolein concentration in the range 0.1 to 15 mM. The strip was used for determination of serum TG. The detection limit of the method was 0.1 mM. Analytical recovery of added triolein was 96 per cent. Within and between batch coefficients of variation (CV) were 2.2 and 3.7 per cent, respectively. A good correlation (r=0.99) was found between TG values by standard enzymic colrimetric method employing free enzymes and the present method. The strip lost 50 per cent of its initial activity after its 200 uses during the span of 100 days, when stored at 4°C.
Interpretation & conclusions:
The nitrating acidic treatment of plasticized PVC strip led to glutaraldehyde coupling of four enzymes used for enzymic colourimetric determination of serum TG. The strip provided 200 reuses of enzymes with only 50 per cent loss of its initial activity. The method could be used for preparation of other enzyme strips also.
Keywords
Co-immobilization
glycerol kinase
glycerol-3-phosphate oxidase
lipase
peroxidase
plasticized PVC strip
triglyceride
Serum triglycerides (TG) level is a valuable indicator of abnormalities in lipid metabolism1. Various methods are available for TG determination such as chemical2, thin layer chromatography, gas liquid chromatography, high performance liquid chromatography34, mass spectrometry5, microtiter plate method6, bioluminescent method7, enzymic bioluminescent8, enzymic colorimetric method9, enzymic flurometric1011 and enzyme sensing methods1213141516. Among all these methods, enzymic colorimetric method employing lipase, glycerol kinase (GK), glycerol-3-phosphate oxidase (GPO) and horse radish peroxidase (HRP) is comparatively simple, sensitive, specific and precise method, and hence used for routine analysis17. However, the method becomes expensive for a large number of samples due to the requirement of bulk quantity of costly enzymes. Immobilization of enzymes provides many advantages over the use of free/native enzymes such as enzyme reusability, continuous operation, controlled product formation, simplified and efficient processing18. Polymeric organic materials are considered to be usual supports due to their unique chemical and physical properties19. Polymers are modified on surface by the introduction of active functional groups for covalent attachment of biomolecules such as protein, DNA and carbohydrates19. Plasticized polyvinyl chloride (PVC) has exceptional properties and its ease of use makes it an ideal support for direct immobilization of enzyme(s) after its chemical cleavage20. We have earlier reported the co-immobilization of lipase, GK, GPO and HRP onto free and affixed alkyl/aryl amine glass beads, through glutraldehyde coupling and their application for determination of triglyceride2122. However, these glass beads are expensive and their handling is cumbersome. The present study describes the co-immobilization of lipase, GK, GPO and HRP onto a cheaper and stable plasticized PVC strip through glutaraldehyde coupling for colorimetric determination of TG, in serum samples.
Material & Methods
This study was conducted in the department of Biochemistry, M.D. University, Rohtak, Haryana, India.
Triton X-100, glutraldehyde and Sephadex G-100 were purchased from Sigma; 3, 5-dichloro-2-hydroxy benzene sulphonic acid (DHBS) was from E-Merk Germany. Triolein and ATP were from SRL Mumbai. Kit for enzymic colorimetric method for TG manufactured by Agappe diagnostic Kit, Ernakulam, Kerala, India was from M/S Haryana Scientific & Engineering Corporation, Rohtak. All other chemicals used were of AR grade. Plasticized PVC sheet (plastic strip) was from the local market.
Preparation of mixture of lipase, GK, GPO and HRP: To prepare mixture of lipase, GK, GPO and HRP 3 mg of Reagent 1 of TG kit consisting of lipase (110U), GK (80U), GPO (500U), HRP (35U) and 4-aminoantipyrine was dissolved in 1 ml of 0.02 M sodium phosphate buffer, pH 7.0 and loaded on a Sephadex G-100 column (24×1cm) pre-equilibrated with 0.02 M sodium phosphate buffer, pH 7.0. The column was run in the same buffer at a flow rate of 0.5 ml/min. Fractions (2 ml each) were collected after passing the void volume and tested for protein by Lowry method23. The fractions showing the presence of protein were pooled and treated as mixture of lipase, GK, GPO and HRP. The mixture was tested for combined activity of lipase, GK, GPO and HRP as given below.
Preparation of triolein solution: Triolein was used as a substrate for lipase, GK GPO and HRP. Triolein emulsion was prepared as described9. Solutions of different concentrations of triolein ranging from 0.1 to 20 mM were prepared in 0.1 M sodium phosphate buffer (pH 7.0) and stored at 4°C until use.
Co-immobilization of lipase, GK GPO and HRP on plastic strip: The mixture of lipase, GK GPO and HRP was co-immobilized covalently onto a plasticized PVC strip (1×12cm) as described20 with slight modification. The plasticized PVC strip was first incubated with nitrating acid (mixture of conc. HNO3 and H2SO4 in 5:1 ratio) at room temperature (30±5°C) for 6 h to cleave oxidatively polyvinyl chloride polymers into small chain polymers having protruding ends toward the surface. This acid-treated plasticized PVC strip was rinsed with distilled water repeatedly and then incubated with 2.5 per cent (w/v) glutraldehyde solution in 0.05 M sodium phosphate buffer, pH 7.0 for 7 h at room temperature (30±2°C). The glutraldehyde activated plasticized PVC strip was washed in distilled water several times to remove excess of glutraldehyde and then incubated with 3 ml of enzyme mixture (6 mg protein) at 4°C for 24 h in dark. The excess of enzyme was decanted off and tested for activity and protein concentration. The plastic strip was also tested for activity and stored at 4°C until use.
Assay of mixture of free and immobilized lipase, GK, GPO and HRP: The assay of mixture of free lipase, GK, GPO and HRP was carried out in a 15 ml conical flask wrapped in a black paper9. The reaction mixture consisted of 0.54 µmol MgCl2, 0.63 µmol ATP, 1.0 µmol potassium ferrocyanide, 0.27 µmol 4-aminophenazone and 1.53 µmol DHBS, 1.0 µmol of Triton X-100/l of 0.1 M sodium phosphate buffer, pH 7.0. To 900 µl reaction mixture, 50 µl of mixture of enzymes in 0.2 M sodium phosphate buffer pH 7.0 (Sephadex G-100 fraction) was added and pre-incubated at 37°C for 5 min. The reaction was started by adding 50 µl of 22.6 mM triolein. After incubation at 37°C for 15 min, A510 was measured. Hydrogen peroxide (H2O2) generated in reaction was calculated from standard curve between A510 versus H2O2 concentration. One unit of combined activity of enzyme is defined as nmoles H2O2 generated/min/ml under standard assay conditions. Assay of immobilized enzymes was also carried out in the same manner as described for combined assay of free lipase, GK, GPO and HRP except that mixture of free enzymes was replaced by plastic strip containing co-immobilized enzymes and volume of reaction buffer was increased by 0.1 ml and the reaction mixture was stirred continuously during incubation, and strip was taken off from the reaction mixture before addition of colour reagent. The strip was stored in 0.05 M sodium phosphate buffer, pH 7.0 at 4°C, when not in use.
Scanning electron microscopy and fourier transform infra-red (FTIR) spectroscopy of plasticized PVC strip: To confirm co-immobilization of enzymes, the scanning electron microscopic (SEM) study of plasticized PVC strip with and without co-immobilized enzymes was carried out, at the sophisticated analytical instrumentation facility (SAIF) of All India Institute of Medical Sciences, New Delhi, using an evaporated gold-carbon film by detectors for processing.
The plasticized PVC membrane (1×1 cm) before and after immobilization of enzymes was placed between two potassium bromide (KBr) disks for the mid-IR characterization, using a FTIR spectrometer (mode iS10, Thermoelectron, USA) instrument.
Kinetic properties of co-immobilized lipase, GK, GPO and HRP: The following kinetic properties of co-immobilized enzymes were studied: Optimum pH, incubation temperature, time of incubation, effect of triolein concentration and calculation of Michaelis-menten constant (Km) and maximal velocity (Vmax) from Lineweaver Burk plot24.
Determination of serum TG with enzyme strip: Blood samples (1 ml each) were drawn from apparently healthy adults (male and female, 20 each) and persons suffering from hypertriglyceridemia (n=20) were provided by Pt. BDS Post Graduate Institute of Medical Sciences, Rohtak, and centrifuged at 4000×g for 5 min and supernatant was collected and stored at 4°C until used. Total triglyceride in serum was determined by the biostrip as described above for testing of enzyme strip under its optimal assay conditions except that triolein solution was replaced by serum. The concentration of triglyceride in serum was extrapolated from standard curve between triolein concentrations vs A510.
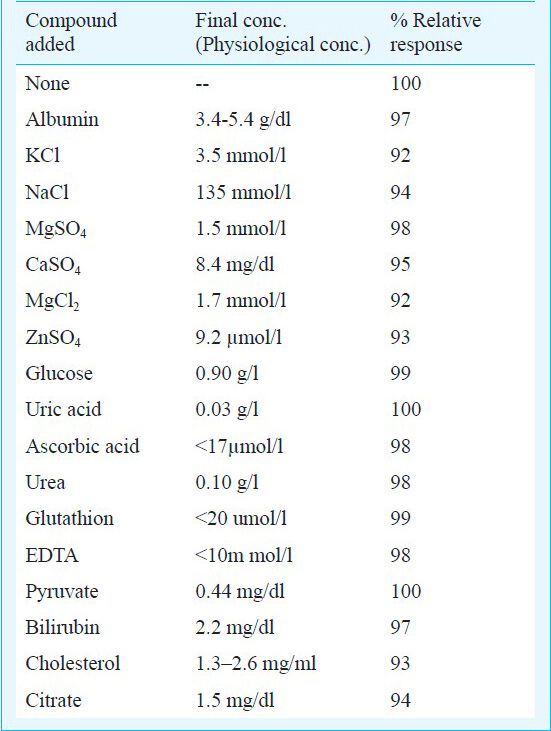
Evaluation of enzyme strip: The present method of enzymic colorimetric determination of TG with enzyme strip was evaluated by studying its analytic recovery, precision and correlation. The limit of detection of the present method was calculated as the lowest quantity of triolein required to give an absorbance to a background (blank) +3 times SD of blank. The possible interference by various substances such as albumin, KCl, NaCl, MgCl2, ZnSO4, MgSO4, CaSO4, glucose, uric acid, ascorbic acid, urea, glutathione, EDTA, pyruvate, bilirubin, cholesterol and citrate were also studied at their physiological concentrations.
Reusability and storage of enzyme strip: The ‘enzyme strip’ was reused by dipping and shaking gently for 10 sec in a series of test tubes containing 3.0 ml of 0.05 M sodium phosphate buffer, pH 7.0. For storage of strip, its active end was kept dipped in reaction buffer (0.05 M, pH 7.0) at 4°C.
Results & Discussion
Co-immobilization of lipase, GK, GPO and HRP onto plastic strip: A mixture of commercial lipase, GK, GPO and HRP was co-immobilized covalently onto plasticized PVC strip with 69.73 per cent retention of initial activity of enzyme and 0.47 mg/cm2 yield.
Scanning electron microscopy (SEM) of plasticized PVC strip: High-resolution scanning electron micrographs of plasticized PVC sheet surface with immobilized enzyme had folds and clusters along some beaded structures that were not observed in plasticized PVC sheet alone (Fig. 1). A change in surface morphology of the support after immobilization confirmed enzyme immobilization20. The presence of these structures indicated immobilization of enzyme on plasticized PVC sheet surface.
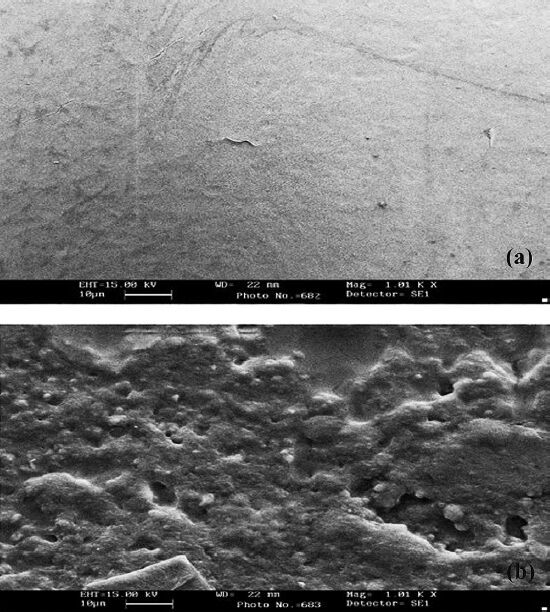
- Scanning electron micrographs of chemically modified plasticized PVC surface without (a) and with (b) immobilized enzymes (lipase, GK, GPO and HRP).
FTIR spectroscopy of plasticized PVC strip: Fig. 2A (curve i) showed the FTIR spectra of plasticized PVC membrane. The characteristic bands of plasticized PVC can be classified into three regions. The first is called the C-Cl stretching region in the range from 600-700/cm. The second region is called C-C stretching in the range from 900-1200/cm. The third region is 1250-2970/cm in plasticized PVC (numerous CH modes)25. Figure 2A (Curve ii) shows plasticized PVC membrane with glutaraldehyde and enzymes.
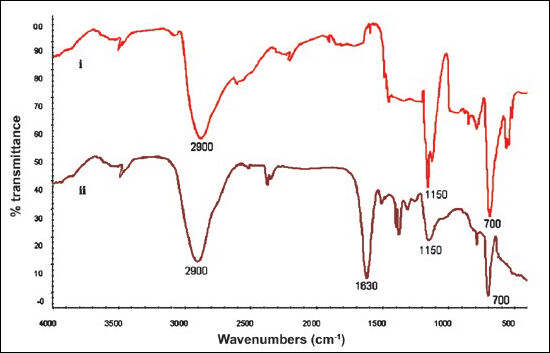
- FTIR spectra of (i) plasticized PVC and (ii) plasticized PVC sheet bound enzymes.
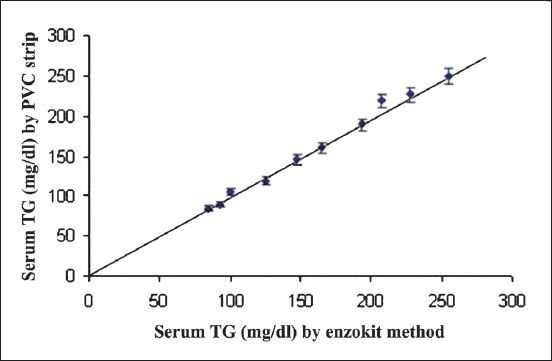
- Correlation of serum TG values in 10 serum samples measured by standard commercial enzymic colorimetric method and present method. Each sample was tested three times and values are plotted as mean ± SD.
The peak at 1725/cm showed –C O– bond of aldehyde, while no peak at 1725/cm in curve ii confirmed that –C O– group of glutaraldehyde got combined with –NH2 groups on the surface of enzyme to form –N=C– bond. Curve ii showed a peak of –N C– bond at 1630/cm, which also revealed that there was no free aldehyde group of glutaraldehyde, as it got cross-linked with enzyme.
Preparation of reusable enzymes strip for TG determination: A reusable enzyme strip was prepared for TG determination employing plasticized membrane bound lipase, GK, GPO and HRP.
Optimization of enzyme strip
Optimum pH - To determine optimum pH of the strip, pH of reaction buffer was varied from pH 5.5 to 9.0 at an interval of pH 0.5, using 0.05 M sodium phosphate buffer. The strip activity increased as the pH increased up to 7.5, after which it decreased. This optimum pH was comparable to that immobilized on alkylamine glass beads (8.0)21 and arylamine glass beads (7.5)22.
Optimum temperature - The activity of strip was tested at different incubation temperatures ranging from 25 to 60°C at an interval of 5°C. The strip response increased as incubation temperature increased up to 35°C for 5 min and thereafter, it declined. Hence all the subsequent assays were carried out at 35°C. The optimal temperature of immobilized enzymes was 35°C, which is slightly lower than that of free enzyme (37°C). This optimum temperature was lower than that conjugated with alkylamine (40°C)21 and arylamine (40°C)22. A comparison of kinetic properties of co-immobilized and of free enzymes is given in Table I.
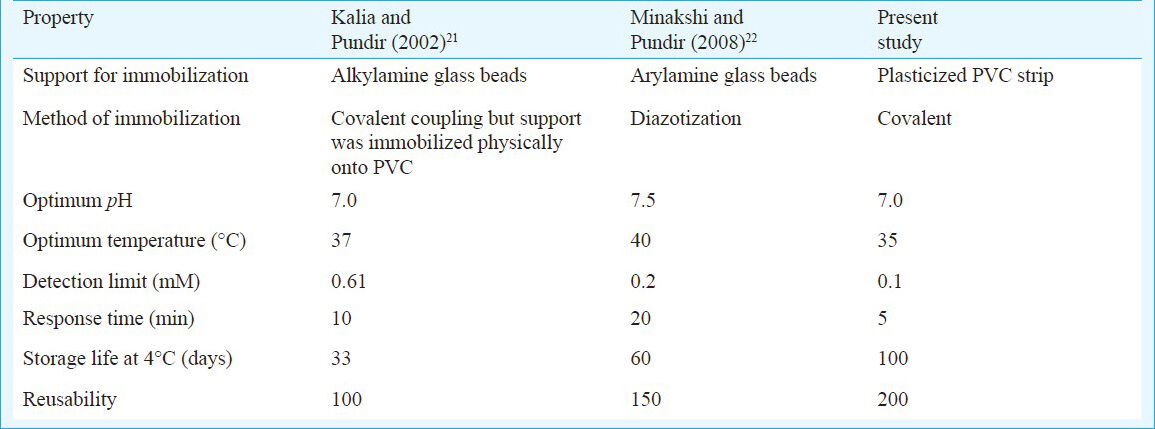
Effect of substrate concentration - To study the effects of substrate concentration on the plasticized PVC strip, the concentration of triolein varied from 0.1 to 20 mM. There was a linear relationship between strip activity and TG concentration ranging from 0.1 to 15 mM. Km for triolein as calculated from Lineweaver Burk plot was 1.2 mM and Vmax was 0.192 mmol/ml/min. Immobilized enzymes had a Km value of 1.2 mM and Vmax was 0.192 mmol/ml/min, which is lower than the native enzyme (21.1 mM). The decrease in Km of enzymes after immobilization might be due to a change in the microenvironment of enzyme. This Km value is lower than that reported for alkylamine (18.6 mM)21 and for arylamine (11.8 mM)22 conjugated enzyme.
Application of enzyme strip - The enzyme strip was employed for determination of TG in serum. The method had the major advantage that it provided the reuse of all four enzymes with ease and these was no leakage of enzymes during repeated use.
Evaluation of enzyme strip: The following criteria were studied to evaluate the performance of this enzyme strip:
Linearity - There was a linear relationship between A510 and triolein concentration ranging from 0.1 to 15 mM in reaction mixture, which was better than that of enzymes co-immobilized onto alkylamine glass beads21 and arylamine glass beads22.
Detection limit - Detection limit of the method was 0.1 mM, which was better than those employing enzymes co-immobilized on to free alkylamine glass beads (0.61 mM)21 and arylamine glass beads (0.2 mM)22.
Recovery: The per cent recovery of added triolein in serum (0.11 and 0.22 mM) as determined by the present method was 95.00 and 96.50 per cent, respectively, which was higher than that affixed alkylamine glass beads (96.3%)21 and arylamine glass beads (87.0%)22 indicating better reliability of the method.
Precision - Within and between batch coefficients of variation (CV) for serum TG determination by the present method were < 2.23 and < 3.7 per cent, respectively, which were lower than that of enzymes co-immobilized onto alkylamine glass beads and arylamine glass beads (< 3.1 and 14.2% and < 7 and < 11%)2122 revealing the better reproducibility of the method.
Accuracy: To study the accuracy of the present method, TG level in 10 serum samples were determined by both the commercial enzymic colorimetric method (x) and the present method (y). The values obtained by both the methods matched with each other with a good correlation (r=0.99) (Fig. 2B).
Interference study - Among the various serum substances tested such as albumin, KCl, NaCl, MgCl2, ZnSO4, MgSO4, CaSO4, glucose, uric acid, ascorbic acid, urea, glutathione, EDTA, pyruvate, bilirubin, cholesterol and citrate at their physiological concentration, none had any interference (Table II).
Determination of serum TG: Serum TG level in apparently healthy adults as measured by the present method was in the range from 63-194 mg/dl, which is in established normal range (40 to 190 mg/dl) TG level in sera of persons suffering from hypertriglyceridemia was in the range of 200-499 mg/dl, similar to earlier reports15.
Reusability and storage: The enzyme strip lost only 50 per cent of its initial activity after its 200 uses over a period of 100 days, when stored in reaction buffer (0.05 M, pH 7.0) at 4°C. Such a high stability of enzyme strip could be attributed to the covalent immobilization of enzymes onto plasticized PVC strip.
Co-immobilizing of lipase, GK, GPO and HRP covalently onto a plasticized PVC strip has not only provided its reuse with ease but also resulted into better performance of the strip compared to earlier reusable strips. The plasticized PVC strip could also be used as support for co-immobilization of enzymes used as analytical reagents. This enzyme strip provided 200 uses with only 50 per cent loss of its initial activity and did not suffer from leakage of enzymes. The method could be used for preparation of other enzyme strips also.
Acknowledgment
The authors thank Pt. BDS Post Graduate Institute of Medical Sciences, Rohtak for providing serum samples.
References
- Hyperlipidemia: diagnosis and therapy. New York: Grune and Stratton Foundation; 1997.
- [Google Scholar]
- Skeggs LT Jr, ed. Automation in analytical chemistry. New York: Mediad; 1966.
- Recent advances in the high performance liquid chromatography of lipids. Prog Lipids Res. 1988;27:5-38.
- [Google Scholar]
- Quantitative analysis of triglycerides using atmospheric pressure chemical ionization – mass spectrometry. Lipids. 1996;31:919-35.
- [Google Scholar]
- Microplate method for determination of serum cholesterol, high-density lipoproteins cholesterol, triglyceride and apolitroproteins. Lipids. 1993;28:51-5.
- [Google Scholar]
- Ultramicro determination of serum triglycerides by bioluminescent assay. Clin Chem. 1981;22:268-71.
- [Google Scholar]
- Simple fully enzymatic biolumininiscent assay for triglycerides in serum. Clin Chem. 1989;28:1742-4.
- [Google Scholar]
- Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem. 1982;28:2077-80.
- [Google Scholar]
- A fluorometric method for the determination of triglycerides in nanomolar quantities. Anal Biochem. 1986;156:386-9.
- [Google Scholar]
- Quantitative determination of serum triglycerides by the use of enzyme. Clin Chem. 1973;19:476-82.
- [Google Scholar]
- Determination of serum triglyceride by enzyme electrode using covalently immobilized enzyme on egg shell membrane. Int J Biol Macromol. 2010;47:691-5.
- [Google Scholar]
- Fabrication of an amperometric triglyceride biosensor based on PVC membrane. Anal lett. 2010;40:1-10.
- [Google Scholar]
- Construction of an amperometric triglyceride biosensor using PVA membrane bound enzymes. Clin Biochem. 2010;43:467-72.
- [Google Scholar]
- Construction of a triglyceride amperometric biosensor based on chitosan-ZnO nanocomposite film. Int J Biol Macromol. 2011;49:707-15.
- [Google Scholar]
- Construction of an amperometric TG biosensor based on AuPPy nanocomposite and poly (indole-5-carboxylic acid) modified Au electrode. Bioprocess Biosyst Eng. 2012;36:425-32.
- [Google Scholar]
- A stable radioactive substrate emulsion for assay of lipoprotein lipase. J Lipid Res. 1976;17:536-46.
- [Google Scholar]
- Wiseman A, ed. Handbook of enzyme biotech. New York: John Wiley & Sons; 1975. p. :147-201.
- Activation of polyvinyl chloride sheet surface for covalent immobilization of oxalate oxidase and its evaluation as inert support in urinary oxalate determination. Anal Biochem. 2008;374:272-7.
- [Google Scholar]
- Evaluation of serum triglyceride determination with lipase, glycerol kinase, glycerol-3-phosphate oxidase and peroxidise co-immobilized onto alkylamine glass beads. Indian J Biomechem Biophys. 2004;41:326-8.
- [Google Scholar]
- Co-immobilization of lipase, glycerol kinase, glycerol- 3-phosphate oxidase and peroxidase onto arylamine glass beads affixed on a plastic strip for determination of triglycerides in serum. Indian J Biochem Biophys. 2008;45:111-5.
- [Google Scholar]
- Characterization of PVC/PEMA based polymer blend electrolytes. Int J Electrochem Sci. 2008;3:282-90.
- [Google Scholar]






