Translate this page into:
Antibiotic susceptibilities, streptococcal pyrogenic exotoxin gene profiles among clinical isolates of group C or G Streptococcus dysgalactiae subsp. equisimilis & of group G S. anginosus group at a tertiary care centre
Reprint requests: Dr Purva Mathur, Associate Professor, Department of Laboratory Medicine, JPNA Trauma Centre All India Institute of Medical Sciences, New Delhi 110 029, India e-mail: purvamathur@yahoo.co.in
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Group C and group G streptococci (together GCGS) are often regarded as commensal bacteria and their role in streptococcal disease burden is under-recognized. While reports of recovery of GCGS from normally sterile body sites are increasing, their resistance to macrolides, fluoroquinolone further warrants all invasive β haemolytic streptococci to be identified to the species level and accurately tested for antimicrobial susceptibility. This study was aimed to determine the prevalence, clinical profile, antimicrobial susceptibility and streptococcal pyrogenic exotoxin gene profile (speA, speB, speC, speF, smeZ, speI, speM, speG, speH and ssa) of GCGS obtained over a period of two years at a tertiary care centre from north India.
Methods:
The clinical samples were processed as per standard microbiological techniques. β-haemolytic streptococci (BHS) were characterized and grouped. Antimicrobial susceptibility of GCGS was performed using disk diffusion method. All GCGS were characterized for the presence of streptococcal pyrogenic exotoxins (spe) and spe genes were amplified by PCR method.
Results:
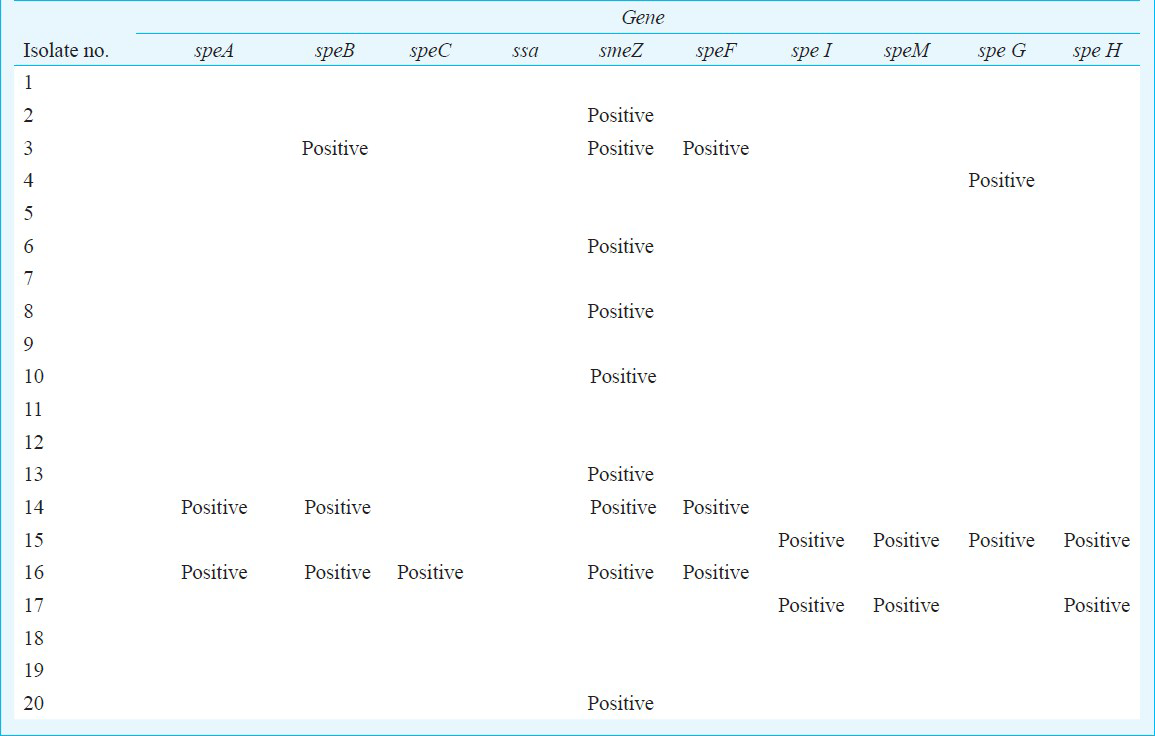
GCGS (23 GGS, 2GCS) comprised 16 per cent of β haemolytic streptococci (25/142 βHS, 16%) isolated over the study period. Of the 25 GCGS, 22 (88%) were recovered from pus, two (8%) from respiratory tract, whereas one isolate was recovered from blood of a fatal case of septicaemia. Of the total 23 GGS isolates, 18 (78%) were identified as Streptococcus dysgalactiae subsp equisimilis (SDSE, large-colony phenotype), five (21%) were Streptococcus anginosus group (SAG, small-colony phenotype). The two GCS were identified as SDSE. All GCGS isolates were susceptible to penicillin, vancomycin, and linezolid. Tetracycline resistance was noted in 50 per cent of SDSE isolates. The rates of macrolide and fluoroquinolone resistance in SDSE were low. Twelve of the 20 SDSE isolates were positive for one or more spe genes, with five of the SDSE isolates simultaneously carrying speA+ speB+ smeZ+ speF or speB+ smeZ+speF, speI+speM+speG+speH or, speI+spe M+speH or speA+ speB+ speC+ smeZ+ speF. One notable finding was the presence of spe B in four of the five isolates of the Streptococcus anginosus group. No isolate was positive for ssa.
Interpretation & conclusions:
Our study showed no association between GCGS isolates harbouring streptococcal pyrogenic exotoxins and disease severity. This might be attributed to the small sample size of spe-positive isolates.
Keywords
β-haemolytic streptococci
groups C and G streptococci
streptococcal pyrogenic exotoxins
superantigens
Streptococcus dysgalactiae subsp. equisimilis
S. anginosus group
Beta-haemolytic streptococci (βHS) of Lancefield groups C and G (together GCGS) are emerging as pathogens of increasing interest world-over1. Traditionally regarded as commensals, the spectrum of human infections caused by GCGS appears to be ever increasing1. While at one end, reports of the recovery of GCGS from various invasive infections like bacteraemia, endocarditis, meningitis, arthritis, osteomyelitis, pneumonia are on rise; a few recent studies have also reported higher GCGS throat colonization rates relative to group A Streptococcus (Streptococcus pyogenes, GAS)123.
In addition to being classified by Lancefield group carbohydrate, GCGS are also distinguished morphologically on the basis of whether they form large or small colonies on sheep blood agar plates4. GCGS large-colony phenotypes are usually associated with human infection and are classified in the same subspecies, Streptococcus dysgalactiae subsp. equisimilis subsp. nov (SDSE)5. After being considered non pathogenic for many years, SDSE is now recognized as an important bacterial pathogen. The clinical spectrum of diseases caused by this species closely resembles S. pyogenes infections, including the occurrence of post streptococcal sequelae6. Small colony forming species are placed in the S. anginosus group (SAG, formerly known as S. milleri) and are less common causes of invasive infections, compared to SDSE. The SAG comprises three species S. anginosus, S. intermedius, and S. constellatus of which two subspecies, S. constellatus subsp. constellatus and S. constellatus subsp. pharyngis, are further distinguished7. Streptococci of the anginosus group can reside commensally in various mucosal sites, such as the intestinal and the genital tracts, in addition to the human oral cavity, but are reported to cause pharyngitis, bacteraemia, and serious purulent infections in the deep neck and soft tissue and in internal organs such as the brain, lung, and liver89. The differences of habitat and pathogenicity among distinct species emphasize the interest of the distinction of S. anginosus from S. constellatus among groups C and G isolates of the SAG.
Although GCGS isolates remain almost uniformly susceptible to penicillin and other β-lactam agents, there are recent reports of isolates with a slightly increased penicillin minimum inhibitory concentration (MIC) of 0.25 μg/ml10. Reports of macrolide and fluoroquinolone resistance in GCGS further warrants all invasive βHS to be identified to the species level and accurately tested for antimicrobial susceptibility10.
Several streptococcal pyrogenic exotoxins (Spe) have been shown to play major roles in invasive GAS infections, some of these effectors belong to the family of superantigens (SAgs)11. Due to their designation as minor human pathogens, the virulence attributes of human GCGS are less extensively studied than those of GAS. A few studies which have examined the presence of the GAS-associated virulence repertoire in human GCGS have substantially aided in understanding the potential role of these SAgs in the pathogenesis of GCS and GGS1213. However, very few studies have looked into the role of various Spe in the pathogenesis of SDSE infections.
This study was aimed to describe the clinical and molecular characteristics of GCGS obtained over a period of two years at a tertiary care hospital of north India.
Material & Methods
During a two year period (from January 2009 to December 2010), various clinical samples including pus, blood, wound, endotracheal aspirates and throat swab. Out of 25 GCGS isolates during the study 22 (85%) were recovered from pus and wound, two (11.5%) were recovered from respiratory tract (endotracheal aspirate and throat swab) and one isolate was recovered from blood of a fatal case of septicemia were received from outpatients and admitted patients in the clinical Microbiology laboratories of the 190-bedded, level-1 Trauma Centre of the All India Institute of Medical Sciences (AIIMS) and the 2000 bedded AIIMS hospital, New Delhi, India. The clinical samples were processed according to standard microbiological techniques14. βHS were initially identified by their ability to lyse red cells on sheep blood agar plates (BioMe΄rieux, France). βHS were further characterized by Gram stain, a negative catalase reaction, zone diameter around bacitracin disk (0.04 units) and colony size (large colony vs small colony βHS). Small colonies were defined as having “sand-like” morphology, with a colony diameter of less than 0.5 mm. Large colonies were defined as having a diameter of ≥1.0 mm. Two clinical isolates were recovered from a private hospital in south India which were sent to us for characterization. The study was approved by the institute's ethical committee.
All the isolates (n=142) were identified by the Vitek -2 system, using GP-61 cards. (BioMerieux Vitek, Hazelwood, Mo, France). Grouping was performed by the agglutination kit (Histrep™, Hi Media laboratories, Mumbai, India) according to the manufacturer's instructions.
Antimicrobial susceptibility testing of GCGS was performed using disk-diffusion methods on sheep blood agar plates according to the Clinical and Laboratory Standards Institute (CLSI) guidelines15. The following antibiotics were tested by the disc diffusion method: Penicillin G (10 units), ampicillin (10 μg), amoxicillin /clavulanic acid (20/10 μg), cefotaxime (30 μg), ceftriaxone (30 μg), ciprofloxacin (5 μg), levofloxacin (5 μg), tetracycline (30 μg), chloramphenicol (30 μg), erythromycin (15 μg), clindamycin (2 μg), vancomycin (30 μg), teicoplanin (30 μg), and linezolid (30 μg) (BBL™BD, USA). The minimum inhibitory concentrations (MICs) of Penicillin G, ampicillin, ceftriaxone, ciprofloxacin, levofloxacin, tetracycline, erythromycin, clindamycin, vancomycin and linezolide were determined by E-test BioMerieux, France according to the manufacturer's instructions. The inhibition zone diameters and MIC breakpoints were adopted according to CLSI guidelines for βHS15. Since CLSI does not provide breakpoints for ciprofloxacin, the levofloxacin/ofloxacin breakpoints were taken for interpretation. S. pneumoniae ATCC 49619 was taken as control for all antimicrobial susceptibility testing methods.
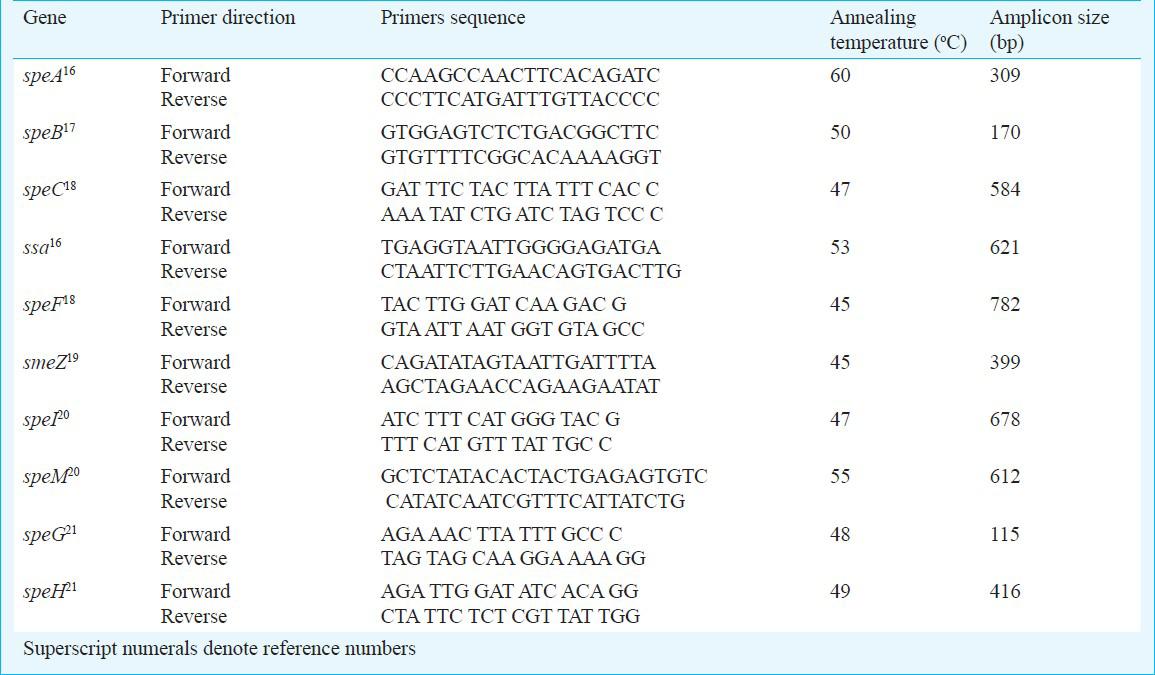
All GCGS were characterized for the presence of streptococcal pyrogenic exotoxins (Spe). For this, template DNAs from GCS and GGS was extracted by using QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany). Primers used for the amplification of (speA, speB, speC, speF, smeZ, speI, speM, speG, speH and ssa) genes are shown in Table I161718192021. S. pyogenes ATCC strains 12351 was used as positive control for speA, speB, speC, speF and smeZ, and S. pyogenes ATCC 12344, 700294 and 51500 were used as positive controls for speA, speC, and ssa, respectively.
Results
A total of 142 isolates of βHS were recovered from various clinical samples (n=13,203) received in the microbiology laboratories of Trauma Centre and AIIMS hospital. Of these 142 isolates, GAS comprised 109 (77%) isolates. Five isolates (3.5%) belonged to group B and two (1.4%) to group F. Twenty (n=20) β-haemolytic streptococcal isolates were of large colony phenotype and five were of small colony phenotype. All the 20 large colony phenotype BHS isolates were unequivocally identified as S. dysgalactiae subsp. equisimilis by the Vitek 2 system with 99% confidence. Eighteen of the 20 S. dysgalactiae subsp. equisimilis isolates belonged to group G, while two belonged to group C. Five small colony phenotype β-haemolytic streptococci were identified as belonging to “Streptococcus anginosus group” by the Vitek system with >95% confidence. All isolates belonged to Lancefield group G (Table II). Of the 25 GCGS (23 GGS, 2 GCS) 22 (85%) were recovered from pus, two (11.5%) from respiratory tract (endotracheal aspirate and throat swab), whereas one isolate was recovered from blood of a fatal case of septicaemia.
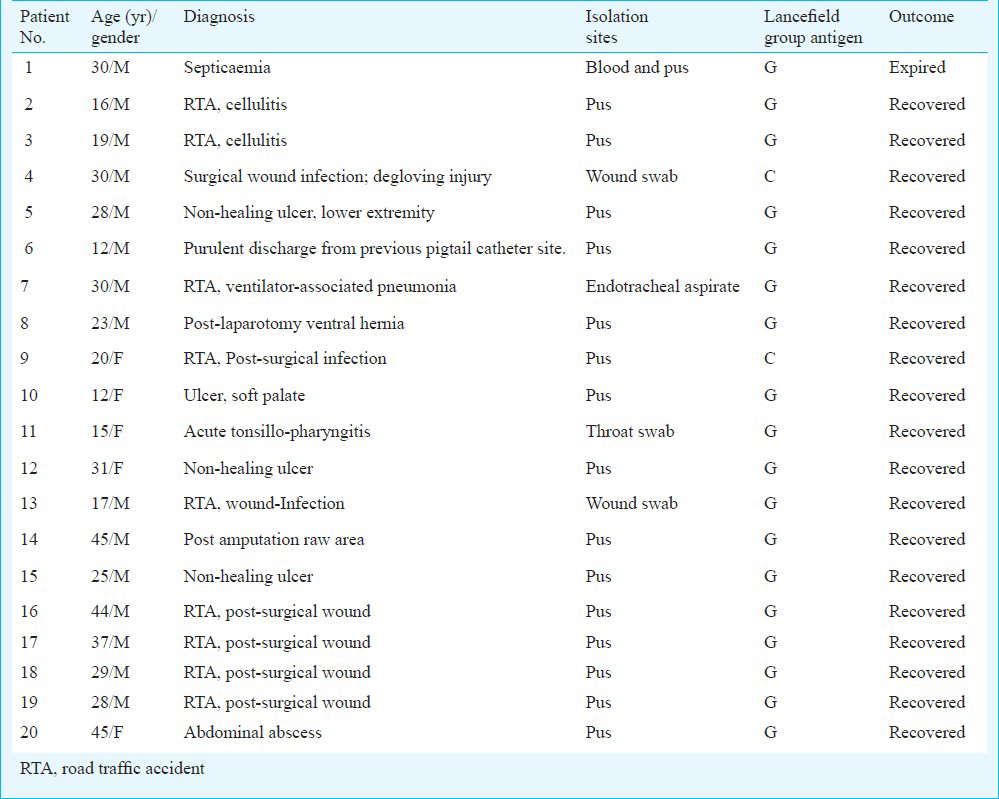
Of the total 23 GGS isolates (large-colony phenotype), eighteen (78%) were identified as SDSE, five (small-colony phenotype) (21%) were identified as SAG. The two GCS (large-colony phenotype) were identified as SDSE. The clinical and microbiological details are shown in Table II.
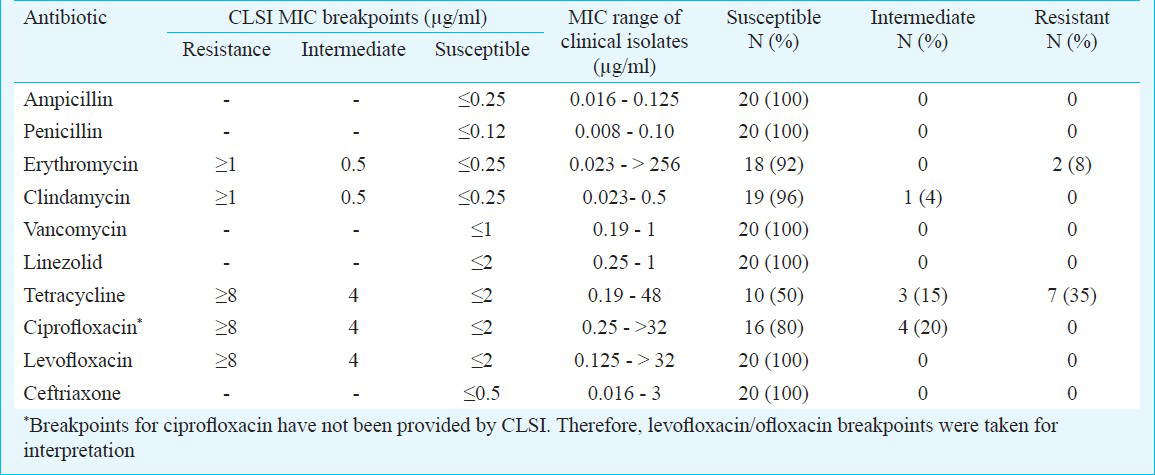
All the 25 GCGS isolates were sensitive to penicillin, ampicillin, vancomycin and linezolid. The antimicrobial susceptibility of SDSE is summarized in Table III.
Twelve of the 20 SDSE isolates screened during this study were positive for one or more spe genes, with five of the SDSE isolates simultaneously carrying spe A+ speB+ sme + speF or speB+ smeZ+speF, spe I+speM+speG+speH or, speI+ speM+ speH or spe A+ spe B+ spe C+ sme Z+ spe F. One notable finding was the presence of speB in four of the five isolates of the Streptococcus anginosus group. No isolate was positive for ssa (Table IV).
Discussion
The prevalence of invasive (e.g. STSS (Streptococcal toxic shock syndrome), necrotizing fasciitis, cellulitis, urosepsis, and pneumonia) and non invasive SDSE infections has increased gradually over the years in India and other Asian countries. In our study, GAS was the predominant βHS, followed by GCGS. In an earlier study from our institute, which examined the prevalence of GGS and GCS in various body samples from outdoor and admitted patients during a five year period, the prevalence of GCGS among βHS was 14.7 per cent. Respiratory tract was the most common source of GGS (92%), followed by soft tissues (5%)22. In the present study, the majority of GCGS were recovered from pus samples of post surgical infections in trauma patients.
Among the GCGS, there was four-fold higher isolation of SDSE with respect to SAG. SDSE remains the most common pathogen among human GCGS. Similar observations have been made in a study from Vellore, south India, which found a high incidence of Streptococcus dysgalactiae subsp. equisimilis (81%) relative to S. anginosus (19%) in a 2-year study period (2006-2007)7.
The usefulness of the Vitek 2 system in species level identification of Group G streptococci has been compared to the molecular gold standard ‘16S rRNA gene sequencing’ by Woo et al23. In that study, Vitek system (GPI) was able to identify 94 per cent group G streptococcal isolates as SDSE with >95% confidence23. In another study24, pyrosequencing methodology was compared to the biochemical systems Vitek 224. Full accordance between pyrosequencing and Vitek 2 was observed for S. dysgalactiae isolates belonging to group G. The streptococcal isolates belonging to anginosus group (S. anginosus, S constellatus) was better resolved with the biochemical systems (Vitek 2) in comparison to pyrosequencing24.
Comprehensive examinations of the GAS-associated virulence repertoire in human GCGS have been attempted by a few investigators1213. Davies et al12 used a group A streptococcal virulence array comprising 60 genes previously described as GAS virulence factors, and another 159 genes predicted to encode for extracellular proteins to examine the extent of their occurrence in a contemporary collection of GCGS isolates, pooled from GAS endemic and non-endemic regions. Between 25 and 50 per cent of the genes represented on the array were present in the GCGS isolates. In relation to the frequency of the known and putative GAS virulence factors, there was no distinction between the isolates whether these were derived from a GAS endemic or a non-endemic region12. In another study by Hashikawa et al13, which characterized 12 GCGS isolates (isolated from cases of streptococcal toxic shock syndrome) for 13 Spe (speA, speB, speC, speF, smeZ, spegg, speH, speI, speJ, speL, mf-2, mf-3, sagA), only sagA gene was found in all of the strains except one. The speG gene was detected in seven S. dysgalactiae isolates, whereas none of the other 11 spe genes could be detected13. Kittang et al25 also concluded that speGdys and other Sags might not play a major role in the pathogenesis of severe human disease caused by SDSE. Kalia and Bessen26 have described the presence of exotoxins A and C in GGS being identical to S. pyogenes genes. A study by Prabu and Menon27, which looked for the presence of speA, speB, speC and speG genes encoding SPEs in 131GCS/GGS isolates from both patients as well as asymptomatic carriers, found 12.97 per cent possessed speG gene, 4.5 per cent possessed speC gene, 2.29 per cent possessed both speC and speG genes and none of the isolates possessed the speA or the speB gene. All the 20 isolates positive for speC/speG genes were GGS27. Another study also supported streptococcal pyrogenic exotoxins gene transfer from GGS to GAS, particularly streptococcal pyrogenic exotoxin G [speG(dys)]28.
In the present study, SDSE isolates harbouring more than one Spe were recovered from non-fatal cases of cellulitis, and had different types of spe combinations. Twelve of the 20 SDSE isolates screened during this study were positive for one or more Spe genes. No isolate was positive for ssa and smeZ was the commonest spe detected. It could be due to sequence variations in the priming sequence or may be the strain harboured a Spe that was not included in the screen such as mf-2. Based on the findings of the present study, there are no clear cut relationships between GCGS isolates harbouring streptococcal pyrogenic exotoxins (Spe) and disease severity, although this may be a reflection of the small sample size of spe-positive isolates. Presence of speB has not been reported in GGS and SAG. We could not ascertain the specificity of the speB PCR by sequencing and that this was a limitation of this study.
According to literature, human GCGS isolates remain almost uniformly susceptible to penicillin and other β-lactam agents, and penicillin is still considered the drug of choice1. Isolates with a slightly increased MIC (0.12 and of 0.25 μg/ml) for penicillin were recently reported in SAG and SDSE2930. Macrolide resistance in SDSE has been shown to be widespread in many countries, with rates up to 16 per cent in Europe, 19 per cent in the United States and 24 per cent in Hong Kong2930. The prevalence of macrolide resistance in members of SAG is low and limited to certain geographical regions2930. Tetracycline no longer represents an option for the empirical treatment of SDSE isolates; resistance rates upto 60 per cent and higher have been reported2930. In North America and Europe, the incidence of fluoroquinolone-resistance among βHS (1%) remains low2930. However, there are occasional reports of fluoroquinolone-resistance in members of SAG2930. In our study, all SAG isolates remained susceptible to all the antimicrobials tested. Tetracycline resistance was noted in 50 per cent of SDSE isolates. The rates of macrolide and fluoroquinolone resistance in SDSE were low. Inducible clindamycin resistance was not detected in erythromycin resistant SDSE isolates.
The role of GCGS in streptococcal disease burden is under-recognized by clinicians and microbiologists. Accumulating indications of the considerable clinical relevance of the GSGS and the propensity of these bacteria to develop antimicrobial drug resistance suggest that these may be a group of emerging pathogens that should be monitored31.
In conclusion, 12 of the 20 SDSE isolates screened during this study were positive for one or more Spe genes. SDSE isolates harbouring more than one Spe were recovered from non-fatal cases of cellulitis. One notable finding was the presence of speB in four of the five isolates of the Streptococcus anginosus group. However, no association could be found between GCGS isolates harbouring streptococcal pyrogenic exotoxins and disease severity in the present study; this might be attributed to the small sample size of spe-positive isolates.
Acknowledgment
Authors thank Shrimati Neelu, Sweety, Rajrani, Shriyut Trilok, Pawan, Vineeth and Miss Deepali, for technical support. The research project was funded by the Indian Council of Medical Research, New Delhi.
References
- Group C and G streptococci infections: emerging challenges. Clin Lab Sci. 2003;16:209-13.
- [Google Scholar]
- Disease burden due to Streptococcus dysgalactiae subsp. equisimilis (group G and C streptococcus) is higher than that due to Streptococcus pyogenes among Mumbai school children. J Med Microbiol. 2010;59:220-3.
- [Google Scholar]
- Epidemiology of Streptococcus dysgalactiae subsp. equisimilis in tropical communities, Northern Australia. Emerg Infect Dis. 2007;13:1694-700.
- [Google Scholar]
- β-hemolytic (groups C and G) streptococci. In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas and Bennett's principles and practices of infectious disease (4th ed). New York: Churchill Livingstone; 1995. p. :1852-60.
- [Google Scholar]
- Taxonomic study of Lancefield streptococcal groups C, G, and L (Streptococcusdys galactiae) and proposal of S. dysgalactiae subsp. Equisimilis subsp. nov. Int J Syst Bacteriol. 1996;46:774-81.
- [Google Scholar]
- Human infections due to Streptococcus dysgalactiae subspecies equisimilis. Clin Infect Dis. 2009;49:766-72.
- [Google Scholar]
- Contribution of Streptococcus anginosus to infections caused by groups C and G streptococci, southern India. Emerg Infect Dis. 2010;16:656-63.
- [Google Scholar]
- Streptococcus intermedius, Streptococcus constellatus, and Streptococcus anginosus (the Streptococcus milleri group): association with different body sites and clinical infections. J Clin Microbiol. 1992;30:243-4.
- [Google Scholar]
- Streptococcus anginosus (“Streptococcus milleri”): the unrecognized pathogen. Clin Microbiol Rev. 1988;1:102-8.
- [Google Scholar]
- Characterization of fluoroquinolone-resistant â -hemolytic Streptococcus spp. isolated in North America and Europe including the first report of fluoroquinolone-resistant Streptococcus dysgalactiae subspecies equisimilis: report from the SENTRY Antimicrobial Surveillance Program (1997-2004) Diagn Microbiol Infect Dis. 2006;55:119-27.
- [Google Scholar]
- Distribution of group A streptococcal virulence genes in group C and G streptococci. Int Congress Ser. 2006;1289:184-7.
- [Google Scholar]
- Characterization of group C and G streptococcal strains that cause streptococcal toxic shock syndrome. J Clin Microbiol. 2004;42:186-92.
- [Google Scholar]
- Tests for the identification of bacteria. In: Collee JG, Fraser AG, Marmion BP, Simmons A, eds. Mackie and McCartney practical medical microbiology (14th ed). Edinburgh: Churchill Livingstone; 1996. p. :131-49.
- [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing; Twenty-first informational supplements. CLSI Document M100-S21. Wayne, PA: CLSI; 2011.
- [Google Scholar]
- Superantigen genes in group A streptococcal isolates and their relationship with emm types. J Med Microbiol. 2008;57:1238-46.
- [Google Scholar]
- Epidemiological analysis of group A streptococci recovered from patients in China. J Med Microbiol. 2006;55:1101-7.
- [Google Scholar]
- Toxin-gene profile heterogeneity among endemic invasive European group A streptococcal isolates. J Infect Dis. 2003;188:1578-86.
- [Google Scholar]
- Molecular and clinical characterization of invasive group A streptococcal infection in Sweden. Clin Infect Dis. 2007;45:450-8.
- [Google Scholar]
- Superantigen gene profile, emm type and antibiotic resistance genes among group A streptococcal isolates from Barcelona, Spain. J Med Microbiol. 2006;55:1115-23.
- [Google Scholar]
- Site-specific manifestations of invasive group A streptococcal disease: Type distribution and corresponding patterns of virulence determinants. J Clin Microbiol. 2003;41:4941-9.
- [Google Scholar]
- Prevalence of group G & group C streptococci at an Indian tertiary care centre. Indian J Med Res. 2004;120:199-200.
- [Google Scholar]
- Group G beta-hemolytic streptococcal bacteremia characterized by 16S ribosomal RNA gene sequencing. J Clin Microbiol. 2001;39:3147-55.
- [Google Scholar]
- Identification of 43 Streptococcus species by pyrosequencing analysis of the rnpB gene. J Clin Microbiol. 2005;43:5983-9.
- [Google Scholar]
- emm gene diversity, superantigen gene profiles and presence of SlaA among clinical isolates of group A, C and G streptococci from western Norway. Eur J Clin Microbiol Infect Dis. 2011;30:423-33.
- [Google Scholar]
- Presence of streptoccal pyrogenic exotoxin A and C genes in human isolates of group G streptococci. FEMS Microbiol Lett. 2003;219:291-5.
- [Google Scholar]
- Genotypic characterization of toxigenic group C and G streptococci isolated in Chennai, South India. Folia Microbiol (Praha). 2011;56:345-8.
- [Google Scholar]
- Superantigen-like gene(s) in human pathogenic Streptococcus dysgalactiae, subsp. equisimilis: genomic localisation of the gene encoding streptococcal pyrogenic exotoxin G (speG(dys)) FEMS Immunol Med Microbiol. 2002;34:159-67.
- [Google Scholar]
- Prevalence of erythromycin and clindamycin resistance among clinical isolates of the Streptococcus anginosus group in Germany. J Med Microbiol. 2009;58:222-7.
- [Google Scholar]
- Non-susceptibility trends among enterococci and non-pneumococcal streptococci from bacteraemias in the UK and Ireland, 2001-06. J Antimicrob Chemother. 2008;62(Suppl 2):75-85.
- [Google Scholar]
- Invasive infection caused by Streptococcus dysgalactiae subsp. equisimilis: characteristics of strains and clinical features. J Infect Chemother. 2011;17:1-10.
- [Google Scholar]






