Translate this page into:
Biapenem versus meropenem in the treatment of bacterial infections: a multicenter, randomized, controlled clinical trial
Reprint requests: Dr Xiaoju Lv, Center of Infectious Diseases, West China Hospital, Sihuan University, Wainan Guoxuexiang 37, Chengdu 610041, PR China e-mail: xhpapertg@gmail.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Biapenem is a newly developed carbapenem to treat moderate and severe bacterial infections. This multicenter, randomized, parallel-controlled clinical trial was conducted to compare the clinical efficacy, bacterial eradication rates and safety of biapenem and meropenem in the treatment of bacterial lower respiratory tract infections and urinary tract infections (UTIs) at nine centres in China.
Methods:
Patients diagnosed with bacterial lower respiratory tract infections or UTIs were randomly assigned to receive either biapenem (300 mg every 12 h) or meropenem (500 mg every 8 h) by intravenous infusion for 7 to 14 days according to their disease severity. The overall clinical efficacy, bacterial eradication rates and drug-related adverse reactions of biapenem and meropenem were analyzed.
Results:
A total of 272 enrolled cases were included in the intent-to-treat (ITT) analysis and safety analysis. There were no differences in demographics and baseline medical characteristics between biapenem group and meropenem group. The overall clinical efficacies of biapenem and meropenem were not significantly different, 94.70 per cent (125/132) vs. 93.94 per cent (124/132). The overall bacterial eradication rates of biapenem and meropenem showed no significant difference, 96.39 per cent (80/83) vs. 93.75 per cent (75/80). Drug-related adverse reactions were comparable in biapenem and meropenem groups with the incidence of 11.76 per cent (16/136) and 15.44 per cent (21/136), respectively. The most common symptoms of biapenem-related adverse reactions were rash (2.2%) and gastrointestinal distress (1.5%).
Interpretation & conclusions:
Biapenem was non-inferior to meropenem and was well-tolerated in the treatment of moderate and severe lower respiratory tract infections and UTIs.
Keywords
Bacterial infection
biapenem
lower respiratory infection
meropenem
treatment
UTI
Biapenem (1β-methyl-carbapenem) is stable to most β-lactamases, including AmpC and extended-spectrum β-lactamases (ESBLs), with a broad spectrum activity against Gram-positive and Gram-negative aerobic and anaerobic bacteria12. It combines with penicillin binding proteins and inhibits bacterial cell wall synthesis. Owing to the 1-β-methyl group, biapenem is more stable against the hydrolysis by human renal dehydropeptidase-I (DHP-I) than is meropenem3. Thus, in contrast to imipenem and panipenem, which must be compounded with a renal dehydropeptidase inhibitor, biapenem could be administrated independently. Meanwhile, its structure of triazole cations enhances its outer membrane permeability to Gram-negative bacteria. It is distributed widely in tissues, especially in the urinary tract, lungs and liver. However, no randomized controlled clinical trials have been published to compare the clinical efficacy, bacterial eradication rates and safety between biapenem and meropenem in the treatment of bacterial infections. We, therefore, conducted a multicenter, randomized, parallel-controlled clinical trial in nine tertiary care teaching hospitals in China to compare biapenem and meropenem in the treatment of bacterial lower respiratory tract infections and urinary tract infections (UTIs).
Material & Methods
Study design: This prospective, multicenter, randomized, parallel-controlled clinical trial was designed to compare the efficacy and safety of biapenem and meropenem in the treatment of bacterial lower respiratory tract infections and UTIs. It was conducted in nine tertiary teaching hospitals in China, between January 5, 2009 and January 29, 2010. The study protocol was approved by the ethics committee of West China Hospital, Sichuan University, Chengdu, which was the principal investigating institution. Patients or their guardians provided written informed consents to participate in the study prior to the enrollment. A stratified block randomization method was used. A computer-generated randomization schedule was used to provide randomization number and medication-kit number for each patient. The patients were randomized to receive biapenem or meropenem at a 1:1 ratio. The sample size was calculated for general infections. Considering the validity and the one-sided test, according to the statistical requirements, α = 0.05, β = 0.2 (efficacy = 80%), and non-inferiority test with expected average efficiency of P = 0.88 (88%), non-inferiority standard of δ= 0.10, the number of each group of patients was estimated to be 131 cases. Considering expulsion cases, a sample size of 272 cases was considered adequate. The data were monitored and retrieved by an assistant research panel. The clinical trial registration number was ChiCTR-TRC-12001943.
Criteria for eligibility: Inpatients and a few outpatients aged 18 to 70 yr, regardless of their gender and ethnicity, who were diagnosed of either lower respiratory tract infections or UTIs caused by bacteria were eligible for the study. Lower respiratory tract infections referred to pneumonia, infection with bronchiectasis, acute exacerbation of chronic obstructive pulmonary disease (AECOPD), pneumonia accompanied with COPD and infection secondary to bronchiectasis with COPD. In additional to these symptoms, signs and laboratory results, lower respiratory infections were diagnosed by radiography with patchy consolidations. UTIs referred to acute pyelonephritis, acute onset of chronic pyelonephritis and complicated urinary tract infection. Complicated urinary tract infection was defined as infection in urinary tract with functional or structural abnormalities, including indwelling catheters and calculi. In addition to symptoms and signs, UTIs were confirmed by pyuria. Patients who had not received antimicrobial therapy within 48 h before the study were enrolled. Moderate and severe cases were identified according to revised rating scales based on the national guidelines and consensus45 for clinical investigation of antimicrobial drug, which were established by the assessment of the patients’ symptoms, physical signs and baseline laboratory results. Patients with any of the following conditions were excluded: history of hypersensitivity to β-lactams; serum creatinine level above the upper limit of the normal range or creatinine clearance <50 ml/min; serum aminotransferase (ALT or AST) >1.5 times of the upper limit of the normal range; severe cardiac or haematological abnormalities; terminal malignancy; central nervous system illness or immunodeficiency; complex infections that needed combination therapy with other antimicrobial drugs; pregnant or lactating woman; psychiatric illness; or non-bacterial infections. Enrollment of patients with healthcare-associated infections was permitted.
Randomization and treatment: Patients were randomly assigned to receive biapenem or meropenem as stratified by the Center, through consecutively opening sealed computer-generated envelopes. Biapenem (300 mg, every 12 h) or meropenem (500 mg, every 8 h) was administrated intravenously and the infusion time was 1 h. For severe infections, the dose was doubled. The duration of therapy was 7 to 14 days, according to their disease severities.
Evaluation and monitoring: Symptoms, physical signs and adverse events of each patient were monitored and recorded on a daily basis during the treatment. Voluntary reports from the patients were also encouraged. Before starting the antimicrobial therapy, a complete medical history, electrocardiogram, complete blood count with differential, urinalysis, routine chemistry and culture of the sputum or urine samples were performed. For patients with lower respiratory tract infection, chest radiography at baseline and at the termination of the therapy was carried out. A complete blood count with differential and urinalysis were also performed on the fourth day of the treatment. Women had a negative pregnancy test. Patients with abnormal laboratory results were followed until they returned normal.
Bacterial identification and susceptibility determination: All isolates, recovered from all cultures were subjected to in vitro susceptibility test for biapenem, meropenem, imipenem, cefepime and piperacillin-tazobactam using the Kirby-Bauer disc diffusion method as recommended by the Clinical and Laboratory Standards Institute (CLSI)6. The minimum inhibitory concentrations (MIC) of all isolates were determined using the agar dilution method in our laboratory following the recommendations of CLSI6.
Clinical and bacteriological efficacy evaluation: The clinical efficacy was defined as cure, marked improvement, improvement or failure. Cure: complete resolution of symptoms and signs, eradication of the pathogen identified by culture, normal laboratory results and improved chest radiography. Marked improvement: only one abnormality of the above remained. Improvement: at least two abnormalities remained at the treatment termination. Failure: clinical signs and symptoms of infection persisted or worsened after 72 h of treatment. The overall efficacy rate was defined as the proportion of the patients cured and markedly improved.
Bacterial efficacy was evaluated based on the following four categories: complete eradication if elimination of the original causative pathogens, persistence if the original causative pathogens were repeatedly isolated, substitution if new organisms were isolated on repeated culture and reinfection if reappearance of the original causative pathogens after eradication and with clinical symptoms of infection.
Safety assessment: All adverse events and their time of occurrence, manifestation, severity, management and outcome throughout the study period were recorded. Suspected adverse reactions were classified into five categories: definitely drug-related, probably drug-related, possibly drug-related, possibly drug-unrelated or definitely drug-unrelated. The former three were considered to be drug-related adverse reactions, for which the incidence was calculated accordingly.
Statistical analysis: All data were carefully checked at the end of the study by the principal investigators of each centre. Each case report form was then systematically reviewed by two of the investigators and the chief clinical research coordinator. All statistical analyses were performed using SAS software version 6.12 (SAS Institute, Cary, N.C., USA). As this was a non-inferiority study, student's t test, χ2 or Fisher's exact test were used to test the hypotheses, according to the type of the variants and the subject of study. An intent-to-treat (ITT) analysis was used to assess efficacy and safety. Data management and statistical analysis were completed by a contract statistical organization of West China College of Public Health, Sichuan University.
Results
A total of 272 cases were enrolled, each was randomly assigned to biapenem or meropenem group. All 136 cases in each group were included in the ITT analysis. Each patient received at least one dose of the study medication, so all of them were included in the safety analysis. Only 132 cases in each group were included in the clinical efficacy assessment because four patients in each group could not be assessed due to various reasons. During the treatment, one patient was found to have HIV co-infection, one was found to have tuberculosis co-infection by additional sputum smears, one sample was cultured positive for carbapenem-resistant Gram-negative bacteria, one had the baseline creatinine clearance <50 ml/min although her serum creatinine level was normal, and four patients were found to be taking other oral antimicrobial drugs unknowingly.
There was no difference in demographics and baseline medical characteristics between the two groups (Table I).
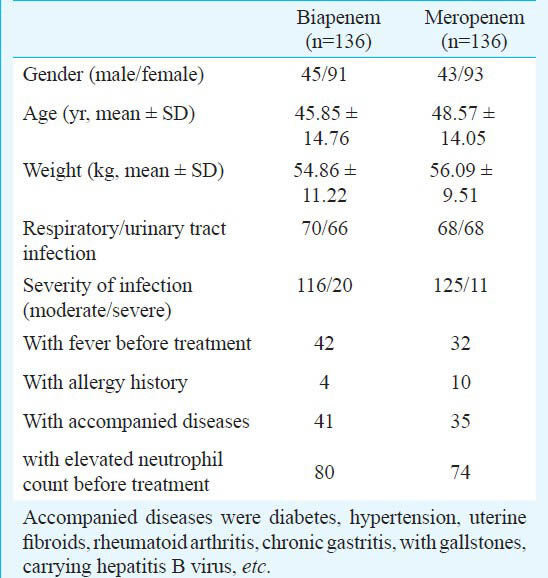
Clinical efficacy assessment: The overall clinical efficacies of biapenem and meropenem were equivalent, 94.70 per cent (125/132) vs. 93.94 per cent (124/132). The clinical efficacies of biapenem and meropenem against lower respiratory tract infections were 92.65 per cent (63/68) and 92.54 per cent (62/67), respectively, and against UTIs were 96.88 per cent (62/64) and 95.38 per cent (62/65), respectively (Table II).
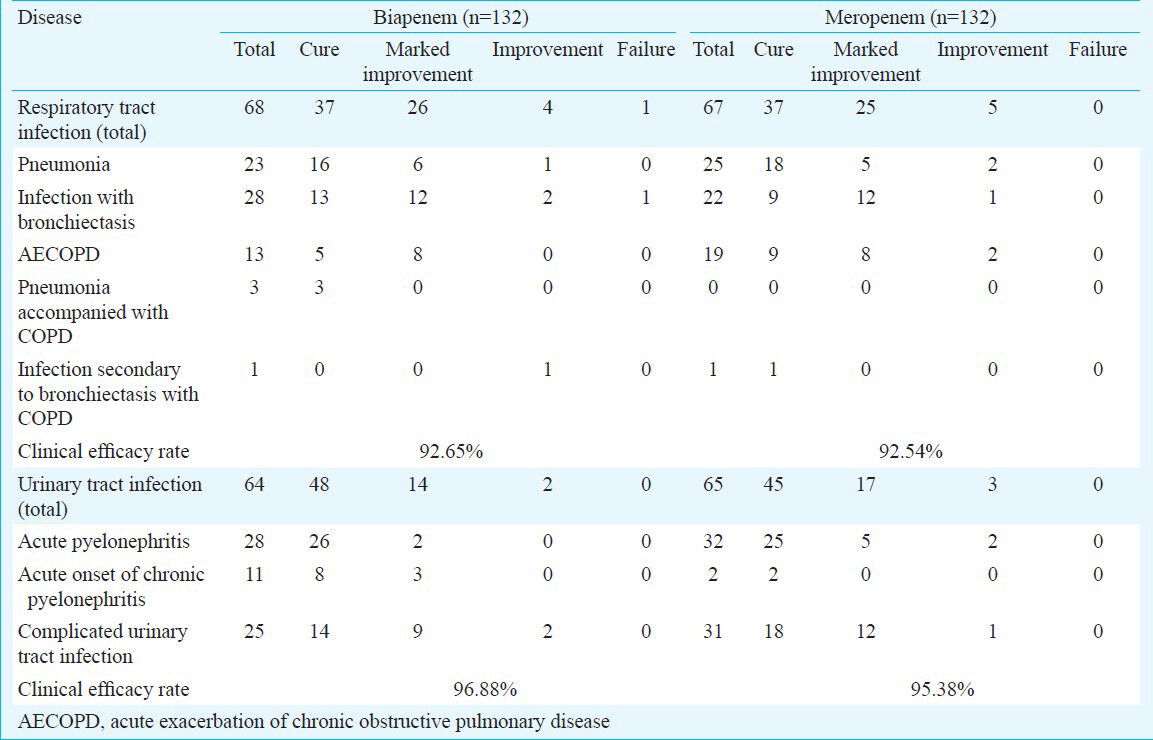
Bacteriological efficacy assessment: A total of 163 isolates were recovered, with 83 in the biapenem group and 80 in the meropenem group. There was no methicillin-resistant Staphylococcus aureus or Streptococcus pneumoniae isolates. Thirty nine isolates (38.61%) of Escherichia coli and Klebsiella isolates were ESBL (extended-spectrum β-lactamase) positive. There was no significant difference between the overall bacterial eradication rates of biapenem and meropenem, 96.39 per cent (80/83) vs. 93.75 per cent (75/80) (Table III). The bacterial eradication rates of biapenem and meropenem against lower respiratory tract infections were 94.74 per cent (36/38) and 87.80 per cent (36/41), respectively. The bacterial eradication rates of biapenem and meropenem against UTIs were 97.78 per cent (44/45) and 100.00 per cent (39/39), respectively.
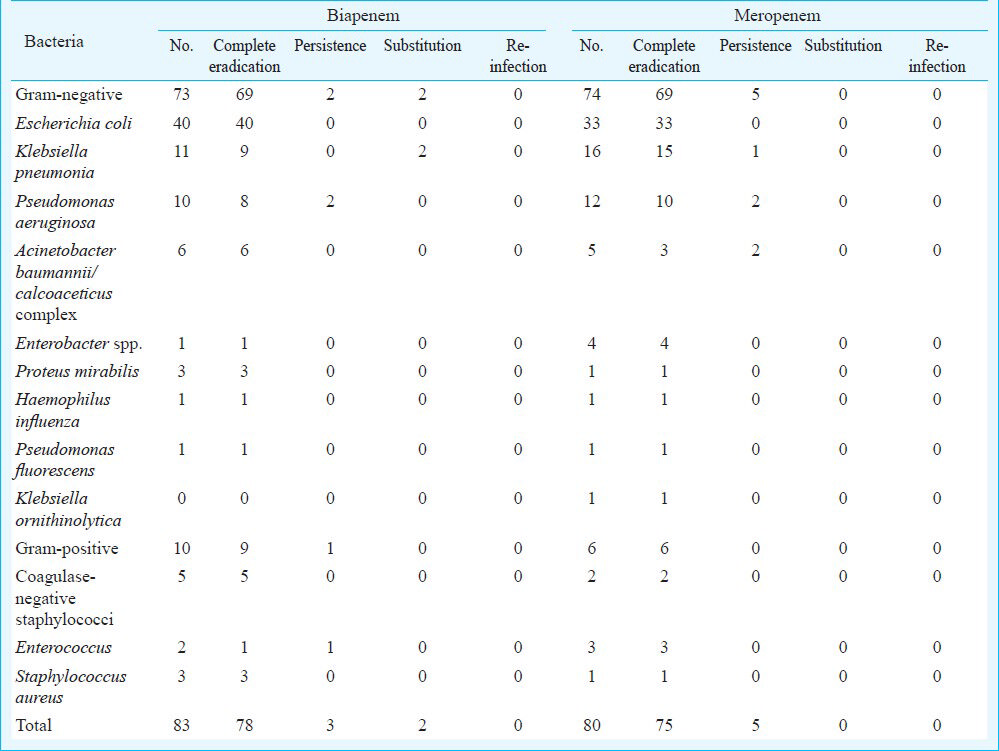
In vitro antimicrobial susceptibility assay: There was no significant difference in the susceptibility of biapenem and meropenem revealed by the drug susceptibility testing. The MICs for all the isolates are shown in Table IV, as the range of MIC (MICr) and the concentration required to inhibit 50 and 90 per cent of the isolates (MIC50 and MIC90). It appeared that biapenem was as active as meropenem against most Gram-negative bacteria, except Klebsiella ornithinolytica and Proteus mirabilis. For Gram-positive bacteria, biapenem was slightly less active against coagulase-negative staphylococci and Enterococcus. However, the difference in the antibacterial susceptibility between these two drugs was not significant (Table IV).
Drug safety: A total of 272 cases received at least one dose of the study medication, with 136 patients in each group; all were included in the safety analysis. The rate of drug-related adverse reactions did not differ significantly in biapenem and meropenem groups with the incidence of 11.76 per cent (16/136) and 15.44 per cent (21/136), respectively. Most of these were mild and transient. The majority of the adverse reactions consisted of abnormal laboratory results, mainly increased serum transaminase levels and decreased white blood cell count without accompanied symptoms and signs, with the incidence of 8.09 per cent (11/136) and 11.03 per cent (15/136), respectively. Follow up study showed that most of the laboratory values normalized within 2 wk of discontinuation of the drug. Additionally, the most common symptoms were rash (2.2%) and gastrointestinal distress (1.5%), with lower frequency. Severe adverse reaction was not observed during the entire trial course neither in the biapenem group nor in the meropenem group.
Discussion
Carbapenems are very potent bactericidal drugs to treat severe or complicated bacterial infections and drug-resistant bacterial infections. Biapenem has broad-spectrum antibacterial activity and a rapid bactericidal effect against Gram-positive and Gram-negative organisms in vitro, including anaerobic bacteria and multiple-drug-resistant Pseudomonas aeruginosa27. Biapenem is stable against the hydrolysis by human renal DHP-I, and could be administrated independently. Although it has been launched in the Japanese market89, it is seldom used outside of Japan, and there is still limited information on the usefulness of this drug. As with meropenem, biapenam belongs to the second generation of carbapenem drugs, with the same action mechanism, similar chemical structure, antimicrobial spectrum and pharmacokinetics. With its excellent tissue distribution ability, biapenem is believed to be able to treat severe infections including septicemia, pneumonia, lung abscess, secondary infections of chronic respiratory diseases, complicated urinary tract infections, pyelonephritis, peritonitis and adnexitis1011.
This nine-center, randomized controlled clinical trial confirmed the clinical efficacy, bacterial efficacy and safety of biapenem. The overall clinical efficacy and the overall bacterial eradication rates of biapenem and meropenem were equivalent. The rate of drug-related adverse reactions did not differ significantly in biapenem and meropenem groups. Our clinical trial verified its clinical usage, in accordance with another clinical trial published recently12. As carbapenems are developed for severe infections, this clinical trial only enrolled cases with moderate or severe infections. Disease condition assessment was conducted strictly. According to the principle of rational use of antimicrobial drugs, patients with chronic bronchitis or cystitis were not included in this clinical trial. It is noteworthy that patients with healthcare-associated lower respiratory tract infections were included in this trial, and more than half of the patients with UTIs had acute onset of chronic pyelonephritis or complicated urinary tract infections. For complicated cases like these, clinicians need evidence when selecting appropriate drugs for the treatment. Thus, our study provided valuable data for clinical practices. However, due to the limitations of clinical trials, multidrug-resistant Gram-negative infections, or severe infection due to immunodeficiency were not included in our study.
For bacteria eradication rates, biapenem was comparable with meropenem against P. aeruginosa. Earlier studies have shown that biapenem was superior to imipenem in the antimicrobial activity against P. aeruginos3. However, our data demonstrated that compared to meropenem, biapenem was not better in the eradication of P. aeruginosa, with the same MIC range in vitro. For the reason that a few patients included in our study had healthcare-associated infections, 11 isolates of Acinetobacter baumannii/calcoaceticus complex were recovered. All these isolates were susceptible to biapenem, with MIC50<0.03 and MIC90= 0.25 μg/ml. This was consistent with an in vitro activity study in which biapenem showed high activity against A. baumannii13. These two species had similar susceptibility to biapenem and meropenem, with similar MIC50 and MIC range, and biapenem showed slightly better eradication rates. However, due to the limited number of samples, these findings need to be further confirmed in larger clinical trials. For the Gram-positive bacteria, difference of MIC between biapenem group and meropenem group was minor.
Our data showed that biapenem was a relatively safe drug. More than a quarter patients had underlying diseases (41 in biapenem group vs. 35 in meropenem group). The occurrence of drug-related adverse reactions was somehow lower in the biapenem group than in the meropenem group, although the difference was not significant. Similar to other carbapenems, the most common adverse effects were rash, gastrointestinal distress, increased alanine transferase, aspartate amino transferase or alkaline phosphatase alone, and decreased white blood cell count alone. Follow up observation and laboratory data demonstrated that all of the clinical and laboratory abnormalities related to the treatment were transient. Although the main route of elimination of biapenem is via renal glomerular filtration, renal impairment was not seen in any of these subjects. Thus, with combined evidence for its efficacy and safety, biapenem could be considered an alternative choice of carbapenem drugs.
There were some limitations of this study. The study was not double-blinded, due to the reason that meropenem should be given three times daily while biapenem could be administered twice daily, according to their pharmacokinetic features. Additionally, heterogeneous population might have limited the use of this study. Further clinical trials should be conducted to assess cost effective advantage of biapenem over meropenem.
In conclusion, this study suggested that biapenem was non-inferior to meropenem and was well-tolerated. Biapenem could be an alternative choice for therapy of moderate and severe lower respiratory tract infections and UTIs.
Acknowledgment
The pharmaceutical industry, Zhuhai United Laboratories Co., Ltd., PR China provided the financial grant to carry out this premarket trial. The authors acknowledge the doctors involved in this group study from the nine hospitals and the staff from the contract statistical organization.
Conflicts of interest: The authors declare that they have no conflicts of interest regarding the content of this article or funding from the industry.
References
- In vitro activity of biapenem (L-627), a new carbapenem, against anaerobes. Antimicrob Agents Chemother. 1994;38:889-93.
- [Google Scholar]
- In vitro activity of biapenem against recent Gram-negative and Gram-positive clinical isolates. Chemotherapy. 1997;43:393-403.
- [Google Scholar]
- In vitro and in vivo activities of LJC10,627, a new carbapenem with stability to dehydropeptidase I. Antimicrob Agents Chemother. 1991;35:203-7.
- [Google Scholar]
- Ministry of Health of the People's Republic of China (MOHPRC). Guidelines for clinical studies of antimicrobials. Beijing: MOHPRC; 1998.
- [Google Scholar]
- Practice of internal medicine. (13th ed). Beijing: People's Medical Publishing House; 2009.
- [Google Scholar]
- CLSI. Performance standards for antimicrobial susceptibility testing; 21st Informational Supplement. Wayne, PA, USA: Clinical and Laboratory Standards Institute; 2011.
- [Google Scholar]
- Antimicrobial susceptibility and molecular epidemiological analysis of clinical strains of Pseudomonas aeruginosa. J Infect Chemother. 2008;14:99-104.
- [Google Scholar]
- Clinical efficacy and safety of biapenem for febrile neutropenia in patients with underlying hematopoietic diseases: a multi-institutional study. J Infect Chemother. 2011;17:58-67.
- [Google Scholar]
- Evaluation of the efficacy and safety of biapenem against pneumonia in the elderly and a study on its pharmacokinetics. J Infect Chemother. 2013;19:98-102.
- [Google Scholar]
- A multicenter, randomized controlled clinical study on biapenem and imipenem/cilastatin injection in the treatment of respiratory and urinary tract infections. Chemotherapy. 2010;56:285-90.
- [Google Scholar]
- The in-vitro activity of biapanem against 964 clinical isolates of aerobic bacteria. J Antimicrob Chemother. 1995;35:681-6.
- [Google Scholar]






