Translate this page into:
Lipidated promiscuous peptide augments the expression of MHC-II molecules on dendritic cells and activates T cells
Reprint requests: Dr Javed N. Agrewala, CSIR-Institute of Microbial Technology, Sector 39A, Chandigarh 160 036, India e-mail: javed@imtech.res.in, jagrewala@gmail.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
In spite of the fact that BCG is the most widely used vaccine, tuberculosis (TB) continues to be a major killer disease in TB-endemic regions. Recently, many emerging evidences from the published literature indicate the role of environmental mycobacteria in blocking the processing and presentation of BCG antigens and thereby impairing with suboptimal generation of protective T cells. To surmount this problem associated with BCG, we constructed a novel lipopeptide (L91) by conjugating a promiscuous peptide consisting of CD4+ T-helper epitope of sequence of 91-110 of 16 kDa antigen of Mycobacterium tuberculosis to Pam2Cys, an agonist of Toll-like receptor-2.
Methods:
Mice were immunized subcutaneously with 20 nmol of L91, followed by a booster with 10 nmol, after an interval of 21 days of primary immunization. Animals were sacrificed after seven days of post-booster immunization. L91 induced immune response was characterized by the expression of MHC-II and CD74 on the surface of dendritic cells (DCs) by flowcytometry. Cytokines (IL-4, IL-10, IFN-γ) secretion and anti-peptide antibodies were measured by ELISA.
Results:
Self-adjuvanting lipopeptide vaccine (L91) was directly bound to MHC-II molecules and without requiring extensive processing for its presentation to T cells. It stimulated and activated dendritic cells and augmented the expression of MHC-II molecules. Further, it activated effector CD4 T cells to mainly secrete interferon (IFN)-γ but not interleukin (IL)-4 and IL-10. L91 did not elicit anti-peptide antibodies.
Interpretation & conclusions:
The findings suggest that L91 evokes maturation and upregulation of MHC class II molecules and promotes better antigen presentation and, therefore, optimum activation of T cells. L91 mainly induces effector Th1 cells, as evidenced by predominant release of IFN-γ, consequently can mount favourable immune response against M. tuberculosis. As L91 does not provoke the generation of anti-peptide antibodies, there is no fear of the efficacy of the vaccine being neutralized by pre-existing anti-mycobacterial antibodies in TB-endemic population. In conclusion, L91 may be considered as a future potential candidate vaccine against TB.
Keywords
BCG
L91
Pam2Cys
promiscuous peptides
Th1 – tuberculosis
Tuberculosis (TB) is a leading major global threat that causes around 2 million deaths annually1. BCG is the only available vaccine against TB, which is widely administered to control the disease2. BCG engenders protection in childhood but fails to do so in adults3. There are considerable hurdles associated with BCG performance viz. interference in antigen processing, masking and blocking due to exposure to environmental bacteria, and pre-existence of cross-reactive antibodies4. Considerable efforts are being made world-wide to develop a better vaccine than BCG and the rewards of an ideal global vaccine against TB are being vigorously pursued. Recently, several promising TB candidate vaccines have shown substantial improvement over BCG. These include recombinant BCG (rBCG), viral vectors, whole proteins and epitope-based vaccines45. In our contribution to this effort, we have developed a novel vaccination strategy that employs a promiscuous peptide epitope that is conjugated to the Toll-like receptor-2 (TLR-2) agonist, Pam2Cys45. Some of the advantages of this lipidated vaccine are that (i) it is totally synthetic; (ii) it has self-adjuvanting properties; (iii) it targets dendritic cells (DCs); (iv) it elicits robust long-lasting memory T cells; and (v) it provides enduring protection against Mycobacterium tuberculosis45.
One of the fundamental reasons considered for BCG failure in TB-endemic population is the interference of non-tuberculous mycobacteria in the processing and presentation machinery of antigen presenting cells (APCs), especially DCs, and the generation of a Th2-like response6. This is also true for BCG, which inhibits its own processing and presentation7. Further, antibodies generated upon exposure to environmental mycobacteria cause the clearance of vaccine antigens during immunization8. To address these problems, the current study was done to demonstrate that the novel vaccine construct L91 augments the surface expression of mature MHC-II molecules and inhibits the manifestation of CD74 (immature MHC molecules) events which contribute to enhanced antigen presentation to CD4 T cells.
Material & Methods
Reagents and antibodies: All standard reagents were purchased from Sigma (St. Louis, MO, USA), unless otherwise stated. All tissue culture plasticware was purchased from BD Falcon (Bedford, MA, USA). Fluorochrome conjugated anti-mouse antibodies to CD11c (HL-3), CD74 (ln-1), IAd (AMS 32.1), isotype controls Hamster IgG1 (G235-2356), IgG2b (A95-1); capture and detection antibodies for ELISA for interferon (IFN)-γ, interleukin (IL)-4, IL-10 were from BD Biosciences (San Diego, CA, USA). Commercial Pam2Cys (used as control) was purchased from Invivogen (San Diego, CA, USA).
Mice: Female BALB/c mice (6-8 wk, 20±2 g) were procured from the Institute of Microbial Technology, Chandigarh, India, National Institute of Pharmaceutical Education and Research, Mohali, India. The study protocol was approved by Institutional Animal Ethics Committee.
Peptides: The promiscuous CD4 T cell peptide epitope SEFAYGSFVRTVSLPVGADE (91-110) from the 16kDa antigen was synthesized and conjugated to TLR2-ligand Pam2Cys, as described elsewhere9. This lipidated peptide is designated as L91 and free peptide as F91.
Immunization: Mice (3-4/group) were immunized with L91 (20 nmol) subcutaneously at the base of tail. Twenty one days later, a booster dose (10 nmol) was administered. Animals were sacrificed seven days after receiving the post booster immunization for immunological studies.
Expression of MHC-II and CD74 on the surface of DCs: The immunophenotyping for the display of surface markers was done as described elsewhere5. Briefly, bone marrow derived cells (BMDC) (2×106) were incubated in Petri plates (60 mm) in the presence of granulocyte macrophage colony-stimulating factor (GM-CSF) (10 ng/ml) and IL-4 (5 ng/ml) in RPMI-1640 containing 10 per cent fetal bovine serum (FBS) for six days. After three days, the cultures were replenished with new medium supplemented with growth factors. The cells were harvested on day 7 and transferred into 24 well plates (2×105 cells/well). The cells were incubated with L91 (3 nmol), F91 (3 nmol) and lipopolysaccharide (LPS) (5 μg/ml). After 12 h, cells were harvested, washed and examined for surface expression of MHC-II and CD74 molecules.
Estimation of IFN-γ, IL-4 and IL-10: Lymphocytes (1.5×105/well) isolated from the lymph nodes of mice immunized with L91 and F91 were cultured in vitro with equimolar (3 nmol) concentrations of either L91 or F91 or Pam2Cys in triplicate in ‘U’ bottomed 96 well microtitre plates for 48 h at 37°C. Later, the supernatants were collected from in vitro stimulated culture and used for the estimation of IFN-γ, IL-4 and IL-10 by sandwich ELISA (BD Biosciences, San Diego, USA), as per manufacturer's instructions. Briefly, anti-cytokine (IFN-γ, IL-4, IL-10) (50 μl/well) antibodies (Abs) were coated in triplicate in Na2HPO4 (0.1M, pH 9.0) buffer in flat bottomed 96 well ELISA plates overnight at 4°C. Later, the wells were blocked with bovine serum albumin (BSA, 1%) and culture supernatants (50 μl/well) were added and incubated overnight at 4°C. Further, biotinylated secondary Abs to the respective cytokine were added and incubated at 37°C for 2 h. Avidin-horse radish peroxidase (HRP) (50μl/well) was added in each well and plates were kept at 37°C for 1 h. Finally, o-phenylenediamine (OPD)/H2O2 was added and the optical density was determined at 492 nm. Regular procedures of washings were followed at each step.
Detection of anti-peptide Abs: Microtitre plates (96 well) were coated with 50 μl/well of F91 (10 μg/ml) in Na2 CO3/NaHCO3 (pH, 9.4) buffer for 16 h at room temperature. The blocking was performed with BSA (1%) for 2 h at 37°C. Serum samples (dilution, 1:100) obtained from L91 and placebo immunized mice were added and plates incubated overnight at 4°C. Later, HRP-conjugated anti-mouse IgG+IgM+IgA Abs were added, followed by incubation at 37°C for 1 h. The colour was developed using OPD/H2O2 for 20 min at 37°C. Regular procedures for washings were followed at each step.
Results & Discussion
L91 promotes the maturation of MHC class II molecules: Recently, many studies have attributed the decreased processing and presentation that is observed with BCG antigens to environmental mycobacteria7 and downregulation in expression of MHC class II molecules on the surface of APCs, which also impairs the immune response10.
Pam2Cys is known to upregulate the expression of MHC molecules5. Hence it became necessary to check the influence of L91 in affecting the expression of MHC molecules on the surface of APCs. In particular, the display of MHC-II molecules on the surface of DCs was monitored, since they play a cardinal role in activating naïve T cells. It was interesting to observe that L91 significantly enhanced the display of MHC-II molecules on the surface of DCs (Fig. 1a). This increase was comparable to that observed with DCs, which were incubated with LPS (positive control). In contrast, F91 failed to induce any enhancement in MHC-II expression. It was also noticed that L91 downregulated the expression of immature MHC-II molecules (CD74) (Fig. 1b). A similar trend was also observed in the case of LPS and F91. The experiments depicting enhancement in the exposition of MHC-II and decrease in CD74 signify that L91 can activate DCs and therefore, may be successful in TB-endemic regions; where exposure to environmental mycobacteria and BCG immunization is known to downregulate MHC molecules. Further, it has also been reported that BCG infected THP1 cells show reduced expression of MHC class II and upregulation of CD74 (immature MHC)11. Furthermore, decreased expression of MHC-II has been reported at the surface of RAW (mouse leukaemic monocyte macrophage cell line) cells infected with M. avium12. A significant difference in the ratio of MHC-II/CD74 molecules on the surface of DCs was observed (Fig. 1c). We have shown earlier that L91 can also induce the expression of CD80, CD86 and CD40 co-stimulatory molecules and activate DCs to produce IL-6 and IL-125.
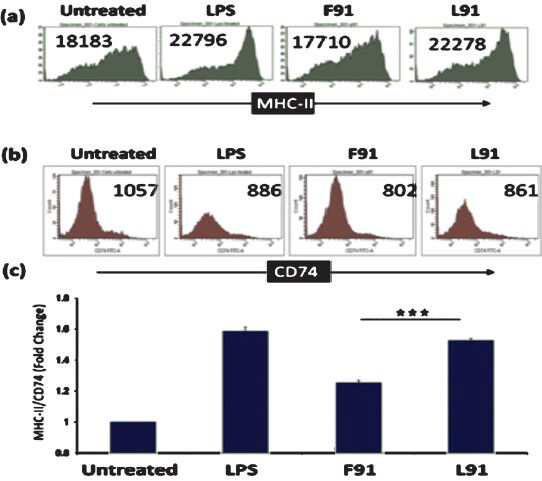
- L91 induces MHC-II but downregulates CD74 expression. Bone marrow derived cells (BMDCs) were cultured in medium containing L91, F91 or lipopolysaccharides (LPS). The CD11c+ cells were analyzed by flowcytometry for the expression of (a) MHC-II; (b) CD74 (immature MHC-II). Numbers in the flow cytometry histograms indicate mean fluorescence intensity (MFI) and are representative of 3 independent experiments; (c) bar graphs depict the change in the ratio of MHC-II to CD74. The cells cultured with medium alone and with LPS were used as negative and positive controls, respectively. Data are shown as the mean±SD are from 3 independent experiments. ***P<0.001.
L91 immunization elicits secretion of IFN-γ: The role of Th1 cells and the cytokine IFN-γ has been very well established in protection against TB13. In contrast, Th2 cells and their associated cytokines (IL-4, IL-10) are correlated with the progression of disease13. Therefore, the production of Th1 and Th2-like cytokines was monitored in the mice immunized with L91 or F91. It was of interest to note that L91 significantly (P<0.003) augmented release of IFN-γ by effector T cells when compared to IL-4 and IL-10 (Fig. 2a, b). We did not observe much secretion of cytokines in the cells pulsed with F91 or Pam2Cys.
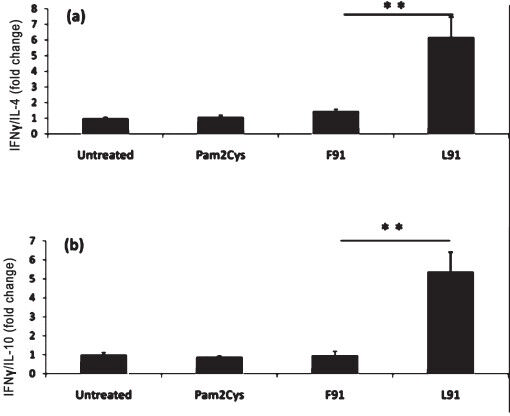
- L91 elicits Th1 but not Th2 response. The cells obtained from immunized mice were cultured with L91, F91, Pam2Cys or medium alone for 48 h. Secretion of cytokines in the culture supernatants was measured by ELISA. The results depicted as bar diagrams show the change in ratio of (a) IFN-γ and IL-4; (b) IFN-γ and IL-10. Data are expressed as the mean ± SD and are pooled data from 3 independent experiments with 3-4 mice per group. **P<0.003.
In TB endemic population, individuals have compromised Th1 and Th2 biased immunity due to helminth and non-tuberculous bacterial infection4. Further, BCG fails to mount Th1 immunity1415. Hence it is crucial that the vaccine developed against this population should evoke chiefly Th1 responses. Our data showed that immunization with L91 provoked robust IFN-γ immunity, which is considered to play a fundamental role in protection against M. tuberculosis.
L91 vaccination fails to provoke Abs: Another important reason suggested for BCG failure in TB population is the masking and blocking of BCG antigens by antibodies elicited by environmental mycobacteria4. Hence in these regions, the efficiency of BCG is impaired by the pre-existing cross-reactive antibodies produced due to exposure to environmental mycobacteria416. Even though L91 is comprised of CD4 T-helper epitope and not a known B cell epitopes, we determined whether L91 evokes an Ab response. No anti-peptide Abs were detected in the serum of animals immunized with L91 based on the very low levels of OD (Fig. 3). Recently, we have also demonstrated the absence of Abs against L91 in the serum of TB patients and PPD+ healthy individuals5. These results taken together suggest that L91 could be usefully employed as a vaccine for the inhabitants of TB-endemic sectors without a fear of its activity being neutralized by anti-peptide Abs.
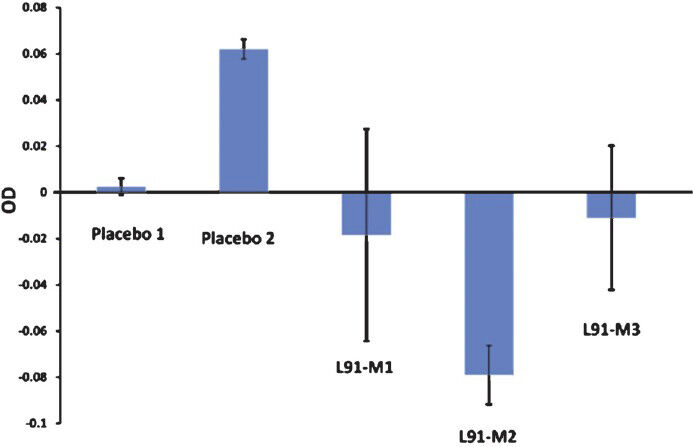
- L91 fails to evoke antibody response upon immunization. The presence or absence of anti-peptide Abs was examined in the serum of mice immunized with L91 or placebo. M1, M2, M3 represent the data from individual mice. The level of OD indicates that the Ab is negative in serum of L91 vaccinated mice. The data (OD) of the bar diagram are represented as the mean ± SD (three independent experiments, **P<0.003).
Our results demonstrate that L91 elicits an immune response, the characteristics of which could be very useful in imparting protective immunity against M. tuberculosis. It is important to note here that L91 has self-adjuvanting properties and it works in genetically distinct populations5. L91 has been designed to overcome the shortcomings that are associated with BCG during vaccination in TB-endemic population. Based on our current results and published work45, it can be speculated that L91 has significant potential to be considered as a future vaccine candidate against M. tuberculosis.
Acknowledgment
Authors acknowledge the CSIR, DBT and ICMR, India for financial support. The first two authors (UG and PKR) were recipients of DBT and CSIR fellowships, respectively. D.C. Jackson and W. Zeng are supported by the National Health and Medical Research Council of Australia.
References
- The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009-21.
- [Google Scholar]
- Coadministration of interleukins 7 and 15 with bacille Calmette-Guerin mounts enduring T cell memory response against Mycobacterium tuberculosis. J Infect Dis. 2010;202:480-9.
- [Google Scholar]
- Lipidated promiscuous peptides vaccine for tuberculosis-endemic regions. Trends Mol Med. 2012;18:607-14.
- [Google Scholar]
- Promiscuous peptide of 16 kDa antigen linked to Pam2Cys protects against Mycobacterium tuberculosis by evoking enduring memory T-cell response. J Infect Dis. 2011;204:1328-38.
- [Google Scholar]
- Dendritic cells derived from BCG-infected precursors induce Th2-like immune response. J Leukoc Biol. 2004;76:827-34.
- [Google Scholar]
- Regulation of antigen presentation by Mycobacterium tuberculosis: a role for Toll-like receptors. Nat Rev Microbiol. 2010;8:296-307.
- [Google Scholar]
- Differential effects of prior exposure to environmental mycobacteria on vaccination with Mycobacterium bovis BCG or a recombinant BCG strain expressing RD1 antigens. Infect Immun. 2005;73:2190-6.
- [Google Scholar]
- A modular approach to assembly of totally synthetic self-adjuvanting lipopeptide-based vaccines allows conformational epitope building. J Biol Chem. 2011;286:12944-51.
- [Google Scholar]
- Mycobacterium bovis BCG decreases MHC-II expression in vivo on murine lung macrophages and dendritic cells during aerosol infection. Cell Immunol. 2009;254:94-104.
- [Google Scholar]
- Mycobacterium bovis BCG urease attenuates major histocompatibility complex class II trafficking to the macrophage cell surface. Infect Immun. 2004;72:4200-9.
- [Google Scholar]
- Mycobacterium avium infection of mouse macrophages inhibits IFN-gamma Janus kinase-STAT signaling and gene induction by down-regulation of the IFN-gamma receptor. J Immunol. 1999;163:2041-8.
- [Google Scholar]
- Contribution of Th1 and Th2 cells to protection and pathology in experimental models of granulomatous lung disease. J Immunol. 2001;166:3432-9.
- [Google Scholar]
- Schistosoma mansoni infection reduces the protective efficacy of BCG vaccination against virulent Mycobacterium tuberculosis. Vaccine. 2005;23:1326-34.
- [Google Scholar]
- Cross-reactive immune responses against Mycobacterium bovis BCG in mice infected with non-tuberculous mycobacteria belonging to the MAIS-Group. Scand J Immunol. 1997;46:16-26.
- [Google Scholar]






