Translate this page into:
Impact of changing over of insecticide from synthetic pyrethroids to DDT for indoor residual spray in a malaria endemic area of Orissa, India
Reprint requests: Dr S.K. Sharma, Scientist - E, National Institute of Malaria Research, Field Station, Sector-5, Rourkela 769 002, India e-mail: suryaksharma@gmail.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Development of insecticide resistance in malaria vectors has been a major problem for achieving effective vector control. Due to limited availability of insecticides, the only option is management of resistance by judiciously using the insecticides and rotating them to maintain their effectiveness. This study was carried out in a malaria endemic area of Sundergarh district in Orissa where synthetic pyrethroids (SP) were in use for the last couple of years. The change-over from SP to DDT was done in one arm of study, and the other two arms remained on SP and insecticide-treated nets (ITN). Entomological and parasitological monitoring was done to assess the impact.
Methods:
The study design comprised of three arms (i) two rounds of indoor residual spraying (IRS) with DDT 1g/m2 as a change-over insecticide in areas previously under synthetic pyrethroids; (ii) two rounds of IRS with synthetic pyrethroid (alphacypermethrin, ACM) @ 25 mg/m2; and (iii) an unsprayed area under ITN/long lasting insecticide nets (LNs). Indoor residual spraying was undertaken under strict supervision to maintain quality and coverage. Contact bioassays were conducted to know the persistence of insecticide on sprayed surfaces and adult vector density was monitored in fixed and randomly selected houses. Malaria incidence was measured through fortnightly domiciliary surveillance under primary health care system in all the study villages.
Results:
The insecticide susceptibility tests showed that An.culicifacies was resistant to DDT but susceptible to malathion and ACM. However, An. fluviatilis was susceptible to all the three insecticides. ACM was effective in killing An. culicifacies on mud and wooden sprayed surfaces and maintained effective bioefficacy ranging from 92 to 100 per cent up to five months, whereas DDT failed to achieve effective mortality in An.culicifacies. However, there was significant decline in the density of An.culicifacies in ACM and DDT areas in comparison to ITNs/LNs. There was 61 per cent reduction in the slide positivity rate in ACM area in comparison to 48 and 51 per cent in DDT and ITN/LNs areas, respectively. The adjusted incidence rate of malaria cases per 1000 population in three study areas also showed significant declines within each group.
Interpretation & conclusions:
The present findings show that the change-over of insecticide from synthetic pyrethroids to DDT brings about the same epidemiological impact as envisaged from continuing SP spray or distributing insecticide treated nets/long-lasting insecticidal nets provided there is a good quality spray and house coverage.
Keywords
Bioassays
DDT
indoor residual spraying
insecticide resistance
malaria incidence
rotation of insecticide
synthetic pyrethroid
vector density
Insecticide based interventions for vector control are the main planks of the anti-malaria campaign in India and have so far remained largely effective for disease management. DDT was the first insecticide to be used in India for indoor residual spraying during 1950s with tremendous success1 and is still in use in many areas. Anopheles culicifacies is the major vector of malaria in the plains and is responsible for transmission of about 65 per cent malaria cases reported in the country. An. fluviatilis is another important vector responsible for transmission of malaria in foot hills and forested region2. Due to development of DDT-resistance in An. culicifacies, in some areas, DDT was replaced by malathion during 1970s, and subsequently with synthetic pyrethroids during 1990s in certain areas where An culicifacies species developed widespread resistance to both DDT and malathion3–5.
Development of insecticide resistance in malaria vector has been a major impediment for successful use of the insecticide for effective vector control. Due to limited availability of insecticides, the only viable option left for effective vector control is management of existing resistance in vectors by judiciously using the insecticides and rotating them to maintain their effectiveness.
Therefore, a study was undertaken to evaluate the impact of indoor residual spraying with alternate insecticide under supervision in malaria endemic area of Sundargarh District in Orissa, India. The study district has distinct geographical characteristics, vector prevalence and varied susceptibility status to insecticides. The present study was conducted in an area where synthetic pyrethroids (SP) were continuously in use for three or more years for indoor residual spraying. The change-over of insecticide from SP to DDT was undertaken in one arm of the study, whereas, the other two arms were kept under SP and insecticide treated nets (ITN)/long-lasting insecticidal nets (LN), respectively. The longitudinal entomological and parasitological monitoring was carried out to see the impact of change-over of insecticide in comparison to synthetic pyrethroid and ITN/LNs.
Material & Methods
Study area: The state of Orissa in the eastern part of India is endemic for malaria. Although the State constitutes only 4 per cent of the total population of India, it accounts for a quarter of all malaria cases, 41 per cent of Plasmodium falciparum cases and 18 per cent of all reported deaths due to malaria in the country6. The trial was conducted in 3 sub-centres of Laing primary health centre (PHC) located in Rajgangpur block of Sundargarh District. Sundargarh District is located in the Garhjat hills of eastern Deccan plateau between 21°35’N and 22°35’N latitudes, and between 83°32’E and 85°22’E longitudes, at an altitude in the range of 200 to 900 m above sea level. Topographically, the area presents ideal ecological conditions for malaria transmission with undulating uplands intersected by forested hills, rocky streams, and paddy fields. The area is characterized by a tropical humid climate and receives rainfall between June and September from the ‘southwest monsoon’ and in December and January from the ‘northeast monsoon’. Average annual rainfall ranges between 160-200 cm and mean annual temperature ranges between 22 to 27°C. The maximum temperature during summer rises to 40-45°C and the minimum temperature during winter falls to 5-10°C. About 40 per cent of the area is covered with forests and is inhabited predominantly by ethnic tribal communities with poor socio-economic status, who constitute 62 per cent of the total population.
Malaria transmission in Laing PHC area is perennial, which peaks during the post-monsoon months of October to December. P. falciparum accounts for more than 80 per cent of malaria cases (unpublished NVBDCP data, District Malaria Officer, Sundargarh District, Orissa). The area is under the influence of two primary malaria vector species An. culicifacies and An. fluviatilis, the former breeds in ponds, pools and rice fields, whereas the later breeds exclusively in slow running streams. An. culicifacies is a predominant species whereas, An. fluviatilis is prevalent at low density throughout the year except during hot dry months of May and June, but it is an efficient vector even at low densities as indicated by its high entomological inoculation rate. The peak malaria transmission season coincides with the peak prevalence of An. fluviatilis7.
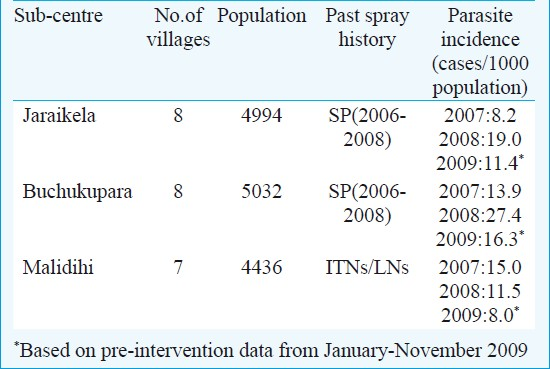
Based on past history of indoor residual spray and epidemiological data, three sub-centres were selected for the present study (Table I). The study villages in Jaraikela and Buchukupara sub-centres were covered with two rounds of indoor residual spray with synthetic pyrethroid alphacypermethrin (ACM, Fendona™ 5%) @ 25 mg/m2 during 2006-2008 as per routine vector control measures undertaken by National Vector Borne Disease Control Programme (NVBDCP). However, these study villages were not covered under government sponsored ITN programme. The third sub-centre Malidihi consisting of seven villages was under ITNs treated with deltamethrin @ 25mg/m2 and factory treated long-lasting insecticidal nets (PermaNets™-2.0) coated with deltamethrin @ 55mg/m2. The District Malaria Officer Sundargarh was requested to exclude the study villages for vector control activities for the duration of the present trial. However, active case detection through fortnightly fever surveillance was continued under primary health care system.
Insecticide susceptibility status of malaria vectors: Insecticide susceptibility status of wild caught malaria vectors An. culicifacies and An. fluviatilis against DDT (4.0%), malathion (5%) and alphacypermethrin (0.1%) was determined as per standard procedure8. The field collected mosquitoes were exposed for one hour to insecticide impregnated papers using WHO adult susceptibility kit. The mosquitoes were kept in the recovery tubes for 24 h and mortality was recorded. In test replicates where mortality in the control tubes was more than 5 per cent, the mortality of the exposed mosquitoes was corrected using Abbott's formula9.
Indoor residual spraying: A three arm study design was prepared as (i) two rounds of indoor residual spraying (IRS) with DDT 1 g/m2 as a change-over insecticide in areas previously under synthetic pyrethroids, (ii) two rounds of IRS with available synthetic pyrethroid (alphacypermethrin) @ 25 mg/m2, and (iii) an unsprayed area under ITN/LNs. Based on the information on past spray history of sub-centres, it was decided that villages under sub-centre Buchukupara, which were continuously under indoor residual spray with synthetic pyrethroids for the last three years (2006-2008) or more, should be sprayed with DDT as a change-over insecticide to see impact on vector density and malaria transmission. Similarly, the villages under sub-centre Jaraikela qualified for the application of synthetic pyrethroid for indoor residual spraying. The third sub-centre Malidihi, which was under conventionally treated insecticidal nets (ITNs) or long-lasting insecticidal nets (LNs) was kept as third arm of the study without indoor residual spray (Table II).
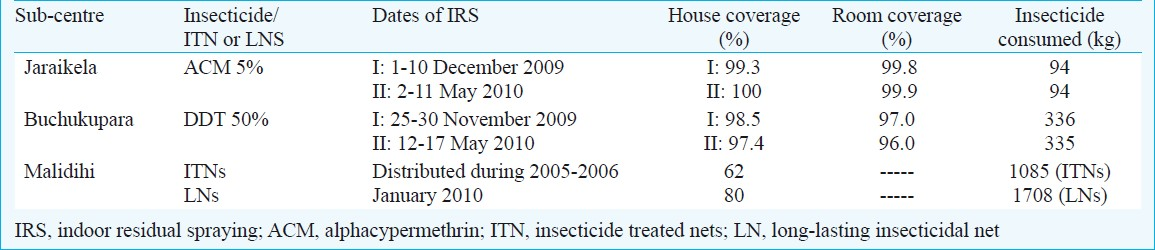
For indoor residual spraying, the services of a non-governmental organization (NGO)- Regional Rural Development Centre (RRDC) based in Rajagangpur block were availed because they had experience of conducting indoor residual spraying (IRS) operations under primary health care system. The stirrup pumps in good working conditions were provided by District Malaria Officer (DMO), Sundargarh. The experienced spraymen engaged by the NGO were given hands on training for proper spraying and were provided with protective gears (hand gloves, goggles and masks) by the study team. DDT 50 per cent WP for the first and second round of IRS was supplied by DMO Sundargarh and alphacypermethrin 5 per cent WP (FENDONA™)for the first round was supplied gratis by BASF India Ltd., and for second round, alphacypermethrin 5 per cent WP (Fendona™) was supplied by DMO Sundargarh. The first and second rounds of IRS were completed between 25 November to 10 December 2009 and 2-17 May 2010, respectively under the close supervision of study team from National Institute of Malaria Research, Field Station, Rourkela.
Contact bioassays: To determine persistence of insecticides on local surfaces, contact (cone) bioassays using the standard WHO procedure was conducted10. Main local surfaces viz., mud-walls and wood surfaces were used for bioassays. Bioassays were carried out on day 1, and then once every month. Wild mosquitoes were used in the contact bioassays because of their susceptibility status determined during baseline studies. Wild caught fully fed An. culicifacies from an unsprayed area were used in five replicates of 10 mosquitoes for each surface. Locations on the surfaces were marked soon after the spraying and bioassays were carried out each time on those fixed spots only. Co-operation of the householders was sought not to disturb, mud-plaster or mutilate these sites until completion of the evaluation. For bioassays, the cones were fixed on the surfaces and 10 mosquitoes were released gently in each of the cones. Mosquitoes were exposed for 30 min on the sprayed surfaces and knockdown time was also recorded intermittently (in SP sprayed area) as recommended by WHO11. After 30 min of exposure to the sprayed surface, mortality was recorded and the mosquitoes were removed gently from the cones and kept in plastic cups with a net on the rim. Mosquitoes were provided with cotton-wool moistened with 10 per cent glucose solution. Mortality in mosquitoes was recorded 24 h post-exposure. Contact bioassays with An. fluviatilis were not done because of insufficient mosquitoes due to seasonality of prevalence of this species.
Mosquito densities: Adult mosquito densities were measured in four fixed houses and four houses selected randomly each in sentinel villages of all the three sub-centres. The indoor resting mosquito collections were made every month in the morning between 0600-0900 h in the fixed and random houses for 15 min in each dwelling with the help of suction tubes using flashlights. The mosquitoes were brought to laboratory in cloth cages, identified and kept under observations for 24 h under optimal conditions. The mean monthly density of indoor-resting mosquitoes was calculated as person-hour density. The spray sheet collections were not done because most of the houses have open eves all around.
Malaria incidence: All the study areas were kept under routine fortnightly domiciliary malaria surveillance with the help of health workers and Accredited Social and Health activists (ASHA) working under primary health care system. The surveillance data were collected village-wise for all the three sub-centres from Laing PHC. In addition to routine parasitological data from PHC, rapid fever surveys were also conducted with the help of local health workers to measure the incidence rate in three clusters of the study area.
Statistical analysis: Data were entered into an excel spreadsheet by month and village. The reduction in slide positivity rate (SPR) was calculated by subtracting post-intervention SPR from baseline SPR and dividing by the baseline SPR. The incidence rate was calculated using STATA (version 11; StataCorp LP, Texas, USA). Poisson regression for each intervention group was used to account for variation in case counts. Cases were the outcome variable and intervention was the main exposure. Monthly blood examination rate (MBER) was considered a confounder and adjusted for in the model, as a higher MBER would increase the number of cases detected independent of any intervention. Ratios of the incidence rate were calculated to determine the proportional reduction of malaria incidence in each group.
Results
The results of the insecticide susceptibility test revealed that An. culicifacies was resistant to DDT but susceptible to malathion and alphacypermethrin. However, An. fluviatilis was susceptible to all the three insecticides.
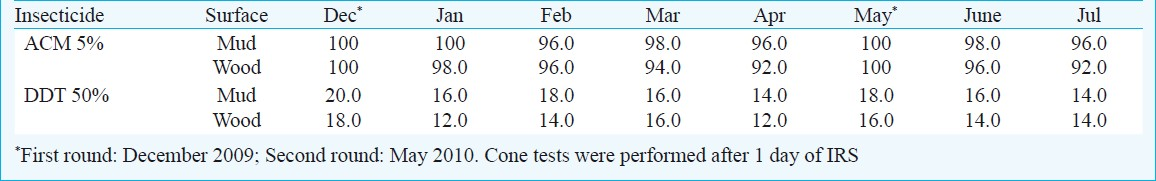
Persistence of insecticide on sprayed surfaces: The results showed that ACM on both the sprayed surfaces was effective in killing the vector mosquito An. culicifacies and maintained effective bioefficacy ranging from 92 to 100 per cent up to five months. However, DDT failed to achieve effective mortality in An.culicifacies. The mortality ranged between 12-18 per cent during different months after indoor spraying (Table III).
Impact on vector density: The first round of spray with ACM and DDT resulted in significant decline in the density of An. culicifacies in comparison to ITNs/LNs (Fig. 1). The density of malaria vector was almost nil in the houses sprayed with ACM. However, after the second round of spray, there was marginal increase in the vector density in ACM and DDT areas. The increase in vector density was also observed in the ITN/LN area.
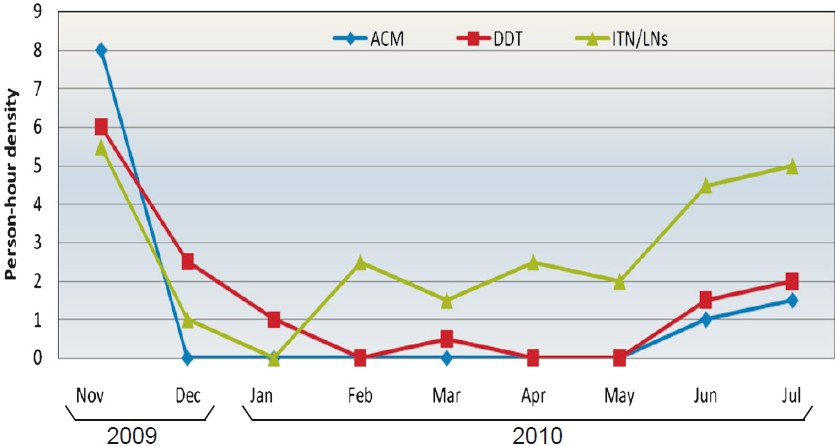
- Density of An. culicifacies in houses sprayed with alphacypermethrin (ACM), DDT and houses with ITN/LNs during intervention period. First round of IRS was carried out in November-December 2009 and second in May 2010.
Impact on malaria incidence: The malaria incidence data for the three study sub-centres were collected from Laing PHC and analyzed to determine the impact of each intervention. The slide positivity rate (SPR) during pre-intervention period in ACM, DDT and ITN/LNs area was 5.3, 4.7 and 4.4, respectively and there was no significant difference between the three areas. ACM and DDT areas prior to change over of insecticide were under synthetic pyrethroid (SP) for the last three years though with different coverage and quality. The change-over of insecticide from SP to DDT was undertaken in Buchukupara sub-centre. After intervention, the SPR was dropped to 2.1, 2.5 and 2.2, respectively and there was 61 per cent reduction in the slide positivity rate in ACM area in comparison to 48 and 51 per cent reduction in DDT and ITN/LNs areas, respectively (Table IV). The adjusted incidence rate of malaria cases per 1000 population per year in three study areas before intervention under ACM, DDT and ITN/LNs was 7.6, 10.1 and 5.8, respectively, and significant declines were observed within each group (Table IV). During intervention, involving change-over of insecticide from SP to DDT in one study area and distribution of long-lasting nets in ITN area during January 2010 and closely supervised IRS, there was decline in crude malaria incidence (P<0.01) in all the three study arms (Fig. 2).
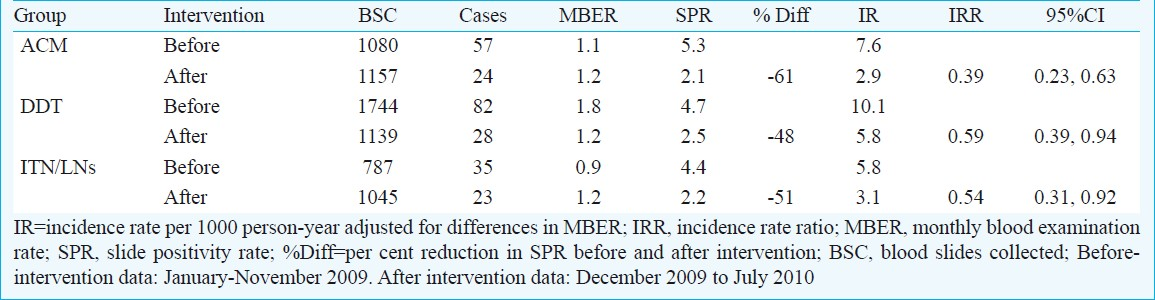
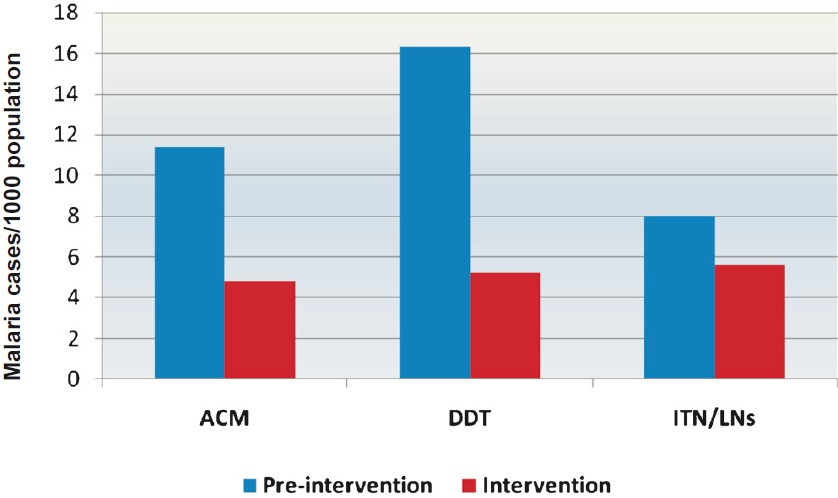
- Comparison of crude malaria incidence (number of cases/1000 population/year) in three study areas during pre-intervention and intervention period. A significant decline was observed in all the three areas (P<0.01).
Discussion
Vector control programme in India relies mostly on indoor residual spraying with DDT. The spectacular success achieved in malaria control between 1958 and 1965 was mainly attributed to DDT. However, this achievement was short-lived and soon after malaria resurgence took place. One of the technical reasons for resurgence was development of DDT resistance in primary malaria vector An. culicifacies. However, DDT continued to be used in larger areas of the country because of its cost effectiveness over organophosphorous insecticides and synthetic pyrethroids and also the availability of limited arsenal of insecticides and formulations recommended by WHO Pesticide Evaluation Scheme (WHOPES)12.
The baseline studies showed that An. culicifacies was resistant to DDT, whereas An. fluviatilis was susceptible to all the insecticides. The susceptibility status of the later species has not changed over a period of time in spite of the fact that majority of the population are endophilic and endophagic13. In Malkangiri and Koraput districts of Orissa, DDT spraying is an effective tool for controlling An. fluviatilis-transmitted malaria though the species is exophilic but its nocturnal resting behaviour facilitates its contact with the sprayed surface14. The results of the cone bioassays on sprayed surfaces revealed that SP was able to achieve significant mortality in the vector species, whereas bioefficacy of DDT was minimal against An.culicifacies represented by two sibling species B (non vector) and C (vector) in the relative proportion of 62 and 38 per cent respectively in the study area7. The marginal increase in the vector density after second round of IRS in both SP and DDT areas was due to onset of monsoon and availability of large numbers of breeding habitats in the study villages. The differential behavioural response of each sibling species to DDT has been documented to produce epidemiological impact on transmission, although it may not be apparent by monitoring of the adult population15.
The impact of the different interventions varied according to the outcome indicator used. Examining the crude incidence in the pre-intervention period and post-intervention follow up between the groups suggested the greatest reduction in DDT sprayed areas, followed by SP, and with minimal effect of ITNs/LNs. However, the dramatic decline in the DDT area, because of a higher pre-intervention incidence of malaria, may be attributable to differences in case detection evident by a monthly blood examination rate almost two times higher. Therefore, in terms of the reduction of the slide positivity rate, SP spray was the most effective followed by similar impact in the DDT and bednet groups. Similar results to the difference in SPR were obtained when analyzing the incidence rate adjusted for differences in blood examination between the groups. Overall, with the exception of the crude incidence outcome, all groups were effective in controlling malaria with similar impact (41-61% reduction). This suggests that proper supervised IRS operations or net distribution coupled with high coverage will bring about reduction in malaria transmission.
There are several methods on the strategic use of available insecticides to delay the onset of resistance1617. In a comparative study on insecticide rotations, mosaics and single use of insecticides in Mexico, the pyrethroid resistance increased markedly in the mosquito populations in all villages, irrespective of insecticide treatment strategy, but was still less in areas under rotation and mosaic treatments compared to single use of insecticide18. However, combination of insecticides and rotation are said to be associated with increased frequency of side effects in the community, which is a limitation for the use of these type of resistance management strategy in public health19. The monitoring of insecticide resistance in local vectors in space and time is also important for planning sustainable large scale insecticide based malaria vector control based on change-over of insecticide over time. The change-over from pyrethroid to carbamate to DDT over a period of time has been successfully implemented in Mozambique to overcome the problem of insecticide resistance20.
The present study revealed that change-over of insecticide from SP to DDT brought about the same impact as envisaged from continuing SP spray or distributing ITNs/LNs. In the present study, internal and external validity of the quality assurance was done through a combined efforts of NGO-district malaria office and technical experts of NIMR, respectively to ensure strict compliance to the protocol and data generation. The synthetic pyrethroids should not be used continuously in the public health programmes because of possibility of developing resistance in the malaria vectors, therefore, it is logical to adopt principle of rotation/change-over of insecticide in order to prolong the effective life of the insecticides for sustainable vector control. The present study area was under the impact of two primary vectors An. culicifacies and An. fluviatilis, which complement each other in maintaining persistent transmission though the malaria peak occurs mainly because of An. fluviatilis because of its high anthropophagic behaviour and entomological inoculation rate (EIR)6. The susceptibility of this primary vector species to all the insecticides was an operational advantage for the change-over of insecticide. Therefore, in such settings where one or more primary vector is susceptible to SP or DDT, the change-over of insecticide either from SP to DDT or vice versa is recommended. Thus, in order to sustain the effectiveness of insecticide based vector control, there is a need to undertake systematic evaluation of the impact of rotation/change-over of insecticide for IRS on malaria transmission in different epidemiological settings.
Acknowledgment
Authors acknowledge the Directorate of National Vector Borne Disease Control Programme, Government of India for funding the study. Authors thank Dr V.K. Dua, Director Incharge and Dr K. Raghavendra of NIMR, New Delhi for co-ordinating the trial, Dr Naman Shah from School of Public Health, University of North Carolina (USA) for help in statistical analysis. The technical support provided by the staff of National Institute of Malaria Research, field station, Rourkela, Orissa is acknowledged. The study was conducted under long-term Integrated Disease Vector Control Project being funded by Indian Council of Medical Research, Department of Health Research, Ministry of Health & Family w0 elfare, Government of India, New Delhi.
References
- The national malaria control programme in India and the possibilities of eradication of malaria in India and the tropics. Bull Nat Soc Mal Mosq Dis. 1958;6:5-6.
- [Google Scholar]
- Malarial morbidity in tribal communities living in the forest and plain ecotypes of Orissa, India. Ann Trop Med Parasitol. 2004;98:459-68.
- [Google Scholar]
- Development of increased tolerance to DDT in Anopheles culicifacies Giles, in the Panchmahal district of Bombay state (India) Indian J Malariol. 1959;12:125-30.
- [Google Scholar]
- Preliminary note on the development of DDT resistance in Anopheles culicifacies Giles in Panchmahal district, Gujarat state, India. Bull World Health Organ. 1962;26:128-34.
- [Google Scholar]
- Development of malathion resistance in a DDT, HCH resistant Anopheles culicifacies population in Thane district (Maharasthra) J Commun Dis. 1983;15:144-5.
- [Google Scholar]
- Epidemiology of malaria transmission and development of natural immunity in a malaria-endemic village, San Dulakudar, in Orissa state, India. Am J Trop Med Hyg. 2004;71:457-65.
- [Google Scholar]
- Epidemiology of malaria transmission in forest and plain ecotype villages in Sundargarh District, Orissa, India. Trans R Soc Trop Med Hyg. 2006;100:917-25.
- [Google Scholar]
- World Health Organization. Manual on practical entomology in malaria. Part II. Methods and Techniques 1975:141-7.
- [Google Scholar]
- World Health Organization. In: Instructions for the bioassay of insecticidal deposits on wall surfaces. Vol 81.5. Geneva: WHO; 1981. WHO/VBC
- [Google Scholar]
- Test procedure for insecticide resistance monitoring in malaria vectors, bio-efficacy and persistence of insecticides on treated surfaces. World Health Organization 1998 WHO/CDS/CPC/MAL/9812, (unpublished document)
- [Google Scholar]
- World Health Organization. Chemistry and specifications of pesticides. World Health Organ Tech Rep Ser. 2001;899:1-68.
- [Google Scholar]
- Insecticide susceptibility status of malaria vectors in some hyperendemic tribal districts of Orissa. Curr Sci. 2004;87:1722-6.
- [Google Scholar]
- DDT indoor residual spray, still an effective tool to control Anopheles fluviatilis-transmitted Plasmodium falciparum malaria in India. Trop Med Int Health. 2005;10:160-8.
- [Google Scholar]
- Response of Anopheles culicifacies sibling species A and B to DDT and HCH in India: Implications in malaria control. Med Vet Entomol. 1988;2:219-23.
- [Google Scholar]
- Insecticide resistance issues in vector-borne disease control. Am J Trop Med Hyg. 1994;50(Suppl 6):21-34.
- [Google Scholar]
- Resistance management strategies in malaria vector mosquito control. A large-scale field trial in Southern Mexico. Pest Sci. 1997;51:375-82.
- [Google Scholar]
- Acceptability and perceived side effects of insecticide indoor residual spraying under different resistance management strategies. Salud Publica Mex. 2006;48:317-24.
- [Google Scholar]
- Monitoring the operational impact of insecticide usage for malaria control on Anopheles funestus from Mozambique. Malar J. 2007;6:142.
- [Google Scholar]






