Translate this page into:
Visceral leishmaniasis: Experimental models for drug discovery
Reprint requests: Dr (Km.) Suman Gupta, Scientist ‘F’, Division of Parasitology, Central Drug Research Institute, Post Box No. 173, Lucknow 226 001, India e-mail: suman_gupta@cdri.res.in
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Visceral leishmaniasis (VL) or kala-azar is a chronic protozoan infection in humans associated with significant global morbidity and mortality. The causative agent is a haemoflagellate protozoan Leishmania donovani, an obligate intracellular parasite that resides and multiplies within macrophages of the reticulo-endothelial system. Most of the existing anti-leishmanial drugs have serious side effects that limit their clinical application. As an alternate strategy, vaccination is also under experimental and clinical trials. The in vitro evaluation designed to facilitate rapid testing of a large number of drugs has been focussed on the promastigotes milt little attention on the clinically relevant parasite stage, amastigotes. Screening designed to closely reflect the situation in vivo is currently time consuming, laborious, and expensive, since it requires intracellular amastigotes and animal model. The ability to select transgenic Leishmania expressing reporter proteins, such as the green fluorescent proteins (GFP) or the luciferase opened up new possibilities for the development of drug screening models. Many experimental animal models like rodents, dogs and monkeys have been developed, each with specific features, but none accurately reproduces what happens in humans. Available in vitro and in vivo methodologies for antileishmanial drug screening and their respective advantages and disadvantages are reviewed.
Keywords
Chemotherapeutic models
primates
rodents
screening assays
visceral leishmaniasis
Introduction
Leishmaniasis is a poverty – associated disease with several different forms, of which the two visceral leishmaniasis (VL) and cutaneous leishmaniasis (CL) are most common. VL is fatal without treatment. CL has a spectrum of presentations; typically with self healing or chronic lesions on the skin. Leishmania spp. is digenetic organisms shuttling between a flagellated promastigote in the gut of the sand fly vector and an intracellular amastigote in the mammalian host. Sand flies are blood feeders and the infectious metacyclic promastigotes are transmitted during a blood feeding meal. Promastigotes attach to mononuclear phagocytes and are taken up by phagocytosis into a phagosome, which fuse with lysosomes to form the phagolysosome. Once inside the macrophage, promastigote undergoes significant biochemical and metabolic changes and differentiate into the obligatory intracellular form of the parasite, the amastigote. Amastigotes are released from macrophages and can re-invade dendritic cells and fibroblasts as well as new macrophages. Over 350 million people are at risk of Leishmania infection, and at least 500,000 new cases of VL and 1.5 million cases of CL with severe morbidity are reported yearly1. Moreover, the rise of leishmaniasis is due to multiple factors including the AIDS epidemic, increase of international travel, lack of effective vaccines, difficulties in controlling vectors, international conflicts and the development of resistance to chemotherapy. The choice of drugs (pentavalent antimonials, amphotericin B, liposomal amphotericin B-Ambiosome, paromomycin, and miltefosine) has increased in the past decade, but there are numerous drawbacks to each of the treatments, such as difficulty to administer, length of treatment, toxicity, cost, and increasing parasitic resistance to treatment. Patients need a treatment which is oral, safe, effective, low cost, and short course (< 10 day course).
Efforts for the development of new therapeutics, essential for the control of leishmaniasis rely mainly on screening of potentially effective compounds in pathogen growth /multiplication assays, both in vitro and in vivo.
There are several in vitro and in vivo systems, each with specific characteristics, available for lead optimization. This review we will focuses on methodologies available for a direct drug screening procedure against the stage present in sand fly gut (promastigotes) and the mammalian stage of the parasite (amastigotes). Leishmania parasite can be grown in vitro as promastigotes and amastigotes in axenic conditions. Both these stages have been exploited for development of primary drug screening procedures. Also higher models used as in vivo models in the drug discovery against visceral leishmaniasis will be described.
In vitro system
The in vitro system may be of potential use for compounds, which have direct lethal action on parasite but compounds, which are effective through their metabolites, or their action is mediated through host defence system will not show any action. Therefore, in vitro testing at times may not be transferable to in vivo situation. However, in vitro drug testing has many advantages, e.g. (i) the parasites from a few animals are sufficient to test many compounds; (ii) the requirement of test compound is very minute; (iii) The turnover of screening results are quick; and (iv) the results are consistent.
Fortunately, in leishmaniasis a close correlation exists between the in vitro and in vivo results2, because the test parasite is the disease-producing organism in human (amastigote) and these are maintained in vitro as axenic amastigotes and in macrophage culture presenting a semi- in vivo condition.
In 1986, Croft3 outlined the requirements for an in vitro assay which include use of mammalian stage of the parasite, a dividing population, quantifiable and reproducible measures of drug activity, and activity of standard drugs in concentrations achievable in serum/tissues.
Recently, assay design has been focused on features that make the system adaptable to high throughput screening (HTS), with additional requirements of (i) small amounts of compound (<1 mg), (ii) quick throughput, and (iii) low cost of tests. However, the test results of in vitro system always need to be verified in animals.
Using promastigotes
The promastigotes (Fig. 1) grown in simple media have been used as test parasite to screen potential antileishmanial agents and the simplicity of this system accounts for its wide popularity. The simplest model to be utilized is the one in which the promastigotes multiply in cell free media4. For drug testing, promastigotes are diluted to a concentration of 1.0-2.0 × 106 per ml of cultivation medium and the drugs in appropriate concentrations are added to the experimental culture. The inhibition of promastigote multiplication is assessed after approximately 3 days, during which the control organisms multiply 3-6 times. The technique is simple and easily applicable.
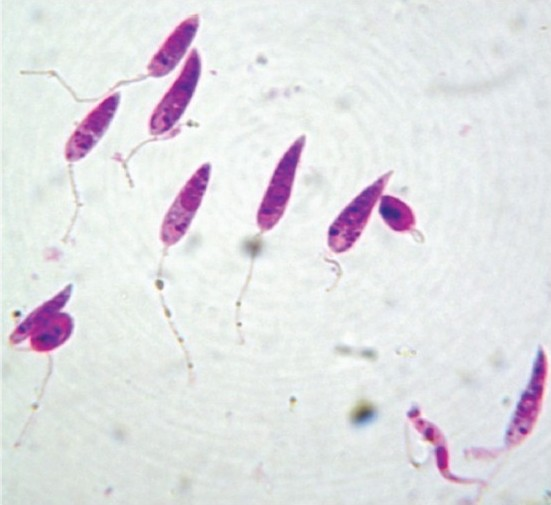
- Stained promastigotes (100 X under oil immersion lens).
However, the metabolism and ecology of promastigote differ so widely from those of amastigote (target form) that screening data obtained from in vitro test with promastigote have very little value in animals56. An other condition which reduces leishmanicidal action in vitro is lower temperature (24 °C) at which the culture normally grows, as opposed to the in vivo temperature of 37 °C. The promastigote in culture at 37 °C will survive but not multiply. Further, the promastigote culture represents an artificial situation and is of little or no value for drug screening. Due to these problems, the use of promastigote for drug testing has been abandoned.
Jackson et al7 developed an in vitro micro test for drug sensitivity, which is quantitative, rapid and readily applicable to parasites isolated from all major forms of human leishmaniasis as it uses promastigotes converted from amastigotes in vitro. The test is done in serum free chemically modified medium containing 120 μg protein/ml. They claimed that Leishmania sensitivity to pentavalent antimonials was detectable at levels below the concentrations achievable in patient’s sera.
Using amastigotes
Ideally to be efficient and exhaustive, a drug screening procedure requires conditions that mimic the environment encountered by the target cell. For Leishmania, intracellular form of the parasite (amastigotes) might represent the ideal conditions. The role played by the host cell on drug mediated toxicity could be important.
Axenic amastigotes
A direct comparison of the drug susceptibility towards standard antileishmnial drugs, between amastigotes and axenic amastigotes, demonstrates that the latter express specific susceptibility to many if, not all the drug tested8. Screening against axenic amastigotes (Fig. 2) presents several advantages; (i) the test is directed against the relevant stage of parasite, (ii) this stage is as easy to manipulate as the promastigote model, and (iii) quantification of drug activity is simple and often inexpensive.
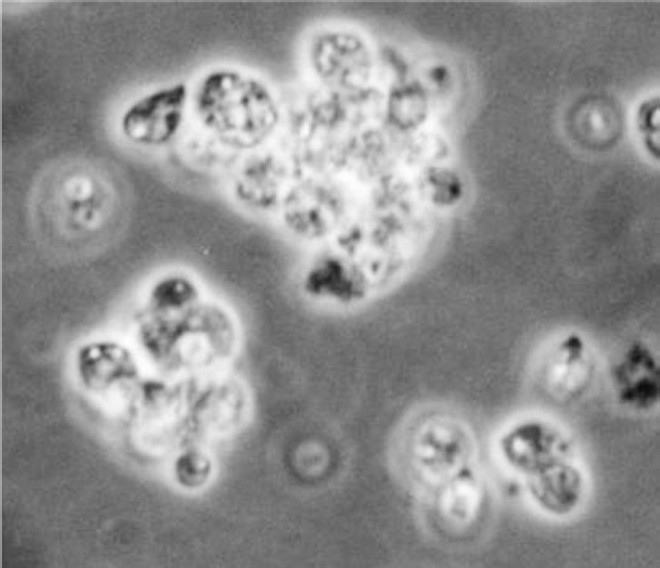
- Axenic amastigotes (100 X under oil immersion lens).
Axenic amastigotes system for drug screening has been used earlier8–10. Several investigators used different methods for evaluating activity of compound against axenic amastigotes such as viability of cell population with a 3-(4-5 dimethylthiazol–2– yl) – 2, 5 diphenyl tetrazolium bromide, Thiazole blue (MTT) based method1112, determining ornithine decarboxylase activity8 or using a fluorescent dye like propidium iodide (PI) and fluorescence-activated-cell-sorter (FACS)1314. Several Leishmania parasites expressing reporter genes have been selected and the capacity of some of them to be used in axenic amastigote drug screening protocol has been assessed (Table I). Sereno et al15 assessed luciferase expressing DNA transformed axenically grown L. infantum amastigotes and showed its use in high-throughput screening for new antileishmanial drugs.
| Strain | Reporter gene | Expression | Screening | Reference no. |
|---|---|---|---|---|
| L. donovani/L.donovaniR | Firefly luciferase | Episomal | Promastigotes Intramacrophagic | 52 |
| L. amazonensis | Firefly luciferase | Integration | Promastigotes Intramacrophagic | 56 |
| L.infantum/L.infantum RSblII | Firefly luciferase | Episomal | Intramacrophagic Axenic amastogotes | 15 |
| L. major | Firefly luciferase | Integration | ND | 38 |
| L. donovani | Firefly luciferase | Integration | ND | 38 |
| L. infantum | GFP | Episomal | Promastigotes | 97 |
| L. donovani | GFP | Episomal | Promastigotes | 36 |
| L. donovani R | Intramacrophagic | 40 | ||
| L. amazonensis | Multimeric GFP | Episomal | Promastigotes | 41 |
| L. amazonensis | eGFP | Episomal | Promastigotes | 39 |
| L. amazonensis | β-Galactosidase | Episomal | Promastigotes | 39 |
| L. amazonensis | B-Lactamase | Episomal | Intramacrophagic | 45 |
| L. major |
ND, not done; GFP, green fluorescent protein
Rapid fluorescent assay using Alamar Blue for screening drugs on axenic amastigotes of L. donovani and L. tropica was done recently by Shimony and Jaffe16. But the assay is semi – predictive, it neither tests for penetration of the compound into the host cell nor for activity in the peculiar environment of the macrophage phagolysosome. In addition, axenic amastigotes may have different metabolic processes than intracellular amastigotes. Also screening with axenic amastigotes from clinical isolates is not possible because they require time to get adapted in the cultures.
Intracellular amastigotes
The most widely used models for testing drugs against Leishmania species have involved either murine peritoneal macrophages or human-monocyte transformed macrophages (THP-1, U937, and HL-60) as host cells (Fig. 3). These models show species/strain variation in drug sensitivity1718. In these differentiated non-dividing macrophages, the rate of amastigote division in host cells and drug activity can be clearly assessed. The activity of test drug is measured by either microscopical counting of percentage of infected cells or number of amastigotes/macrophage19 or by colorimetric or fluorometric methods. The slow rate of division of L. donovani and L. infantum amastigotes in this model is a limitation. Assays that use dividing host cells must ensure that the confounding effects of drug activity on both parasite and host cell number are considered6.
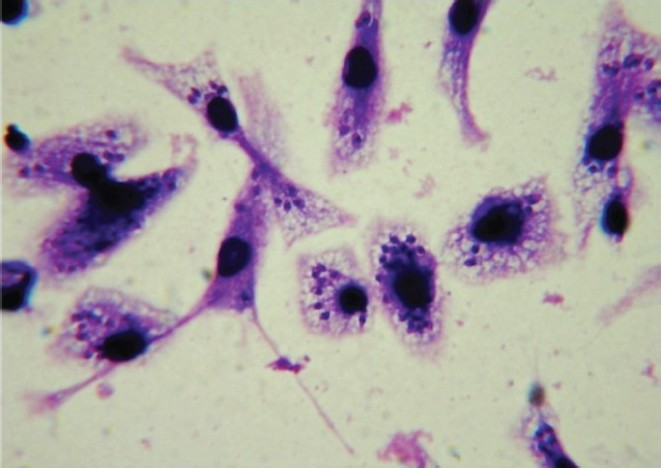
- Stained (with giemsa stain) infected macrophages bearing amastigotes (100 X under oil immersion lens).
Classical methods
Classical screening methods are labour intensive and do not support automation. Direct counting assays are used for evaluating drug activity towards intracellular amastigotes1019–25. Counting cells is time consuming labour intensive, subjective, and incompatible with high-throughput screening and may give inaccurate determination of IC50 since determination of the parasite viability through a staining procedure is difficult.
Reporter gene assays
A more efficient method for quantifying growth of intracellular Leishmania amastigotes would help to remove the drawbacks of current screening methods. The term reporter gene is used to define a gene with a readily measurable phenotype that can be distinguished easily over a background of endogenous proteins26. The use of a reporter gene to monitor intracellular proliferation of micro-organisms has been effectively applied for bacteria, viruses and other parasites27–29. Such methods produce objective quantitative data, increase throughput, and decrease manual labour. Several reporter genes have been effectively used in biological screens including green fluorescent protein (GFP), chloramphenicol acetyl transferase (CAT), β-galactosidase, firefly luciferase, and alkaline phosphatase30.
Various recombinant parasites carrying a reporter gene either as an episomal copy or after its integration in a defined locus, generally the rDNA locus against leishmaniasis are currently available (Table I).
GFP fluorescent assay:
GFP is an autofluorescent and stable protein, which originates from the jellyfish Aequorea victoria103132. GFP based assays offer several advantages over other non-reporter or reporter gene-based assays including greater simplicity, easier kinetic monitoring, low cost and enhanced biosafety33. Expression of GFP in several parasite species has been achieved and applied for drug evaluating studies10. GFP Leishmanial fusion proteins have been synthesized for localization and trafficking analysis34.
GFP expression in Leishmania was first achieved by Ha et al35. Since then its expression by episomal vector has been carried out in several species of Leishmania3637 wherein the fluorescence intensity in parasites decreased with time in the absence of geneticin sulphate (antibiotic G 418), thereby necessitating its regular addition38 (Table II). Okuno et al39 used the recombinant L. amazonensis expressing egfp or the beta-galactosidase gene in drug screening. Dube et al40 have also described the advantages of GFP based intramacrophagic amastigote screening assay using transfected field isolates of L. donovani strains.
| In vitro models | Merits | Demerits |
|---|---|---|
| Promastigote | Rapid method and very little amount of test compounds are required for screening. | Not relevant life cycle stage for mammalian leishmanial infection. |
| Data correlation with amastigote screening is unreliable. | ||
| Axenic amastigotes | Test is direct on relevant stage of the parasite. | The assay is semi – predictive. |
| This stage is as easy to manipulate as the promastigotes. | It neither tests for penetration of compound into host cell nor for activity in peculiar environment of the macrophage phagolysosome. | |
| Quantification of drug activity is simple and often inexpensive. | Different metabolic processes than intracellular amastigotes. Screening of axenic amastigotes from clinical isolates is not possible as they require time to get adapted in the cultures. | |
| Intracellular amastigotes | Effective screening method. | Labour intensive and subjective. |
| Mimic the environment encountered by the target cell. | Provide an approximation of the macrophages that are counted. Rendered difficult the screening of several drugs at a time and incompatible with HTS. | |
| Shows the effect of drug mediated toxicity on host cell. | ||
| Reporter gene assays: | ||
| (GFP) Green fluorescent protein | Simple. | Fluorescence intensity in parasites decreased with time in the absence of geneticin sulphate (antibiotic G 418), thereby necessitating its regular addition. |
| Easier kinetic monitoring. | Application for drug-drug screening is limited to promastigotes. | |
| Low cost and enhanced biosafety. | ||
| β -galactosidase | Colorimetric detection can be performed | Large size (the monomer is 116 kDa) |
| Low sensibility. | ||
| Endogenous expression of β-galactosidase by some mammalian cell types including macrophages. | ||
| β–lactamase | Simple colorimetric β-lactamase assay for quantifying Leishmania amastigotes grown in micotiter plates. | Not very sensitive. |
| High-level stable expression of the enzyme | ||
| Luciferase | The method is rapid. | The only drawback of this system is the use of expensive substrate and cell lysis buffer. |
| Very sensitive. | ||
| Highly reproducible. | Luminescent read out transient. Mixing of the samples and reagents needs to be timed with entering samples into the luminometer. | |
| Does not require any very specialized instrument or training. | ||
| Detection of only live, metabolically active cells by biphotonic imaging. | ||
| Absence of background activity in the host cell. | ||
| Compatible with HTS. | ||
| HTS, high throughtput screening; Source: Ref 56 | ||
Generally transfectants do not express sufficient levels of fluorescence for spectrofluorometric measurement on micro plate. To overcome this problem Chan et al41 have developed a spectrofluorometric assay wherein multimeric form of the GFP was engineered and expressed in L. amazonensis promastigotes. As expected, parasites expressing the multimeric GFP form bear fluorescence quantifiable in 96 wells with spectrofluorometric analysis. The integration of the GFP gene downstream of the 18 S rRNA gene promoters was done by Singh et al42 at the ribosomal locus within the genome of the parasite, which also represents a valuable tool for drug screening in macrophages.
Generally, methods that use catalytic reporter genes technology like luciferase, β-galactosidase, and β-lactamase are more sensitive than methods based on fluorescent proteins4344.
β - galactosidase
β-galactosidase presents the advantage that colorimetric detection can be performed. Okuno et al39 selected Leishmania Promastigotes expressing β-galactosidase and evaluated their use in drug screening procedures. Expression of lacZ in both promastigotes and amastigotes could be clearly visualized by fluorescence microscopy or by light microscopy with 5-bromo-4-chloro-3-indolyl- β-D-galactopyranoside (CPRG). Fluorescence signal and beta galactosidase activity measured by a colorimetric reaction with chlorophenol red beta D galactopyranoside. The inhibitory concentration (IC50) of a leishmanicidal drug, amphotericin B, in L. amazonensis promastigotes measured using La/lacZ was similar to that measured by conventional methods such as cell counting, thymidine incorporation and colorimetric assays. Further, the fluorescence signal and absorbance of CPRG correlated well with the numbers of Lz/lacZ amastigotes in macrophages, respectively, suggesting La/lacZ can be a convenient and useful tool for drug screening not only in promastigotes, but also in amastigotes of L. amazonensis. La/lacZ collected from mouse tissues four weeks after the parasite infection were stained well with X-Gal. La/lacZ allowed parasite detection at high sensitivity in the tissues of infected mice and will be useful for following infections in the macrophages in vivo. However, some commonly cited drawbacks of β-galactosidase include its large size (the monomer is 116 kDa), sensibility and the endogenous expression of β-galactosidase by some mammalian cell types including macrophages (Table II).
β–lactamase
To circumvent above shortcomings, a catalytic reporter system based on β-lactamase was developed. Two species of Leishmania: L. major and L. amazonensis expressing β-lactamase were engineered and overall, the antileishmanial results obtained demonstrate that this methodology could be valuable for drug screening procedures4546. A simple colorimetric β-lactamase assay for quantifying Leishmania amastigotes grown in microtiter plates has also been reported47. The β-lactamase gene was integrated into rRNA region of the genome, thereby allowing for high-level stable expression of the enzyme. Both visceral leishmaniasis and post- kala-azar dermal leishmaniasis isolates were transfected with β-lactamase gene. Results obtained demonstrate that this methodology could be a valuable high-throughput screening assay for checking efficacy of anti-leismanial drugs in the clinical isolates4647.
Luciferase assay
The luciferase reporter gene technology is being widely used to monitor cell growth and proliferation under in vitro culture systems and to monitor the cellular events associated with gene expression48 and signal transduction. The use of firefly luciferase reporter genes in a number of intracellular microorganisms including Mycobacterium tuberculosis49 has facilitated antimicrobial drug testing and discovery. The firefly luciferase50 represents one of the most efficient biological reporter molecules, which allows monitoring host-microbe interactions51, rapid testing of cellular viability, and thus is most suitable for biological screening. The method is rapid, very sensitive, and highly reproducible and does not require expensive specialized instrument or training. Main drawback of this system is the use of expensive substrate and lyses buffer (Table II).
Various species of Leishmania parasites expressing luciferase were recently developed and their susceptibility towards classical antileishmanial agents investigated153852. L. donovani cell lines expressing firefly luciferase reporter gene (luc.) have been developed as a part of episomal vector and suitability of these cell lines for in vitro screening of antileishmanial agents has been establised52. This system has been adapted to evaluate compounds in a 96 well micro plate format and is being employed53–55 for primary screening of novel synthetic compounds and marine extracts.
For assessing the activity of compounds against amastigote stage of the parasite, mouse macrophage cell line (J-774A.1) infected with promastigotes expressing luciferase firefly reporter gene is used5253. The main advantages of this technology include high sensitivity of the test and absence of background activity in the host cell.
Recently, a refined work performed by Lang and co-workers56 demonstrated that L. amazonensis parasites expressing firefly luciferase could be used to monitor Leishmania infection in real time, through imaging analysis. These parasites produced significant bioluminescent signals for both in vitro studies and the development of an in vivo model. First, a model was established, using parasite-infected mouse macrophages for rapidly determining the activity of drugs against intracellular amastigotes. Results indicated that recombinant Leishmania can be reliably and confidently used to monitor compounds acting on intracellular amastigote-harbouring macrophages. Secondly, temporal analyses were performed following inoculation of metacyclic promastigotes into the ear dermis of BALB/c mice and the bioluminescent light transmitted through the tissue was imaged externally using a charge coupled device (CCD) camera. Bioluminescent signals, measured at the inoculation site and in the draining lymph node of mice containing these parasites correlated well with the more classical quantification of parasites. This proves that the real-time bioluminescent assay is not only sensitive but also more rapid than culture-base techniques allowing monitoring parasite-load before any clinical signs of Leishmaniasis are detectable. In short, the luciferase imaging study is useful to monitor the efficacy of antiLeishmanial drugs on live cell culture and to trace Leishmanial infection in animal models.
Limitations
Reporter genes present several limitations (Table II). Cross-resistance conferred by the presence of the antibiotic resistance is one of those. Neomycin confers resistance toward paromomycin57. Development of a method to create defined mutants lacking selectable markers could help to overcome this problem58.
The way by which the reporter gene is introduced could also have an impact on the throughput of the screen. When reporters are part of plasmids, the relative output of reporter may depend on the copy number of the transfected plasmid (which vary from cell to cell) rather than on the activity of the drug. Secondly, transforming parasites could have biological consequences either by disrupting the genomic architecture or just by the presence of the foreign reporter gene product. Thirdly, for the β-galactosidase technology, the reporter could have by itself some limitations (i.e. sensibility, background activity from host macrophages) that make it inaccurate for an in vitro determination of drug activity against intracellular parasites10.
Multiplexing
A versatile methodology that allows for multiple quantifications of drug toxicity against both the host cells and the intracellular amastigotes (multiplexing) could represent a useful tool in the field of parasite pharmacology. To simultaneously gather information on the viability of the host cell and the parasite, working with a combination of parasites and macrophages expressing different reporters can be envisioned. To achieve this goal, reporters must use distinguishable signal from each other and compatible chemistries, like fluorophores emitting different wavelengths. Currently, there have been a growing number of examples using luminescence for multiplexing either in combination with: other luminescent signals, fluorescence or β–galactosidase assay5960. Such methods could also help to directly compare experiments since the results are expressed as a ratio of the output signal emitted by the host cell on the one emitted by the parasites. The usefulness of these approaches for drug screening need to be evaluated on intracellular parasites like Leishmania or T. cruzi10.
High- content/high- throughput screening for the discovery of new anti-leishmanial drugs
High content screening combined with high-throughput screening (HCS/HTS) and automated image analysis considerably speed up the drug discovery process and allow for the screening of a large number of compounds of single measurements of unknown samples to positive and negative control samples in complex phenotypic assays involving whole cells. As the prerequisite for HTS is large sample size, there is a need for statistical measurement of its effect size via Z factor. The Z-factor is defined in terms of four parameters: the means and standard deviations of both the positive and negative controls. Z-factors can never exceed 1 for ideal experiments61.
Siqueira-Neto et al62 with the aim to develop new anti-Leishmanials, have adapted L. donovani intra-macrophagic amastigote culture to a HCS/HTS assay as a cellular model for leishmaniasis. They optimized infection of the human macrophage cell line THP-1 by L. donovani metacyclic promastigotes in order to obtain very high yields of amastigotes-infected macrophages. The infected culture was seeded onto 384-well plates and incubated in the presence of serially diluted miltefosine or amphotericin B, as positive control, and in the absence of drugs, as negative control. After incubation period, parasites and cells were fixed, and DNA was stained with Draq 5 for reading in the automated confocal Opera.
In vivo assays
Animal models are expected to mimic the pathological features and immunological responses observed in humans when exposed to a variety of Leishmania spp. with different pathogenic characteristics. Many experimental models have been developed, each with specific features, but none accurately reproduces what happens in humans. For in vivo testing of new compounds several animal species have served as experimental host for VL. Important among them are BALB/c mice and Syrian golden hamster (primary tests), dogs (secondary tests) and monkeys viz., squirrel, vervet and Indian languor monkeys as tertiary screens (Table III). Animal models enable drug activity to be determined in relation to absorption (route of administration), distribution (different sites of infection), metabolism (pro-drugs, immuno-modulators), and excretion and to give an early indication of the toxicity. A suitable laboratory host for the target parasite (L. donovani) is very important from the point of view of conducting research on various aspects including host-parasite interactions, pathogenesis, biochemical changes, prophylaxis, and maintenance of parasites and above all evaluation of antileishmanial action of newer compounds for development of new drugs.
| Animal species | Examples | Main strength | Reference no. |
|---|---|---|---|
| Mice | BALB/c | Immunology, Vaccines, Chemotherapy | 67,70 |
| C57BL/6 | Negative model-Immunology, Vaccines, Chemotherapy | 63,65 | |
| Transgenic mice | Immunology | 87 | |
| Hamster | Syrian golden hamster | Pathology, Chemotherapy | 81,86 |
| Dogs | Different breeds | Pathology, Vaccine, Chemotherapy | 89,90,87 |
| Non human primates | Langurs, vervet monkey, rhesus monkey, mandrills, owl monkey, baboon, marmoset, squirrel, Sykes monkey | Vaccine, Pathogenesis, Chemotherapy, Pathology | 63,91,92,96,99 |
The aim of using the animal model is to find a drug that can be administered orally, be effective in a short course (< 10 days) and have no indication of toxicity at the highest doses tested (100 mg/kg).
Rodents models
Several attempts were made in the past to use small rodents for L. donovani infection. These includes hamster (European, Chinese and Syrian); mouse (BALB/c, NMRI, DBA/1, C57BL/6) rat, mastomys, squirrel, gerbil, etc63. Of the various animals tried, BALB/c mice and Syrian golden hamsters are the commonest and currently used animal models for drug and vaccine testing against VL. These models also facilitate pre-formulation design, pharmacokinetics and regulatory submission of novel chemical entit active compound64.
Mouse model
Murine models of leishmaniasis have been extensively used to study the pathogenesis of the disease and to test novel therapeutic and immunoprophylactic agents65, where a relatively low amount of compound is required. These are available as SPF and inbred strains enabling reproducible results with five animals per group. Mice are susceptible to most strains and species of Leishmania in both non-cure and self cure models6667.
Outbred mice are generally resistant to infection with L. donovani (visceral Leishmaniasis) but inbred strains of mice are widely used with susceptible, resistant and intermediate strains that share some similarities with human visceral Leishmaniasis. There is a generic basis for susceptibility to infection with L. donovani, based on the presence of Sc11 1a1 locus: mice with a wild type locus (CBA) have an earlier (more vigorous) parasite growth than those with the mutated Scl 1 1a1 locus (BALB/c and C57BL/6)68–70. The infection in each mouse strain needs to be characterized for each parasite strain used to ensure that drugs are tested appropriately. Athymic and SCID mice provide a model for treatment of VL in immunosuppressed cases6.
The BALB/c mouse is a commonly used strain, at 18-20 g, with highly reproducible levels of infection when an amastigote inoculum is administered intervenously. An assay in week one/two after infection examines the activity of the drug against the liver infection but not the spleen infection2571. Briefly, BALB/c mice (both sexes) are infected intravenously with 2 × 107 L. donovani amastigotes and randomly sorted into groups of five. For routine in vivo screening of newly synthesised compound/extracted plant materials are diluted three-fold to obtain three different dose levels. Mice are dosed (by ip or oral route) 7 days post-infection for five consecutive days and sacrificed 3 days after the completion of treatment (day 14 post-infection). Groups of mice are weighed before and after treatment, and the per cent weight change is recorded. Impression smears are prepared from weighed livers; methanol fixed, and stained with 10 per cent Giemsa stain in water. The number of amastigotes per 500 liver cell nuclei is determined and multiplied by the liver weight in milligrams to obtain Leishman-Donovan (LD) units. The per cent inhibition was calculated for all drug-treated groups in relation to a non treated group, and ED50 values are calculated by sigmoidal regression analysis using MicroSoftxlfit (ID Business Solution, Guildford, United Kingdom). For routine in vivo screening of newly synthesised/extracted synthetic compound, plant extract are diluted three-fold to obtain three different dose levels.
Real time GFP imaging of a murine leishmaniasis model
Mehta et al37 used a Leishmania mutant episomally transfected with enhanced green fluorescent protein, enabling in vivo real-time whole-body fluorescence imaging, to follow the progression of Leishmania infection in parasitized tissues. Fluorescence correlated with the number of Leishmania parasites in the tissue and demonstrated the real-time efficacy of a therapeutic vaccine. This approach provides several substantial advantages over currently available animal model systems for in vivo study of immunopathogenesis, prevention, and therapy of leishmaniasis. These include improvements in sensitivity and the ability to acquire real-time data on progression and spread of the infection.
Rat model
The cotton rat (Sigmodon hispidus) represents one of the most susceptible animal hosts for L. donovani72. The infection remains 3-4 months and after the appearance of initial clinical signs, the disease progresses rapidly leading to death of the host. Earlier investigetons7273 infected the African white tailed rat (Mastomys albicandatus) which proved to be an excellent host for in vivo maintenance and long term experiments with L. donovani and L. braziliensis. Nolan & Farrell75 used M. natalensis, a multi-mammate rat as an experimental model for L. donovani and L. Major, and Dwivedi et al76 successfully used this model for L. donovani.
Hamster model
Although many hamster species are susceptible to L. donovani infection77, the Syrian golden hamster (Mesocricetus auratus) establishes a good model for VL and provides a more synchronous infection in the liver and spleen that can develop into a chronic non-cure infection more similar to human VL637879.
For in vivo evaluation of chemotherapeutic agents generally the hamsters are infected intra-cardiacally. Many workers have chosen different days (day 1, 3 and 15) for initiation of drug testing. Duration of treatment (ranges from 5 to10 days) and autopsy days after treatment also differ80–82. A critical appraisal of the screening techniques by Gupta et al83 shows that none of the above is able to provide comprehensive information about the total efficacy of the potential drug. This is because the total effect of a drug depends on the parasites, and host immune system. Many drugs are known to act through the immune machinery of the host.
Methods described by Beveridge84 are more logical as the pre-treatment parasitic burden is assessed by spleen biopsy to select experimental animals carrying similar parasitic load. However, the animals are sacrificed on day 7 post-treatment. Major drawback of these methods is the inability to assess the delayed action of drugs.
Bhatnagar et al2 modified the technique where the delayed action of drugs can also be assessed conducting repeated spleen biopsies on the same animal at different intervals of day 7, 14, and 28, thus making it suitable for studying the sequential effects of drug in the model. This is more rational as it gives all information regarding cure and survival time of treated animals and allowed sufficient time to the host immunity to play, if any, a role2.
Gupta and Tiwari85 reported the suitability and susceptibility of inbred hamsters in terms of parasite establishment and longer survival period as compared to outbred hamsters. Dea-Ayuela et al86 studied its suitability and established suitable immunobiological parameters for in vivo testing of new antileishmanial compounds in the golden hamster model of visceral leishmaniasis. The clinico-pathological features of the hamster model of VL closely mimic active human disease. Systemic infection of the hamster with L. donovani results in a relentless increase in visceral parasite burden, progressive cachexia, hepatosplenomegaly, pancytopenia, hypergamma-globulinaemia and ultimately death78. The advantage is that biopsy is possible to monitor pre- and post treatment infection status and all antileishmanials are active against liver as well as spleen parasites. De-Oliveira et al87 demonstrated that the golden hamster is the best experimental model to study VL, because it reproduces the clinical and pathogenesis of the disease, as seen in humans and dogs. Unfortunately, the wide use of hamsters is still limited due to lack of available reagents such as antibodies to cell markers and cytokines.
Dog model
Dogs have been used as an experimental model for Leishmania infections since the beginning of the century and experimental infections have also been achieved with Leishmania spp. for which dog is not a natural reservoir, e.g., L. donovani from India88. The infection of dogs with L. infantum or L. chagasi is an important laboratory model because it reproduces the natural infection similar to human infections89. German shepherd dogs are reported to give better results than beagles90, but some workers claim highly successful infection rate with mixed breeds91.
Non-human primate model
Some of the observations made in rodent models might not be similar or relevant to human hosts due to distance in phylogeny. The development of a non-human primate model of leishmaniasis, which largely mimics the human situation, is described for studies of different aspects of the disease that would not be possible in humans for ethical reasons. This would also complement studies in other model systems. However, for financial and ethical reasons, the use of primates in biomedical research is limited. Studies involving these animals have, therefore, been tailored to solve questions that cannot be answered in other animals. Monkeys are normally the final experimental animals to be used in studies of the safety and efficacy of vaccines and drugs developed in other laboratory animals. Earlier efforts in establishing VL in New and Old World monkeys demonstrated that Aotus trivirgatus (owl monkeys)92 and Saimiri sciureus (squirrel monkey)93 developed an acute and fulminating, but short-lived, infection. AntiLeishmanial screening was performed in owl and squirrel monkeys. Old World monkeys such as Macaca sp. viz., M. mulatta, M. fascicularis and M. nemestrina, and African vervet monkeys developed low and/or inconsistent infections63. Attempts to establish VL in Presbytis entellus showed that this species was highly susceptible to single intravenous inoculation of hamster-spleen-derived L. donovani amastigotes, which invariably produced consistent and progressive acute fatal infection, leading to death between 110 to 150 days post-infection. The infected animals presented all the clinicoimmunopathological features as observed in human kala-azar9495. The Indian languor has also been used for preclinical evaluation of potential antileishmanial drugs and vaccine9697.
Concluding remarks
Screening on intramacrophagic model may give essential information on drug efficacy in the parasite natural environment. The classical methods of screening like direct counting assays using Giemsa stained chamber slides having infected macrophages (microscopical method) are time consuming, laborious and cumbersome, which limit their use for high throughput screening. Methodologies that increase the throughput of drug screening against intracellular parasites are coming up. The capacity of multiple gene reporter technologies to be used in multiplexing experiments have to be evaluated, as these may represent valuable tool in the pharmacology. For confirmation of in vivo activity of new compounds, several animal species have served as experimental host for VL. Important among them are BALB/c mice and Syrian golden hamster (primary tests), dogs (secondary tests) and monkeys viz., squirrel, vervet and Indian languor monkeys as tertiary screens99.
References
- World Health Organization. In: The leishmaniases and Leishmania/HIV co-infections. Geneva, Switzerland: World Health Organization; 2002.
- [Google Scholar]
- Exploration of antileishmanial activity in heterocycles; results of their in vivo and in vitro bioevaluations. Indian J Med Res. 1989;89:439-44.
- [Google Scholar]
- In vitro screens in the experimental chemotherapy of leishmaniasis and trypanosomiasis. Parasitol Today. 1986;2:64-9.
- [Google Scholar]
- Leishmania major: culture media, mouse strains, and promastigote virulence and infectivity. Exp Parasitol. 1984;57:269-73.
- [Google Scholar]
- Importance of parasite identification in case of leishmaniasis. J R Soc Med. 1983;76:540-2.
- [Google Scholar]
- Current scenario of drug development for 1eishmaniasis. Indian J Med Res. 2006;123:399-410.
- [Google Scholar]
- An in vitro micro method for drug sensitivity testing of leishmania. Am J Trop Med Hyg. 1989;41:318-30.
- [Google Scholar]
- An Axenic amastigote system for drug screening. Antimicrob Agents Chemother. 1997;41:818-22.
- [Google Scholar]
- Stage-specific activity of pentavalent antimony against Leishmania donovani axenic amastigotes. Antimicrob Agents Chemother. 1999;43:278-82.
- [Google Scholar]
- Advances and perspectives in Leishmania cell based drug-screening procedures. Parasitol Int. 2007;56:3-7.
- [Google Scholar]
- Use of an enzymatic micro method to quantify amastigote stage of Leishmania amazonensis in vitro. Parasitol Res. 1997;83:401-3.
- [Google Scholar]
- Development of a semi automated colorimetric assay for screening anti-Leishmanial agents. J Microbiol Methods. 2005;66:78-86.
- [Google Scholar]
- In vitro antiLeishmanial activity of nicotinamide. Antimicrob Agents Chemother. 2005;49:808-12.
- [Google Scholar]
- Stage specific antileishmanial activity of an inhibitor of SIR2 histone deacetylase. Acta Trop. 2005;94:107-15.
- [Google Scholar]
- DNA transformation of Leishmania infantum axenic amastigotes and their use in drug screening. Antimicrob Agents Chemother. 2001;45:1168-73.
- [Google Scholar]
- Rapid fluorescent assay for screening drugs on Leishmania amastigotes. J Microbiol Methods. 2008;75:196-200.
- [Google Scholar]
- Sensitivities of Leishmania species to hexadecylphosphocholine (miltefosine), ET-18-OCH (3) (edelfosine) and amphotericin B. Acta Trop. 2002;81:151-7.
- [Google Scholar]
- The sensitivity of clinical isolates of Leishmania from Peru and Nepal to Miltefosine. Am J Trop Med Hyg. 2005;73:272-5.
- [Google Scholar]
- An in-vitro system for determining the activity of compounds against the intracellular amastigote form of Leishmania donovani. J Antimicrob Chemother. 1984;14:463-75.
- [Google Scholar]
- Leishmania tropica: quantitation of in vitro activity of antiLeishmanial agents by Giemsa staining, viability and 3H-formycin B incorporation. J Parasitol. 1984;70:561-2.
- [Google Scholar]
- Activity of antileishmanial agents against amastigotes inhuman monocyte-derived macrophages and in mouser peritoneal macrophages. J Parasitol. 1984;70:220-5.
- [Google Scholar]
- An in vitro model for investigation of chemotherapeutic agents in leishmaniasis. J Infect Dis. 1980;142:83-6.
- [Google Scholar]
- Growth of Leishmania donovani amastigotes in the continuous human macrophages cell line U937: Studies on drug efficacy and metabolism. J Infect Dis. 1986;154:323-7.
- [Google Scholar]
- An in vitro model for screening antileishmanial drugs: the human leukaemia monocyte cell line, THP1. Acta Trop. 1992;51:237-45.
- [Google Scholar]
- In vitro and in vivo interactions between miltefosine and other antileishmanial drugs. Antimicrob Agents Chemother. 2006;50:73-9.
- [Google Scholar]
- Leishmania amastigotes as targets for drug screening. Kinetoplastid Biol Dis. 2006;5:6.
- [Google Scholar]
- Improved green fluorescent protein reporter gene-based micro plate screening for anti tuberculosis compounds by utilizing an acetamidase promoter. Antimicrob Agents Chemother. 2003;47:3682-7.
- [Google Scholar]
- Detection of HIV-I infection with a green fluorescent protein reporter system. J Acquir Immune Defic Syndr Hum Retroviral. 1996;13:308-13.
- [Google Scholar]
- Efficient technique for screening drugs for activity against Trypanosoma cruzi using parasites expressing β-galactosidase. Antimicrob Agents Chemother. 1996;40:2592-7.
- [Google Scholar]
- Reporter gene technology: the future looks bright. Biochem Pharmacol. 1999;58:749-57.
- [Google Scholar]
- Primary structure of the Aequorea victoria green - fluorescent protein. Gene. 1992;111:229-33.
- [Google Scholar]
- Green fluorescent protein (GFP): applications in cell-based assays for drug discovery. Drug Discov Today. 1999;77:57-64.
- [Google Scholar]
- Dissection of the functional domains of the Leishmania surface membrane 3‘-nucleotidase/nuclease, a unique member of the class I nuclease family. J Biol Chem. 2000;275:16366-72.
- [Google Scholar]
- Use of the green fluorescent protein as a marker in transfected Leishmania. Mol Biochem Parasitol. 1996;77:57-64.
- [Google Scholar]
- Fluorescent leishmania: application to anti-leishmanial drug testing. Am J Trop Med Hyg. 2004;71:400-2.
- [Google Scholar]
- Real-time in vivo green fluorescent protein imaging of a murine leishmaniasis model as a new tool for leishmania vaccine and drug discovery. Clinical Vacc Immunol. 2008;15:1764-70.
- [Google Scholar]
- Episomal and stable expression of the luciferase reporter gene for quantifying Leishmania spp. infections in macrophages and in animal models. Mol Biochem Parasitol. 2000;110:195-206.
- [Google Scholar]
- Applications of recombinant Leishmania amazonensis expressing egfp or the beta-galactosidase gene for drug screening and histopathological analysis. Exp Anim. 2003;52:109-18.
- [Google Scholar]
- Refractoriness to the treatment of sodium stibogluconate in Indian kala-azar field isolates persists in in vitro and in vivo experimental models. Parasitol Res. 2005;96:216-23.
- [Google Scholar]
- Micro plate assay for Leishmania amazonensis promastigotes expressing multimeric green fluorescent protein. Parasitol Res. 2003;89:266-71.
- [Google Scholar]
- Transgenic Leishmania donovani clinical isolates expressing green fluorescent protein constitutively for rapid and reliable ex vivo drug screening. J Antimicrob Chemother. 2009;64:370-4.
- [Google Scholar]
- Escherichia coli beta-galactosidase as an in vitro and in vivo reporter enzyme and stable transfection marker in the intracellular protozoan parasite Toxoplasma gondii. Gene. 1996;169:39-45.
- [Google Scholar]
- Realization of β-lactamase as a versatile fluorogenic reporter. Trends Biotechnol. 2005;22:208-11.
- [Google Scholar]
- Colorimetric assay for screening compounds against Leishmania amastigotes grown in macrophages. Am J Trop Med Hyg. 2005;72:600-5.
- [Google Scholar]
- Quantification of transcriptional and clonal selection of single living cells with β-lactamase as reporter. Science. 1998;279:84-8.
- [Google Scholar]
- High throughput screening of amastigotes of Leishmania donovani clinical isolates against drugs using colorimetric β-lactamase assay. Indian J Exp Biol. 2009;47:475-9.
- [Google Scholar]
- Reporter gene expression for monitoring gene transfer. Curr Opin Biotechnol. 1997;8:617-22.
- [Google Scholar]
- Rapid assessment of drug susceptibilities of Mycobacterium tuberculosis by means of Luciferase Reporter Phages. Science. 1993;260:819-22.
- [Google Scholar]
- Firefly luciferase as a tool in molecular and cell biology. Anal Biochem. 1988;175:5-13.
- [Google Scholar]
- Probing bacterial gene expression within host cells. Trends Microbiol. 1997;5:360-3.
- [Google Scholar]
- Use of Leishmania donovani field isolates expressing the luciferase reporter gene in in vitro drug screening. Antimicrob Agents Chemother. 2005;49:3776-83.
- [Google Scholar]
- Chemotherapy of leishmaniasis Part V: Synthesis and in vitro bio evaluation of novel pyridinone derivatives. Euro J Med Chem. 2006;42:669-74.
- [Google Scholar]
- Synthesis of marine alkaloid: 8, 9-dihydrocoscinamide B and its analogues as novel class of antileishmanial agents. Bioorg Med Chem Lett. 2007;17:4075-79.
- [Google Scholar]
- Synthesis and antileishmanial activity of novel 2,4,6-trisubstituted pyrimidines and 1,3,5-triazines. Europ J Med Chem. 2009;44:2473-81.
- [Google Scholar]
- Bioluminescent Leishmania expressing luciferase for rapid and high throughput screening of drugs acting on amastigote-harbouring macrophages and for quantitative real-time monitoring of parasitism features in living mice. Cell Microbiol. 2005;7:383-92.
- [Google Scholar]
- Extra chromosomal inheritance of paromomycin resistance in Saccharomyces cerevisiae. Mol Genet Genomics. 1973;125:91-8.
- [Google Scholar]
- Generation of Leishmania mutants lacking antibiotic resistance genes using a versatile hit-and-run targeting strategy. FEMS Microbiol Lett. 2004;235:89-94.
- [Google Scholar]
- Multiplexing nuclear receptors for agonist identification in a cell based reporter gene high-throughput screen. J Biomol Screen. 2003;8:239-46.
- [Google Scholar]
- Yeast-based screening for inhibitors of RGS proteins. Methods Enzymol. 2004;389:277-301.
- [Google Scholar]
- http://en.wikipedia.org/wiki/z_factor, accessed on…
- Antileishmanial high-throughput drug screening reveals drug candidates with new scaffolds. PLoS Negl Trop Dis. 2010;4:e675.
- [Google Scholar]
- Experimental models for leishmaniasis and for testing anti-leishmanial vaccines. Ann Trop Med Parasitol. 1995;89(Suppl. 1):55-73.
- [Google Scholar]
- Liposomal amphotericin B in the treatment of visceral leishmaniasis. J Antimicrob Chemother. 1991;28:111-8.
- [Google Scholar]
- Immuno-enhancement combined with amphotericin B as treatment for experimental visceral leishmaniasis. Antimicrob Agents Chemother. 2003;47:2513-7.
- [Google Scholar]
- Experimental cutaneous leishmaniasis: a powerful models to study in vivo the mechanisms underlying genetic differences in Th subset differentiation. Eur J Dermatol. 2002;12:316-8.
- [Google Scholar]
- Intradermal inoculations of low doses of Leishmania major and Leishmania amazonensis metacyclic promastigotes induce different immuno-parasitic processes and status of protection in BALB/c mice. Int J Parasitol. 2003;33:1373-83.
- [Google Scholar]
- Letter. Genetic control of natural resistance to Leishmania donovani. Nature. 1974;250:353-4.
- [Google Scholar]
- Genetic susceptibility to Leishmanial infections: studies in mice and man. Parasitology. 1996;112:S67-74.
- [Google Scholar]
- Variation in susceptibility of mouse strains to Leishmania donovani infection. Trans R Soc Trop Med Hyg. 1972;66:527-8.
- [Google Scholar]
- Mystromys albicaudatus, the African white-tailed rat, as an experimental host for Leishmania donovani. J Parasitol. 1973;59:1085-7.
- [Google Scholar]
- Experimental infection of Mystromys albicaudatus with Leishmania braziliensis: pathology. Am J Trop Med Hyg. 1980;29:753-60.
- [Google Scholar]
- Experimental infections of the multi-mammate rat (Mastomys natalensis) with Leishmania donovani and Leishmania major. Am J Trop Med Hyg. 1987;36:264-9.
- [Google Scholar]
- Comparative susceptibility of Mastomys natalensis and golden hamsters (Mesocricetus auratus) to Leishmania donovani. Presented in the symposium on Fifth National Congress of Parasitology 1983 June 25-27, Tirupati, India
- [Google Scholar]
- The experimental transmission of leishmaniasis to animals. Proc Soc Exp Bio Med. 1924;21:354-6.
- [Google Scholar]
- Leishmania donovani: acquired resistance to visceral leishmaniasis in the golden hamster. Exp Parasitol. 1976;40:89-94.
- [Google Scholar]
- Comparison of T-cell responses in self-limiting versus progressive visceral Leishmania donovani infections in golden hamsters. Infect Immun. 1989;57:3091-6.
- [Google Scholar]
- An eight day method for screening of compounds against Leishmania donovani in the golden hamster. J Protozool. 1958;5:269-73.
- [Google Scholar]
- Leishmania donovani: therapeutic and prophylactic action of antimony dextran glycoside (RL-712) in the golden hamster. Exp Parasitol. 1975;37:348-52.
- [Google Scholar]
- Testing of drugs for antileishmanial activity in golden hamsters infected with Leishmania donovani. Int J Parasitol. 1977;7:443-7.
- [Google Scholar]
- Antileishmanial drug testing: Appraisal on existing techniques. Indian J Parasitol. 1992;16:1-7.
- [Google Scholar]
- Chemotherapy of leishmaniasis. In: Schnitzer RJ, Hawking F, eds. Experimental chemotherapy. Vol vol.1. New York: Academic Press; 1963. p. :257-80.
- [Google Scholar]
- Leishmania donovani infected inbred Golden Hamster as experimental model for visceral leishmaniasis. J Parasitic Dis. 2000;24:211-3.
- [Google Scholar]
- Setting new immuno-biological parameters in the hamster model of visceral leishmaniasis for in vivo testing of antileishmanial compounds. Vet Res Commun. 2007;31:703-17.
- [Google Scholar]
- Animal models for infectious diseases caused by parasites: Leishmaniasis. Drug Dis Today: Dis Mod. 2004;1:81-6.
- [Google Scholar]
- Antileishmanial activity of selected compounds in dogs experimentally infected with Leishmania donovani. Rev Inst Med Trop Sao Paulo. 1979;21:189-93.
- [Google Scholar]
- Leishmaniases in the Mediterranean “Midi”: results of an ecologic survey. Bull Soc Pathol Exot Filiales. 1969;62:332-3.
- [Google Scholar]
- Visceral leishmaniasis in the German shepherd dog. I. Infection, clinical disease, and clinical pathology. Vet Pathol. 1984;21:74-9.
- [Google Scholar]
- An experimental model for canine visceral leishmaniasis. Parasite Immunol. 1991;13:537-50.
- [Google Scholar]
- Toxicity and efficacy of the antileishmanial drug meglumine antimoniate in the owl monkey (Aotus trivirgatus) J Parasitol. 1983;69:1176-7.
- [Google Scholar]
- Visceral leishmaniasis in the squirrel monkey (Saimiri sciurea) J Parasitol. 1981;67:740-1.
- [Google Scholar]
- The Indian langur: preliminary report of a new nonhuman primate host for visceral Leishmaniasis. Bull World Health Organ. 1992;70:63-72.
- [Google Scholar]
- Leishmania donovani: cellular and humoral immune responses in Indian languor monkey Presbytis entellus. Acta Trop. 1999;73:37-48.
- [Google Scholar]
- Vaccination of languor monkeys (Presbytus entellus) against Leishmania donovani with autoclaved L major plus BCG. Parasitology. 1998;116:219-22.
- [Google Scholar]
- Successful vaccination against Leishmania donovani infection in Indian languor using alum-precipitated autoclaved Leishmania major with BCG. Vaccine. 2001;19:3485-92.
- [Google Scholar]
- Expression of green fluorescent protein as a marker for effect of antileishmanial compounds in vitro. Antimicrob Agents Chemother. 2001;45:3654-6.
- [Google Scholar]
- Animal models for vaccine studies for visceral leishmaniasis. Indian J Med Res. 2006;123:439-54.
- [Google Scholar]






