Translate this page into:
Use of succinic & oxalic acid in reducing the dosage of colistin against New Delhi metallo-β-lactamase-1 bacteria
For correspondence: Dr Madasamy Parani, Department of Genetic Engineering, SRM University, Kattankulathur, Chennai 603 203, Tamil Nadu, India e-mail: parani.m@ktr.srmuniv.ac.in
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
New Delhi metallo-β-lactamase 1 (NDM-1) cleaves the beta-lactam ring, and confers bacterial resistance against most of the beta-lactam antibiotics, except tigecycline and colistin. Among these two antibiotics, colistin is considered toxic, and therefore, its clinical use and dosage need cautious approach. In the present study, six organic acids were screened individually and in combination of two acids for their effectiveness against NDM-1 Escherichia coli and a combination of colistin and oxalic or succinic acid was tested to find out the potential of combination therapy for reducing the dose of toxic colistin.
Methods:
Antibacterial activity of the organic acid and their combinations was tested by disc diffusion method against NDM-1 E. coli, and minimum inhibitory concentration (MIC) was determined by broth dilution method. Synergistic effect between organic acids and colistin was tested by checkerboard method.
Results:
Oxalic acid showed the highest zone of inhibition (15±1 mm) followed by succinic acid, tartaric acid, fumaric acid, citric acid and malic acid. The combination of two acids did not increase the zone of inhibition significantly. MIC was found to be the lowest with oxalic acid and succinic acid (320 μg/ml). In the presence of 160 μg/ml oxalic acid or succinic acid, MIC of colistin was reduced from 8 to 4 μg/ml, indicating synergistic effect.
Interpretation & conclusions:
Our findings showed that combination therapy using colistin and oxalic acid or succinic acid might find safe clinical application of this antibiotic in controlling infections due to NDM-1 bacteria.
Keywords
Antimicrobial activity
Escherichia coli
inhibition zone
minimum inhibitory concentration
New Delhi metallo-β
lactamase-1
organic acid
Prevalence of multidrug-resistant bacteria limits the effectiveness of almost all the antibiotics and causes public health concern worldwide1. Even though pharmaceutical industries have developed several antibiotics in the past, bacterial resistance to these drugs is increasing. Multidrug-resistant bacteria have evolved due to the indiscriminate use of broad-spectrum antimicrobial drugs for the treatment of various infectious diseases2. Infections due to such multidrug-resistant bacteria are highly challenging to control using the existing antibiotics3. Bacteria not only acquire resistance to the drugs but also have the ability to transmit the same to other bacteria4. The β-lactams antibiotics such as penicillins, cephalosporins, carbapenems and monobactams are an important class of antibiotics for treating the infections by multidrug-resistant bacteria5. Strains of Escherichia coli and Klebsiella pneumonia, which are highly resistant to all antibiotics, except tigecycline and colistin, were identified from hospital-derived infections6. These strains produced metallo-β-lactamase (MBL) enzyme but were negative for the previously known MBL genes. A new MBL enzyme encoded by blaNDM-1 gene, which shared very little identity with other MBLs, was found in these strains, and was named as New Delhi metallo-β-lactamase 1 (NDM-1)7.
Several antimicrobial compounds have been tested against NDM-1 bacteria. Captopril with the 3-mercapto-2-methylpropanoyl fragment and a proline residue effectively inhibited NDM-1 with an IC50 value of 7.9 μM8. One of the captopril derivatives and its structural analogue were also found to inhibit NDM-1 with an IC50 value of 15 and 10 μM, respectively. The captopril structural analogue compound, being a clinically used antidote for metal poisoning, was proposed as a potential safe chemical to treat bacterial infections due to NDM-1 bacteria9. Aspergillomarasmine A, a fungal natural product, was found to inhibit the activity of NDM-1 and restore the activity of meropenem against Enterobacteriaceae, Acinetobacter sp. and Pseudomonas sp10.
Among the 109 antibacterial drugs that were approved from 1981 to 2010, 69 per cent were derived from natural products11. Organic acids have played a significant role in food preservation by their ability to control microbial growth12. These acids may act by acidification of the cytoplasm or accumulation of the dissociated acid anions to a toxic level13. Antimicrobial effect of the organic acids is mainly associated with the ratio of undissociated forms and reduction in pH14. Microbial toxicity of organic acids is based on the export of protons and depletion of the energy of microbial cells. Organic acids may damage outer or cytoplasmic membrane, which inhibits the macromolecular synthesis or denature the nucleic acids and proteins15. Less direct antibacterial activity of organic acids includes interference with nutrient transport, cytoplasmic membrane damage resulting in leakage, disruption of the outer membrane and influencing macromolecular synthesis1617. Benzoic acid and ascorbic acid have been reported as active inhibitors of bacterial growth18. Acetic acid, fumaric acid, propionic acid and lactic acid have been shown to significantly delay the microbial growth1920. Organic acids such as acetic acid, citric acid and lactic acid were found to inhibit Shigella species of bacteria effectively21. Salmonella species in meat and poultry products, which are responsible for salmonellosis, could be controlled using acetic acid, citric acid, lactic acid, propionic acid, succinic acid, tartaric acid, and malic acid22. In this study, antimicrobial property of citric acid, fumaric acid, malic acid, oxalic acid, succinic acid and tartaric acid was tested against NDM-1 E. coli. Subsequently, combination of colistin and oxalic acid or succinic acid was studied to explore the potential of combination therapy for reducing the dosage of colistin for its safe clinical use.
Material & Methods
The study was conducted in the Genomics laboratory of SRM University, Chennai, India, during January to July 2016.
NDM-1-positive E. coli strain (NDM-1 E. coli) was obtained from Dr David Livermore, UK (through Dr Karthikeyan Kumarasamy, Department of Microbiology, Institute of Basic Medical Sciences, Taramani, Chennai). DNA sequencing of 16S rDNA and NDM-1 gene was done to verify the strain. Nutrient broth and Mueller-Hinton agar and MacConkey agar were purchased from HiMedia Laboratories Pvt. Ltd., Mumbai, India. The strain was sub-cultured on MacConkey agar plates and maintained at 4°C. Citric acid was purchased from Sigma, USA; L-malic acid, fumaric acid, succinic acid, oxalic acid, L-tartaric acid and dimethyl sulphoxide were purchased from HiMedia.
Determination of antimicrobial activity: Antibacterial activity of the organic acid and their combinations was tested using the disc diffusion method23. Organic acids (1.0 mg/disc) were applied on 6 mm sterile discs and placed on the agar plates, which were inoculated with NDM-1 E. coli. The plates were incubated at 37°C for overnight (16 h); the zone of inhibition was measured in millimetres to determine the antimicrobial activity. Colistin (Sigma-Aldrich, USA) was used as positive control at a concentration 10 μg/disc.
Determination of minimum inhibitory concentration (MIC): Minimum inhibitory concentration of the organic acids alone and in combination with colistin against NDM-1 E. coli was determined by broth dilution method24. Briefly, organic acids (1.0 mg/ml) were serially diluted with the nutrient broth and inoculated with 1.0×108 colony-forming units (cfu)/ml of NDM-1 E. coli. The plates were incubated at 37°C for 24 h. MIC was calculated as the lowest acid concentration at which no growth of NDM-1 E. coli was observed.
Synergistic effect of organic acids with antibiotic: The bacterial cultures were grown to OD 0.5 in nutrient broth at 37°C, and used for determining the synergistic effect between organic acids and colistin. The least MIC value of organic acids (i.e. 160 μg/ml) was considered and tested in combination with colistin, which was serially diluted from its MIC (8 μg/ml).
Checkerboard method: Standard forms of succinic acid and oxalic acid were freshly prepared. The stock solutions and serial two-fold dilutions of each drug to at least double the MIC were prepared according to the recommendations of Clinical and Laboratory Standards Institute (CLSI) immediately before testing25. A total of 50 μl of Mueller-Hinton broth was distributed into each well of the microdilution plates. The first combination of organic acid was serially diluted along the ordinate, while the antibiotics were diluted along the abscissa. An inoculum of 0.5 McFarland turbidity was prepared from NDM-1 E. coli isolate in Mueller-Hinton broth. Each microtitre well was inoculated with 3 μl of a bacterial inoculum of 5×105 cfu/ml, and the plates were incubated at 37°C for 48 h under aerobic conditions. The resulting checkerboard contains each combination of organic acids and antibiotics, with tubes that contain the highest concentration of each antibiotic at opposite corners.
Statistical analysis: All the experiments were performed in triplicates. Statistical analysis was done by one-way ANOVA, and P<0.05 was considered as significant.
Results
Antimicrobial activity of citric acid, fumaric acid, malic acid, oxalic acid, succinic acid and tartaric acid was tested individually and in a combination of two acids against NDM-1 E. coli using colistin as positive control. All the acids showed a clear zone of inhibition, and three acids showed a zone of inhibition larger than that observed with colistin. For quantitative assessment, the experiment was done in triplicates, and the zone of inhibition was analyzed. Oxalic acid, succinic acid and tartaric acid at a concentration of 1.0 mg/disc showed a significantly (P<0.05) larger zone of inhibition when compared to colistin at a concentration of 10 μg/disc. Effect of the combination of two organic acids was studied by combining 0.5 mg/disc of each organic acid to achieve a total organic acid concentration of 1.0 mg/disc. The zone of inhibition for the combination of two organic acids was found to be not significantly different when compared with either of the individual organic acids present in the combination. This indicated the absence of additive, synergistic or antagonistic effect between the acids that were tested in combinations.
MIC for the organic acids against NDM-1 E. coli ranged between 320 and 640 μg/ml. Oxalic acid and succinic acid showed the lowest MIC of 320 μg/ml. These two acids were individually combined with colistin to understand the effect of the combinations. Half the MIC of oxalic acid or succinic acid (160 μg/ml) was combined with MIC of colistin (8 μg/ml), and MIC for the combinations was determined by broth dilution method. In colistin plus oxalic acid as well as colistin plus succinic acid combinations, the MIC of colistin was reduced from 8 to 4 μg/ml, which indicated the synergistic effect between the acids and colistin against NDM-1 E. coli. Synergy between the organic acids and colistin was also evaluated using checkerboard assay. Complete growth inhibition of NDM-1 E. coli was observed in 0.5 and 2.0 μg/ml colistin when it was combined with 320 and 80 μg/ml organic acids, respectively Table.
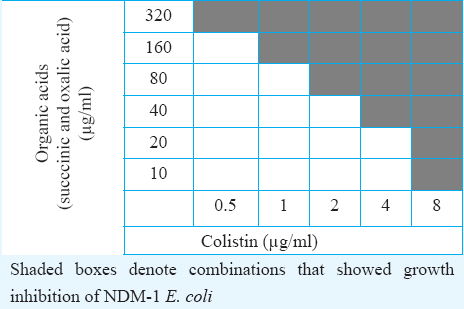
Discussion
NDM-1 bacteria are resistant to most of the current generation of antibiotics, except colistin and tigecycline. Use of colistin was abandoned in the 1970s due to nephrotoxicity but it re-emerged as life-saving antibiotic to combat multidrug-resistant NDM-1 bacteria2627. However, its clinical application requires extreme caution and careful consideration of safety and efficacy28. Therefore, there is an urgent need to identify new and safe antimicrobial compounds, which can control the growth of NDM-1 bacteria. Organic acids are known for their antimicrobial activity and therefore, are regularly used in food preservation1529. Synergistic effect of antibiotics has been shown to reduce the usage and toxicity of antibiotics. In this study, the dosage of colistin was drastically reduced from 8 to 0.5 μg/ml by its synergistic action with organic acids, which in turn could decrease the toxicity of colistin during treatment. Further delivery modes and evaluation of dosage are the future prospects for safer treatments. Organic acids have been reported to control the growth of several bacterial species with varying levels of efficiency30. Since organic acids are naturally present in the fruits and vegetables, these are suitable for human use without any concern about toxicity.
In conclusion, the present study demonstrated that organic acids, which naturally prevent bacterial growth, could be a source to reduce the antibiotic usage. A possibility of combination therapy using colistin and oxalic or succinic acid is indicated by the synergistic effect between the antibiotics and organic acids against NDM-1 bacteria, which may help in the control of misuse of antibiotics in the future.
Acknowledgment
Authors thank the SRM University, Chennai, for providing the well-equipped laboratory facility.
Financial support & sponsorship: This study was funded and supported by the Indian Council of Medical Research (ICMR), New Delhi, India (AMR/22/2011-ECD-I).
Conflicts of Interest: None.
References
- Antibacterial activities of selected edible plants extracts against multidrug-resistant gram-negative bacteria. BMC Complement Altern Med. 2013;13:164.
- [Google Scholar]
- Antibacterial activity of plant extracts and phytochemicals on antibiotic-resistant bacteria. Braz J Microbiol. 2000;31:247-56.
- [Google Scholar]
- Antibiotics and bacterial resistance in the 21st century. Perspect Medicin Chem. 2014;6:25-64.
- [Google Scholar]
- Characterization of a new metallo-β-lactamase gene, blaNDM-1, and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother. 2009;53:5046-54.
- [Google Scholar]
- Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: A molecular, biological, and epidemiological study. Lancet Infect Dis. 2010;10:597-602.
- [Google Scholar]
- A structural view of the antibiotic degradation enzyme NDM-1 from a superbug. Protein Cell. 2011;2:384-94.
- [Google Scholar]
- Simplified captopril analogues as NDM-1 inhibitors. Bioorg Med Chem Lett. 2014;24:386-9.
- [Google Scholar]
- Aspergillomarasmine A overcomes metallo-β-lactamase antibiotic resistance. Nature. 2014;510:503-6.
- [Google Scholar]
- Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75:311-35.
- [Google Scholar]
- Alternatives to traditional antimicrobials for organically processed meat and poultry. In: Ricke SC, ed. Organic meat production and processing. Ames: Iowa State University Press; 2012. p. :211-30.
- [Google Scholar]
- Perspectives on the use of organic acids and short chain fatty acids as antimicrobials. Poult Sci. 2003;82:632-9.
- [Google Scholar]
- Effect of short-chain organic acids on macromolecular synthesis in Escherichia coli. J Bacteriol. 1990;68:69-74.
- [Google Scholar]
- Lactic acid permeabilizes gram-negative bacteria by disrupting the outer membrane. Appl Environ Microbiol. 2000;66:2001-5.
- [Google Scholar]
- Preservatives antimicrobial agents. A mean toward products stability. Food Technol. 1986;40:104-11.
- [Google Scholar]
- Lactic acid inhibition of the growth of spoilage bacteria and cold tolerant pathogens on pork. Int J Food Microbiol. 1995;25:141-51.
- [Google Scholar]
- Inhibition of Listeria monocytogenes and Escherichia coli O157:H7 on beef by application of organic acids. J Food Prot. 1996;59:370-3.
- [Google Scholar]
- Antimicrobial activities of acetic acid, citric acid and lactic acid against Shigella species. J Food Saf. 2013;33:79-85.
- [Google Scholar]
- Organic acids as antimicrobials to control Salmonella in meat and poultry products. Food Res Int. 2011;10:1016-25.
- [Google Scholar]
- Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45:493-6.
- [Google Scholar]
- Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc. 2008;3:163-75.
- [Google Scholar]
- Clinical and Laboratory Standards Institute: Performance standards for antimicrobial susceptibility testing: Twenty-fourth information supplement, M100-S24. . 2014;34 No.1
- [Google Scholar]
- Toxicity of polymyxins; a systematic review of the evidence from old and recent studies. Crit Care. 2006;10:R27.
- [Google Scholar]
- Breakthrough bacteraemia due to tigecycline-resistant Escherichia coli with New Delhi metallo-beta-lactamase (NDM)-1 successfully treated with colistin in a patient with calciphylaxis. J Antimicrob Chemother. 2011;66:2677-8.
- [Google Scholar]
- Strategies for the safe use of colistin. Expert Rev Anti Infect Ther. 2015;13:1237-47.
- [Google Scholar]
- Susceptibility of Escherichia coli, Salmonella sp. and Clostridium perfringens to organic acids and monolaurin. Vet Med. 2006;51:81-8.
- [Google Scholar]
- Organic acids: chemistry, antibacterial activity and practical application. Adv Microb Physiol. 1991;32:87-108.
- [Google Scholar]






