Translate this page into:
Use of first line antiretroviral therapy from a free ART programme clinic in Pune, India - A preliminary report
Reprint requests: Dr Manisha V. Ghate, Scientist D, National AIDS Research Institute (ICMR) Post Box 1895, G-73, MIDC, Bhosari, Pune 411 026, India e-mail: mghate@nariindia.org
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
The treatment outcomes under national antiretroviral therapy (ART) programme are being evaluated in some ART centres in the country. We carried out this study to analyze the impact of first line antiretroviral therapy in HIV infected patients attending a free ART roll out national programme clinic in Pune, India.
Methods:
Antiretroviral naive HIV infected patients attending the clinic between December 2005 and April 2008 and followed up till March 31, 2011 were included in the analysis. The enrolment and follow up of these patients were done as per the national guidelines. Viral load estimations were done in a subset of patients.
Results:
One hundred and forty two patients with median CD4 count of 109 cells/μl (IQR: 60-160) were initiated on treatment. The median follow up was 44 months (IQR: 37-53.3 months). Survival analysis showed that the probability of being alive at the end of 5 years was 85 per cent. Overall increase in the median CD4 count was statistically significant (P<0.001). It was significant in patients with >95 per cent adherence (P<0.001). In 14 per cent patients, the absolute CD4 count did not increase by 100 or more cells/μl at the end of 12 months. Viral load estimation in a subset of 68 patients showed undetectable levels in 61 (89.7%) patients after a median duration of 46 months (IQR: 38.3-54.8).
Interpretation & conclusions:
The first line treatment was effective in patients attending the programme clinic. The adherence level influenced immunological and virological outcomes of patients.
Keywords
AIDS
ART Centre
CD4 counts
HIV
viral load
The benefits of highly active antiretroviral therapy (HAART) have been well documented in the literature globally123 and free antiretroviral therapy (ART) programmes have been rolled out in many countries456. India has an estimated population of 2.39 million HIV infected individuals with an adult prevalence of 0.31 per cent7. The free ART programme was rolled out on April 1, 2004. As on March 2012, a total of 355 ART Centres are functional in 31 States and Union Territories and more than 5,16,000 patients are receiving antiretroviral treatment (ART) at these centres8. Several investigators have studied the treatment response of ART at individual ART centres910. Evaluation of ART roll out programme carried out in south11 and north India9 has documented good clinical response to therapy, as indicated by significant weight gain and improvement in CD4 count especially for patients with higher baseline CD4 count1011. Low retention rate, low adherence and drug resistance are some of the concerns identified in these studies.
Here we report the evaluation findings of effectiveness of national programme in HIV infected patients attending the free ART roll out programme clinic at National AIDS Research Institute (NARI) in Pune, India.
Material & Methods
Study site: The National AIDS Control Organization (NACO) extended free ART roll out programme in December 2005 to one of the NARI clinics at Pune to provide care and support to HIV infected participants from NARI research studies who are eligible to receive free ART as per the National guidelines12. All antiretroviral treatment naive adult patients registered between December 2005 and April 2008 were included in the analysis.
Patients and clinical services: Initially, baseline investigations of HIV infected patients were done as per the national treatment guidelines12 and the eligible patients were initiated on one of the following combination of first-line regimens after obtaining their consent.
I. Zidovudine (300 mg) + lamivudine (150 mg) + nevirapine (200 mg)
I (a) Stavudine (30 mg) + lamivudine (150 mg) + nevirapine (200 mg)
II. Zidovudine (300 mg) + lamivudine (150 mg) + efavirenz (600 mg)
II (a) Stavudine (30 mg) + lamivudine (150 mg) + efavirenz (600 mg)
The patients were provided drugs for one month and were required to come back after a month for follow up visit and to collect drugs for the next one month. The patients who were on zidovudine and nevirapine based regimen were requested to come after every 15 days initially for one month to get tested for haemoglobin and alanine aminotransferase (ALT). The patients were clinically evaluated on each follow up visit. A patient centred approach was used to promote adherence which was assessed by pill counts with counselling reinforced at every follow up visit. The patients who did not turn up for collecting the drugs at their scheduled visits were either contacted telephonically or by home visit. Chemoprophylaxis was given as per the national guidelines12. The referral of patients was done to local tertiary care government hospital for inpatient care and tuberculosis treatment, if required.
Laboratory investigations: The CD4 counts were estimated using the standard flow cytometer (FACSCalibur, Becton Dickinson, USA) using panleukogating and lyse no wash techniques and expressed as cells/μl.
Viral load estimation: A proportion of patients who came during follow ups were tested for HIV viral load using in house assay based on TaqMan Real Time PCR (ABI7900HT). The PCR cycling condition was essentially done as reported by Kamat et al13. Briefly, 140 μl of plasma samples were used to extract viral RNA using QIAamp viral RNA mini kit (Qiagen Inc., USA) following manufacturer's protocol. The RT-PCR was carried out in a 20 μl reaction, containing 5μl of viral RNA, RNase-free water, 1μl of random hexamers and 1μl of RNase Inhibitor. The cDNA synthesized from the above step was used to assess the viral load. In absolute quantification by Real Time PCR assay, determination of viral load was done by running unknown samples along with a standard series. Here, linearised plasmid DNA containing HIV-1 Gag was used to form standard curve. The variability precision results of intra-assay (CV%=3.33, 1.90 & 1.90%) and inter-assay (CV%=13.21%) were acceptable.
Statistical analysis: Data were generated for each patient at baseline and month 6, year 1, year 2, year 3, year 4 and year 5. The clinical and laboratory data analyzed included absolute CD4 counts, proportion of opportunistic infections and body mass index (BMI). Patients alive and in care till March 31, 2011 were right-censored on the date of their last visit prior to this date. Kaplan-Meier survival analysis was performed to determine survival probability. Only reported deaths were considered in the analysis. Log rank statistics were used to test the equality of survival distribution in above and below baseline CD4 count of 100 cells/μl. The time was measured from the start of ART and ended at the earliest of: the date of death, the date of last follow up or at 5 years after start of treatment.
Median and Inter quartile ranges were calculated for CD4 and BMI. The change in absolute median CD4 lymphocyte counts from baseline to end of every year was assessed using Mann Whitney test. CD4 counts that fall within the window of 30 days (±30 days) of the scheduled 6 monthly visits were considered for analysis.
Adherence of the patients was measured at each visit as >95, 80-95 and <80. The adherence of the patient at the end of year was considered as above 95% if he/she had sustained adherence of above 95% for every month. It was considered to be below 95% if it decreased at any time point.
As the patients were enrolled from various research studies, including HIV-TB co infection studies; clinical diagnosis of the patients from TB study was not included while calculating proportion of opportunistic infections (OIs) at baseline to avoid selection bias. Data analysis was carried out using SPSS (version 15.0, SPSS Inc., USA)
Results
Between December 2005 and April 2008, 270 patients were registered in the ART clinic. Of these, 142 (52.6%) were naive to ART. The baseline characteristics of these naive patients are shown in the Table. Male: Female ratio was 1.5:1. Patients had a mean age of 31.9 ± 6.78 yr, a mean body mass index of 19.6 ± 3.35 kg/m2 (Males: 20.1 ± 3.26, Females: 18.9 ± 3.39 kg/m2), a mean weight of 50.80 ± 11.21 (Males: 55.69 ± 10.31, Females: 43.5 ± 8.2 kg). Median CD4 count was 109 cells/μl (IQR: 60-160). All patients were followed up till March 31, 2011 and the median follow up duration was 44 months (IQR: 37-53.3 months).
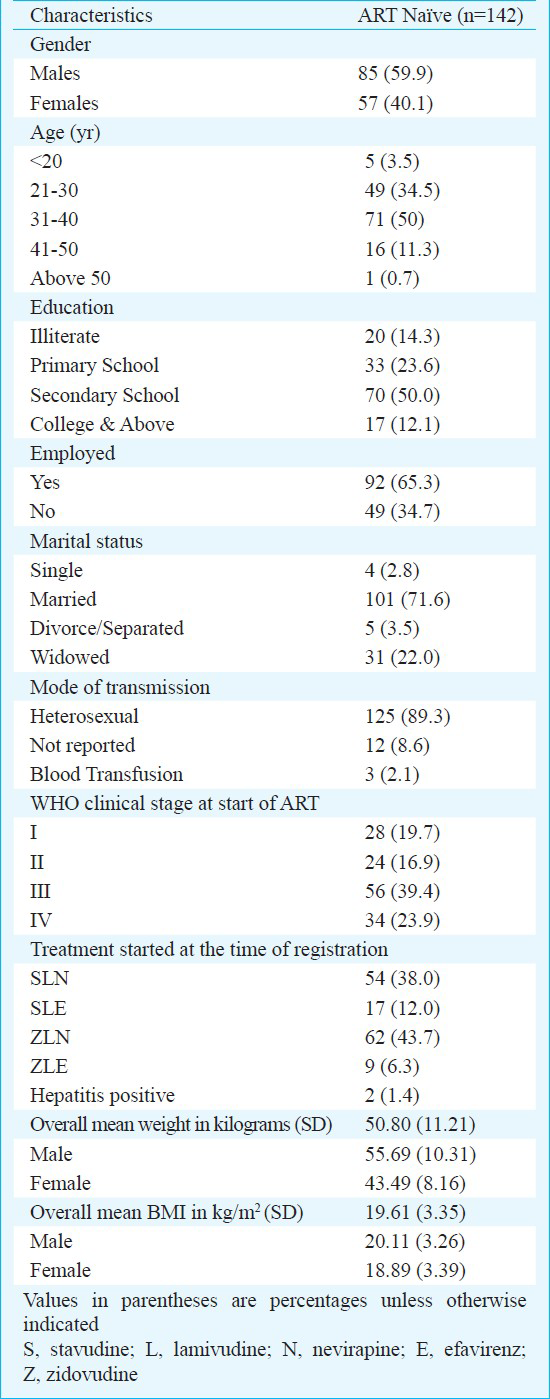
Two patients were positive for hepatitis B surface antigen. Sixteen patients were transferred out to other ART centres and six patients were lost to follow up.
Survival and other clinical outcomes: Seventeen patients (12%) died after ART initiation and six of them died within first 6 months. Survival analysis showed that the probability of being alive at the end of one year was 94 per cent, 92 per cent at the end of 2 years, 91 per cent at the end of 3 years, 87 per cent at the end of 4 years and 85 per cent at the end of 5 years (Fig. 1). Survival of patients stratified by CD4 counts at baseline showed that the probability of being alive in patients with CD4 count <100 and > 100 cells/μl at the end of five years was 79 and 92 per cent, respectively (P=0.011) (Fig. 2).
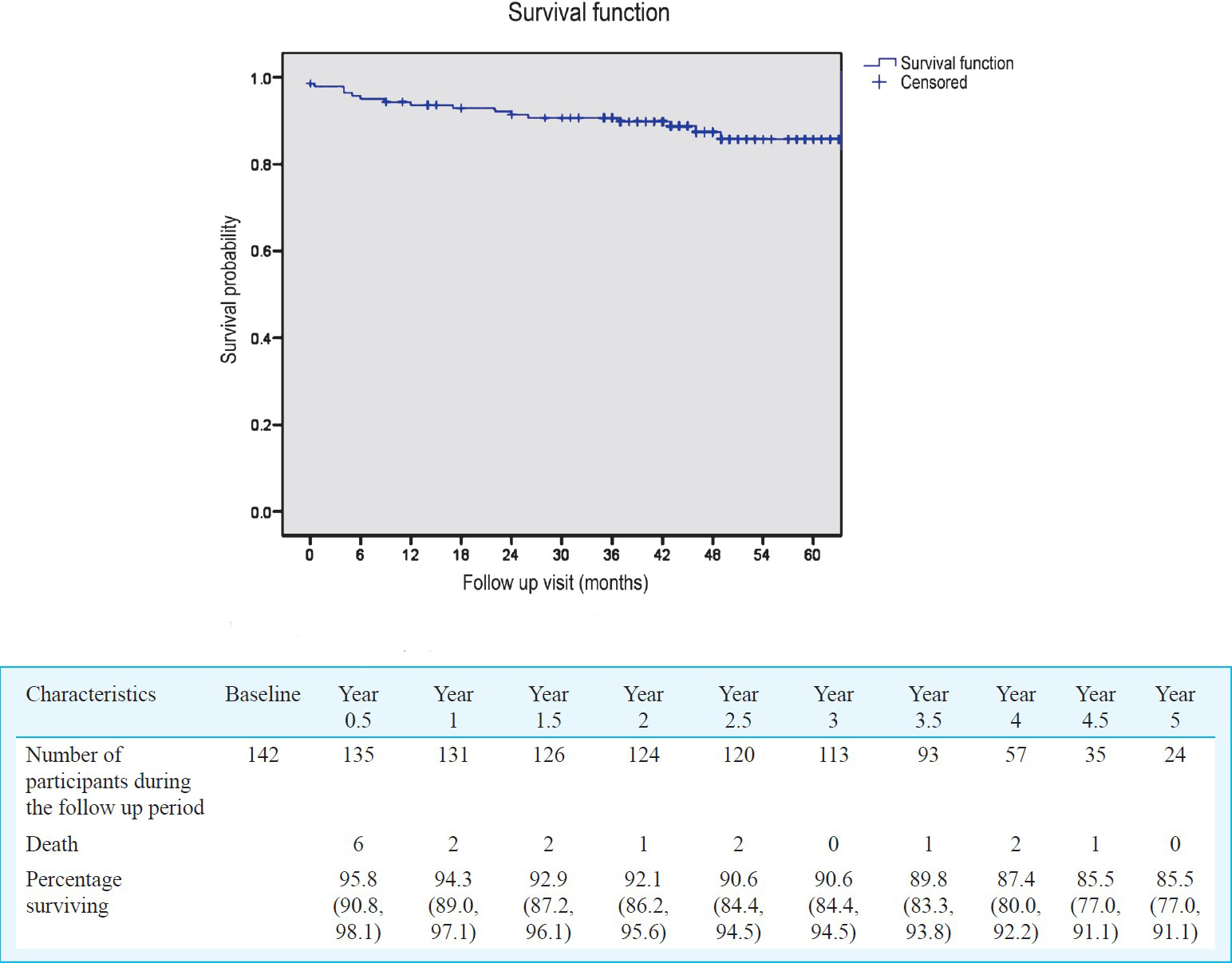
- Survival of ART naive patients attending NARI ART Centre (n=142).
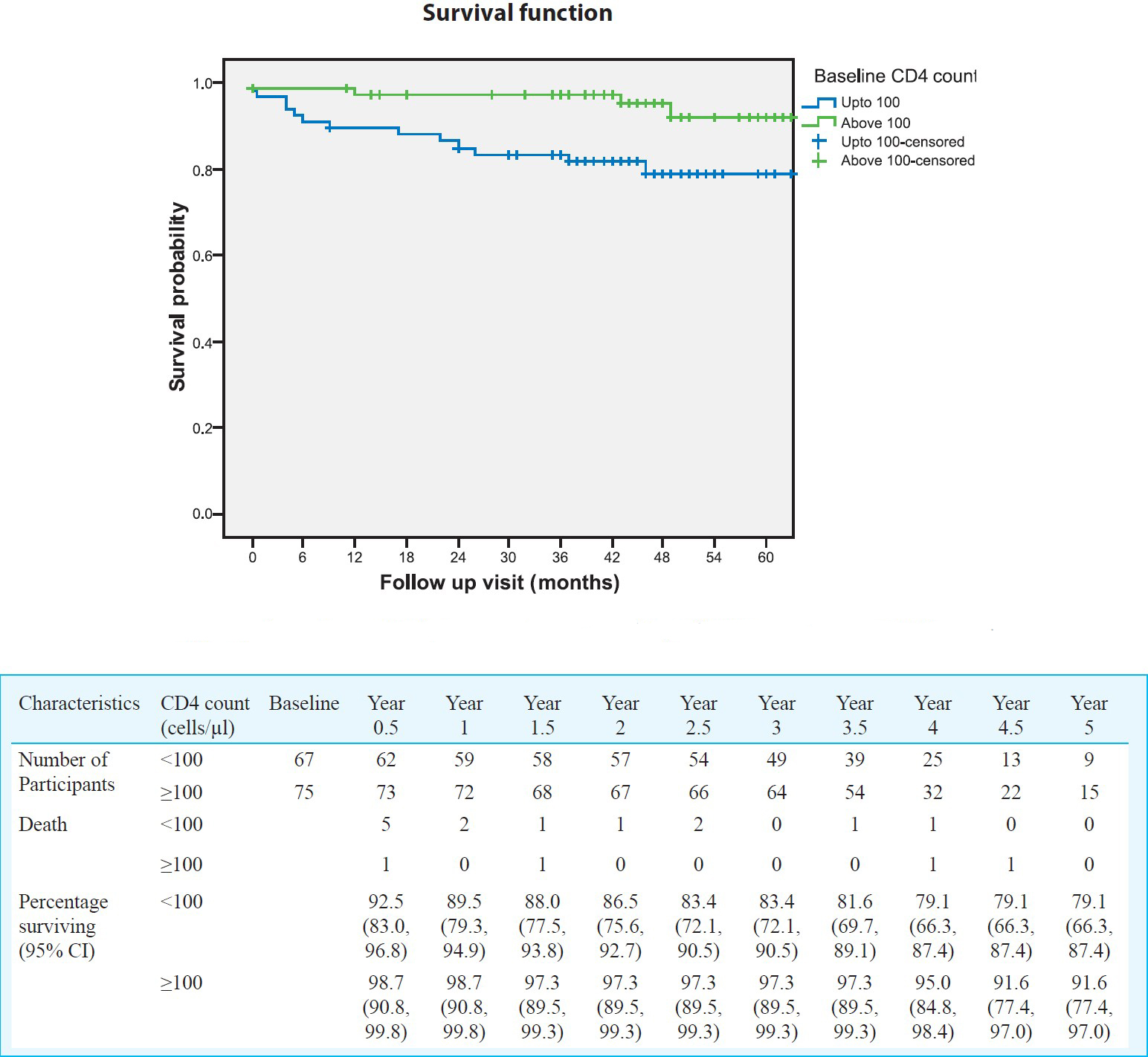
- Survival of patients on antiretroviral drugs stratified by baseline CD4 lymphocyte count (n=142).
The CD4 cell count of ART naive patients showed increase at all monitoring points after initiation of the treatment (Fig. 3). The median CD4 count of ART naive patients increased from 109 cells/μl at baseline to 290 cells/μl at 1 year, 408 cells/μl at 2 years, 429 cells/μl at 3 years, 482 cells/μl at 4 years and 561 cells/μl at the end of 5 years. The increase in CD4 cell/μl count was statistically significant at all yearly follow up visits (P<0.001).
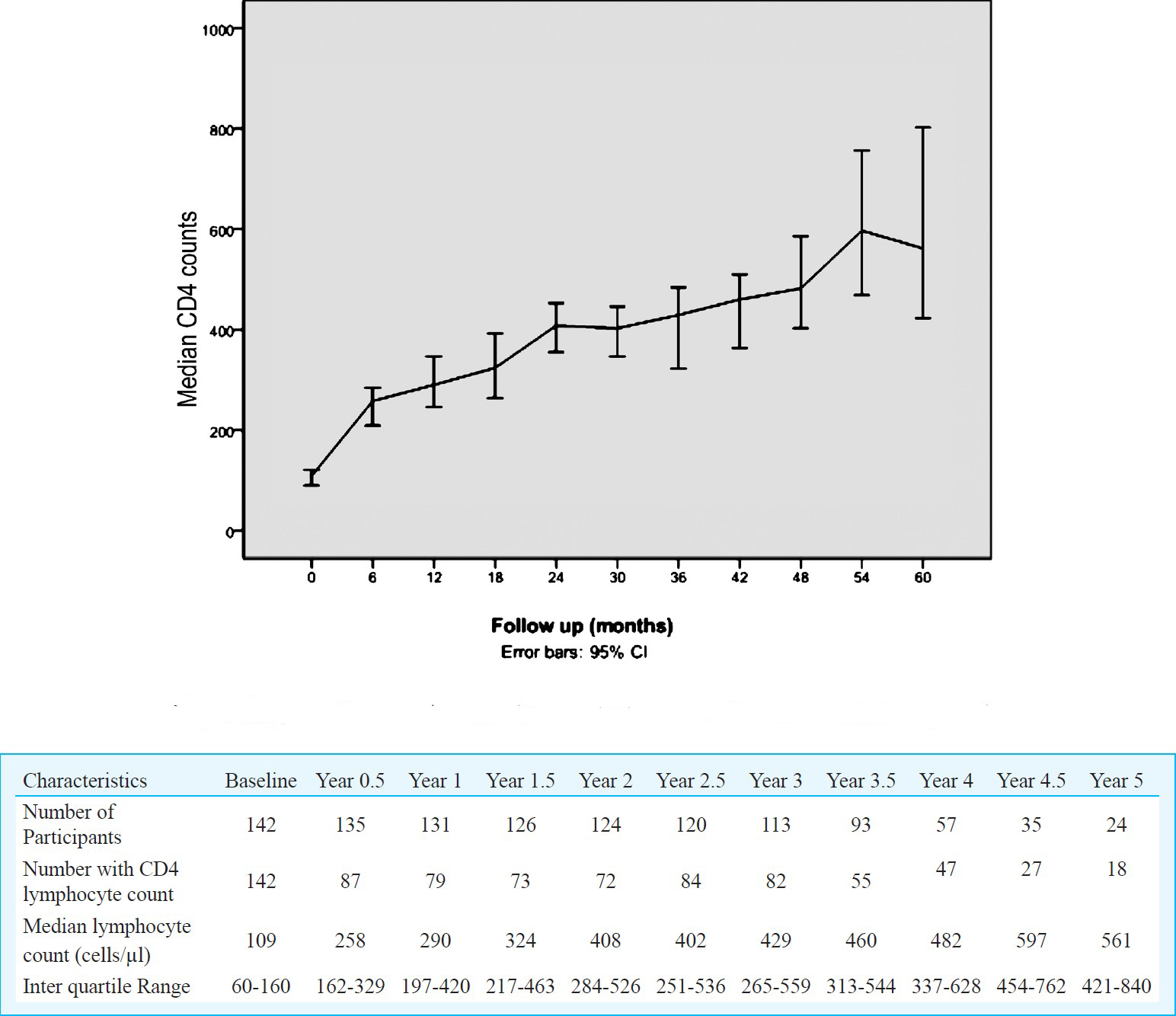
- Changes in CD4 count in ART naive patients attending NARI ART centre in Pune (n=142).
Though there was overall increase in median CD4 counts at different time points, 8 per cent (7/87) patients did not show increase in cell counts by 50 cells/μl at 6 months. In 14 per cent (11/79) patients, there was no rise in CD4 counts by 100 cells/μl from baseline at the end of one year in spite of >95 per cent adherence.
Viral loads were estimated in a subset of 68 (47.8%) patients after a median duration of 46 months (IQR: 38.3-54.8). It was undetectable in 61 (89.7%) patients and of them, the drug adherence was more than 95 per cent in 43 (70.5%) patients. Of the seven patients with detectable viral load, three (6.5%) reported more than 95 per cent adherence.
The data showed that number of opportunistic infections decreased as the CD4 counts increased over the period of time. At baseline, 14 per cent patients presented with single or multiple opportunistic infections with tuberculosis being the most prevalent. However, as the treatment progressed, the proportion of patients presenting with opportunistic infections decreased to 4 per cent at 5 years.
The mean body mass index increased from 19.6 ± 3.35 at baseline, to 21.2 ± 3.01 at 1 year, 21 ± 3.10 at 2 years, 21.5 ± 3.02 at 3 years, 21.9 ± 2.98) at 4 years and 20.7 ± 5.7 kg/m2 at 5 years.
The treatment substitution was done in 33 (23.2%) patients due to side effects or drug toxicities. The reasons for substitution for nevirapine-based regimen were skin rash (3.4%), neurological side effects for stavudine-based regimen (5.6%) and anaemia in zidovudine-based regimen (11%).
Analysis of the data on cumulative adherence showed that 91 (66%) patients reported adherence of >95 per cent during all their follow up visits. The median CD4 counts in these patients increased by 490 cells/μl at the end of 5 years from baseline which was statistically significant (P<0.001).
Discussion
The data collected from ART programme clinic at Pune provided evidence supporting that the first line therapy was effective in HIV infected patients attending the clinic. The probabilities of survival at the end of one and five years were 94 and 85 per cent, respectively. This was higher than reports from other Indian studies910. This may be due to good follow up rates and high adherence to antiretroviral drugs. As number of patients in the clinic was small, it was possible to give more time to each patient and motivate them for adherence. The results were similar to reports from south Africa14. The survival analysis was carried out using CD4 counts stratification and the results showed that patients with advanced disease had probability of less survival as compared to those with ART initiation at CD4 counts of >100 cells/μl. This finding was similar to the studies done elsewhere1115. It emphasizes the importance of creating awareness about early diagnosis so that the eligible patients can be initiated on ART earlier.
The optimal response to ART therapy is indicated by a median rise of CD4 count of 50 cells/μl at the end of 6 months or 100 cells/μl at one year of treatment16. The studies conducted in different countries have documented different levels of rise in CD4 counts after the treatment. Our results on rise in CD4 counts are similar to that observed in other studies in India as well as in other countries9101718.
Though overall immunological response was satisfactory, three patients did not show increase in CD4 cell count at 6 months and one year follow up. Viral load assays were not performed at scheduled follow up visits as these were not included under the ‘essential’ investigation in the programme. So it was not clear whether the patients had suboptimal response to antiretroviral therapy or virological failure. A study from Germany has documented that compared with immune responders, patients with immuno-virological discordance seem to remain at increased risk for AIDS, and absolute risk is greatly reduced after the first six months of complete viral suppression19. It would be helpful to perform viral load assays in patients with suboptimal response to antiretroviral therapy to differentiate them from virological failure.
Viral load estimations in a subset of 68 patients showed viral suppression in about 90 per cent patients and the drug adherence was more than 95 per cent in 70.5 per cent patients. Most of the study patients for whom viral loads were available had virologic suppression though only 70 per cent of them had reported their adherence over 95 per cent. A study on non-nucleoside reverse transcriptase inhibitor (NNRTI) outcomes among combination ART-treated adults in Botswana has shown 6.6 to 9.6 per cent failure in patients on efavirenz and nevirapine based regimens, respectively after 3 years of treatment20. Another study from South Africa has shown improvement in the virologic outcomes in a linear dose-response manner as adherence to NNRTI-based regimens increases beyond 50 per cent21. A study conducted in United Kingdom on long term trends in adherence to ART has reported that adherence does not decrease on average over more than a decade from start of HAART which is beneficial for patients as they could potentially maintain viral suppression for many years22. This also emphasizes the importance of adherence counselling in the ART centres.
The opportunistic infections were decreased significantly from the baseline and BMI showed increase at the end of five years indicating the clinical improvement in addition to the immunological restoration. These findings are similar with the data published from other free ART centres in India910. In our analysis 70 per cent patients had adherence of > 95 per cent throughout their follow up visits. The adherence is reflected in the effective rise in the CD4 counts. Higher levels of adherence were possible as they had excellent rapport with the clinic counsellors. They approached the clinic in case of problems like side effects, clinical problems or drug reactions and received counselling and care at that time point.
This study had certain limitations. The clinic provided free antiretroviral drugs to patients who participated in the institutional research studies and hence the number of patients was less as compared to other Programme ART centres in the country. Some opportunistic infections may have been missed in the follow up as the patients got admitted in the private hospitals and the actual number may be underestimated though every effort was made to record any such episode of hospitalization or treatment in each follow up visit. The opportunistic infections diagnosed within three months were not considered as immune reconstitution inflammatory syndrome (IRIS) in this analysis as CD4 counts were not performed and hence could not be shown as immune reconstitution at that time point. Viral load was estimated only in a subset of patients during a defined period and there could be bias in the results. Since the regimens in the national ART programme under good adherence and minimal attrition rate have shown good response up to five years, there is a strong case for greater efforts for better follow up, retention and drug adherence.
In conclusion, though our results are based on a limited number of patients, our analysis has documented the efficacy of ART regimens in the Government roll out Programme for clinical management of patients. It is necessary that Programme invests in decreasing loss to follow up and increasing the adherence to antiretroviral drugs to achieve the success in virus suppression.
References
- A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med. 1997;337:725-33.
- [Google Scholar]
- Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853-60.
- [Google Scholar]
- The changing natural history of HIV disease: Before and after the introduction of generic antiretroviral therapy in southern India. Clin Infect Dis. 2005;41:1525-8.
- [Google Scholar]
- The Senegalese government's highly active antiretroviral therapy initiative: an 18-month follow-up study. AIDS. 2002;16:1363-70.
- [Google Scholar]
- Scale-up of national antiretroviral therapy programs: progress and challenges in the Asia Pacific region. AIDS. 2010;24(Suppl 3):S62-71.
- [Google Scholar]
- Available from: http://www.unaids.org/globalreport/global_report.htm
- Outcomes of antiretroviral therapy in a northern Indian urban clinic. Bull World Health Organ. 2010;88:222-6.
- [Google Scholar]
- Two-year treatment outcomes of patients enrolled in India's national first-line antiretroviral therapy programme. Natl Med J India. 2010;23:7-12.
- [Google Scholar]
- Increase in CD4 cell counts between 2 and 3.5 years after initiation of antiretroviral therapy and determinants of CD4 progression in India. J Postgrad Med. 2009;55:261-6.
- [Google Scholar]
- Quantitation of HIV-1 RNA levels in plasma and CSF of asymptomatic HIV-1 infected patients from South India using a TaqMan real time PCR assay. J Clin Virol. 2007;39:9-15.
- [Google Scholar]
- Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa. AIDS. 2004;18:887-95.
- [Google Scholar]
- Overview of the effectiveness of triple combination therapy in antiretroviral-naive HIV-1 infected adults. AIDS. 2001;15:1369-77.
- [Google Scholar]
- WHO/UNAIDS/IAS. Safe and Effective Use of Antiretroviral Treatment in Adults With Particular Reference to Resource Limited Settings. Geneva, Switzerland: WHO/AIS; 2000. p. :1-31.
- [Google Scholar]
- Once-a-day highly active antiretroviral therapy in treatment-naive HIV-1-infected adults in Senegal. AIDS. 2003;17:1017-22.
- [Google Scholar]
- Evaluation of a 6-year highly active antiretroviral therapy in Chinese HIV-1-infected patients. Intervirology. 2010;53:240-6.
- [Google Scholar]
- Clinical outcome of HIV-infected patients with discordant virological and immunological response to antiretroviral therapy. J Infect Dis. 2011;203:364-71.
- [Google Scholar]
- Non-nucleoside reverse transcriptase inhibitor outcomes among combination antiretroviral therapy-treated adults in Botswana. AIDS. 2010;24(Suppl 1):S27-36.
- [Google Scholar]
- Adherence to nonnucleoside reverse transcriptase inhibitor-based HIV therapy and virologic outcomes. Ann Intern Med. 2007;146:564-73.
- [Google Scholar]
- Long-term trends in adherence to antiretroviral therapy from start of HAART. AIDS. 2010;24:1153-62.
- [Google Scholar]






