Translate this page into:
Upper limb muscle strength & endurance in chronic obstructive pulmonary disease
Reprint requests: Dr Swati H. Shah, C-201, Element-5, Behind Shivar Garden Hotel, Rahatani, Aundh Annex, Pune 411 017, India e-mail: sshah282@gmail.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
There are very few studies that have investigated the muscle strength and endurance of upper limbs (UL) in chronic obstructive pulmonary disease (COPD). We undertook this study to measure and compare the skeletal muscle strength and endurance of UL in COPD patients and age matched healthy controls and to study the association between lung function parameters and UL muscle strength and endurance.
Methods:
Forty one COPD patients and 45 height and weight matched healthy subjects of the same age group were studied. UL skeletal muscle strength and endurance were measured using the hand grip dynamometer test. Forced vital capacity (FVC), forced expiratory volume in 1 sec (FEV1), forced expiratory flow during 25-75% FVC (FEF25-75%) and peak expiratory flow rate (PEFR) were measured. The handgrip muscle strength and endurance between the two groups were compared and correlations between FVC and FEV1 with muscle strength and endurance were analyzed.
Results:
The mean handgrip strength and mean muscle endurance in COPD patients were significantly lesser than the normal subjects in both males and females (P<0.001). There was significant positive correlation between muscle strength and FVC in males (r2=0.32, P<0.05); and between muscle strength and FEV1 in females (r2=0.20, P<0.05).
Interpretation & conclusion:
The study showed that the handgrip muscle strength decreases as the FVC and FEV1 decrease in patients with COPD. Identifying those patients who have reduced strength and endurance will allow early interventions targeted at improving the quality of life of the patient.
Keywords
Chronic obstructive pulmonary disease
hand grip spirometry
skeletal muscle dysfunction
Chronic obstructive pulmonary disease (COPD) is a chronic inflammatory disease of the airways and lung parenchyma associated with airway narrowing, alveolar wall destruction and systemic hypoxaemia. It is believed that the cellular mediators of inflammation present in the airways and alveolar walls spill over into the circulation and contribute to skeletal muscle dysfunction, cardiac failure, atherosclerosis and osteoporosis leading to poor quality of life and increased morbidity and mortality in COPD patients1. It is, therefore, important to treat the systemic consequences also whenever possible to improve the quality of life and reduce mortality.
Skeletal muscles are widely affected in COPD and several mechanisms have been postulated viz: (i) systemic hypoxia which causes a change in the muscle metabolic phenotype from oxidative metabolism to anaerobic metabolism as an adaptive process2, and (ii) increased circulating levels of inflammatory mediators such as interleukin-6 (IL-6), tumour necrosis factor-α (TNF-α) and C-reactive protein (CRP)3.
Importance of dysfunction of peripheral skeletal muscles on exercise capacity in patients with COPD was first suggested by Killian and coworkers in 19924. A few years later Hamilton and colleagues showed that approximately 70 per cent of patients with chronic lung disease had poorer quadriceps strength when compared to age matched healthy subjects5. Thereafter, there have been many studies showing reduced skeletal muscle strength and endurance, especially in the lower limbs of COPD patients6789. However, there has been little research into the upper limb skeletal muscle dysfunction in COPD patients. If upper limb muscles show reduced strength and endurance, clinicians can plan out better pulmonary rehabilitation activities which could include upper limb muscle exercise training. We hypothesized that COPD patients would have poor hand grip strength and endurance as compared to age matched healthy subjects and that this would correlate with lung function parameters. The aim of this study was to compare the hand grip muscle strength and endurance of the distal skeletal muscles of the upper limb in patients with COPD with age and gender matched healthy controls. Further, their association with spirometric lung function parameters viz: forced vital capacity (FVC) and forced expiratory volume in one second (FEV1) in COPD patients was also studied.
Material & Methods
This case control study was conducted in the department of Physiology at B. J. Medical College, and the Respiratory Medicine Outpatient Department at the Sassoon General Hospital, Pune, India, during March to August in 2009-2010.
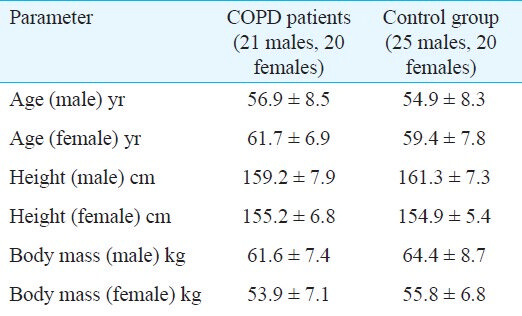
A sample size of 41 for both COPD patient group as well as normal control group was calculated assuming power more than 80 and 5 per cent level of significance. Twenty one male and 20 female patients aged 40-70 yr attending the Respiratory Medicine outpatient department at Sassoon General Hospital were randomly selected for this study. They were all diagnosed COPD patients based on history and a post bronchodilator FEV1/FVC ratio of <70 per cent on spirometry10. All were nonsmokers. Patients with serious concomitant illnesses such as major cardiovascular, neurological and musculoskeletal diseases were excluded from the study. Twenty five male and 20 female sedentary healthy volunteers of the same age group with normal lung function were selected randomly in control group. Since it was difficult to find normal controls with similar physical activity as that of the COPD patients, it was ensured that they had physical activity status almost similar to the patients i.e. having a sedentary life-style. We selected the normal controls from the workers of the Sassoon General Hospitals, so that they will have similar socio-economic status as that of the patients, which was also confirmed by verbal interview. Those with cardio-respiratory, musculoskeletal or endocrine diseases were excluded from the study.
The study was approved by the Institutional Ethics Committee. After obtaining a written informed consent, all study subjects were administered a questionnaire which captured demographic details, medical history, personal history and diagnosis as per the GOLD (Global Initiative for Obstructive lung disease) guidelines and duration of COPD with current symptoms10.
A handgrip dynamometer (INCO India Ltd., Ambala) was used to measure the muscle strength and endurance of the upper limbs according to the technique described and validated by Madanmohan et al11. After explaining the procedure to the study subject and giving a demonstration, they were asked to hold the handgrip dynamometer in the dominant hand in sitting position. The forearm was extended over a table and elbow flexed at 90°. Subjects were asked to hold the dynamometer in such a way that the second phalanx was against the inner stirrup, and were then asked to grip the dynamometer handle with as much force as they possibly could apply. The handgrip muscle strength was recorded in kilograms as indicated by the pointer on the dynamometer. Three recordings were taken with a gap of two minutes between each effort and the maximum value was recorded for the analysis.
The handgrip endurance was measured by asking the subjects to maintain their grip on the handgrip dynamometer at ⅓rd of their maximum handgrip strength for as long as they could. The duration for which they could maintain the grip strength was noted in seconds. Two recordings were obtained with a gap of five minutes between each effort and the maximum value was recorded for the analysis.
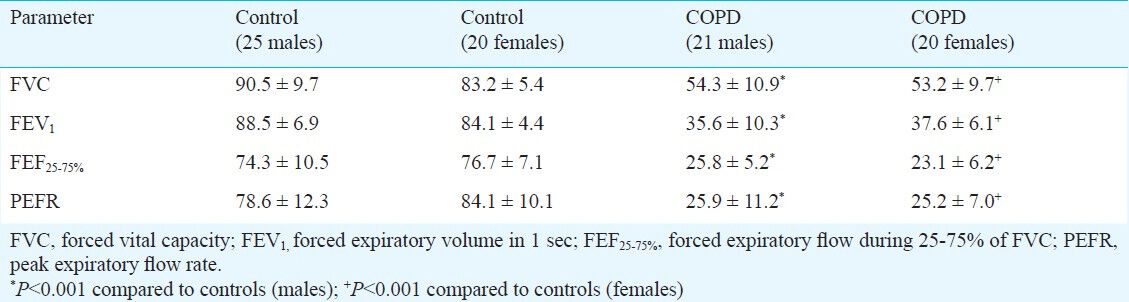
Spirometry was performed using the turbine flow-sensor based MIR Spirolab II (Italy) by trained personnel in a quiet room as per the guidelines of the American Thoracic Society and European Respiratory Society (ATS/ERS)12. All spirometries were performed between 1230 to 1330 h to avoid diurnal variations. The parameters measured were forced vital capacity (FVC) in liters, forced expiratory volume in 1 sec (FEV1), forced expiratory flow during 25-75% of FVC (FEF25-75%) and peak expiratory flow rate (PEFR).
All data were collected in a Data Collection Form and then transferred to an Excel sheet by two independent data entry operators. Discrepant values were corrected by checking the data collection form. Clean data were then analyzed statistically.
Statistical analysis: All the variables were expressed as mean ± standard deviation (SD). Handgrip muscle strength and handgrip muscle endurance were compared between COPD patients and normal subjects by using the unpaired Student's t test for males and females separately. The correlations between FVC and FEV1 with muscle strength and muscle endurance were analyzed by using the Pearson's coefficient correlation. All statistical analyses were performed by using SPSS software version 11.0 (Chicago, USA) and Graphic Pad Prism software version 6.0.
Results
The study population included 45 healthy controls and 41 COPD patients. There were no significant differences between the mean ages, heights and weights of the control subjects and COPD patients (Table I). The respiratory parameters (FVC, FEV1, FEF25-75, PEFR) were significantly lower in COPD patients when compared with control population (P<0.001), (Table II).
The mean handgrip strength in male COPD patients was 21.8±4.7 kg and was significantly lesser than the normal males (31.2±4.3 kg, P<0.001). In females, the mean handgrip strength in COPD patients was 19.2±3.4 kg and was significantly lesser than the normal controls (23.0±1.9 kg, P<0.001). The mean muscle endurance in male COPD patients was 48.9 ± 20.6 sec and was significantly lesser than the normal male subjects (108.3±33.7 sec, P<0.001). The mean muscle endurance in female COPD patients was 37.4±11.2 sec and was significantly lesser than the normal subjects (99.1±9.1 sec, P<0.001). On comparing the percentage reduction in COPD patients compared to controls, muscle endurance was more affected than muscle strength (muscle strength reduction-31% in males, 17% in females and muscle endurance reduction-56% in males and 63% in females). FVC showed a positive correlation with muscle strength in males (r2=0.32, P<0.05), while in females FEV1 was positively correlated with muscle strength (r2=0.20, P<0.05). Muscle endurance did not show any correlation with either FEV1 or FVC both in males and females.
Discussion
In this study, patients with COPD showed a significantly lower handgrip muscle strength and endurance compared to age matched controls. The reduced handgrip muscle strength correlated positively and with both FEV1 and FVC% predicted values. Skeletal muscle dysfunction has been reported in COPD. Gosselink et al13 showed that in patients with COPD handgrip force decreased significantly. Clark and colleagues6 showed that in COPD patients upper limb muscle strength (biceps and triceps) was reduced but sustained performance was not reduced. Some studies have shown reduced strength of shoulder girdle and latissimus dorsi muscle8, and reduced endurance of the adductor pollicis muscle14. Another study showed that peak and average force of elbow flexor muscles was reduced in patients with COPD, but the difference was not statistically significant. Heijdra et al16 showed that there was no change in the handgrip strength in COPD patients compared to age matched healthy control group. This is in contrast to our study. They have taken the average of three measurements of right and left handgrip strength, while in the present study the best of three measurements of the dominant hand was taken.
There are many studies which have shown significant positive correlation between quadriceps strength/endurance and FEV17813. El-Din et al17 showed no correlation between the FVC, FEV1 and the upper limb muscle strength, but they found significant positive correlation between FVC, FEV1 and quantitative interference pattern EMG parameters. In our study, a significant positive correlation was found between percentage predicted FVC and handgrip strength and between percentage predicted FEV1 and muscle strength. This indicates that pulmonary functions may have direct impact on skeletal muscle strength. Severity of the disease affects the extent to which the skeletal muscle functions are compromised.
Structural and biochemical abnormalities have been reported in the skeletal muscles of COPD patients such as lower fraction of type I fibers and higher fraction of type II fibers18. A high fraction of myosin heavy chain type 2B Isoforms have also been found19. Concentration of aerobic but not glycolytic enzymes is reduced in COPD patients20. This indicates a switch over from aerobic to anaerobic metabolism. In our study the muscle endurance was more affected than the muscle strength. This finding suggests a shift from aerobic to anaerobic metabolism in COPD patients. The capillary density in the skeletal muscles is often reduced which results in increased diffusion distances for oxygen transport and thereby reduced oxygen utilization. Several studies have shown that lactic acidosis occurs at much lower work rates than in healthy subjects21.
Deconditioning is a major contributor to the skeletal muscle dysfunction seen in patients with COPD. These patients generally assume a sedentary lifestyle to avoid the dyspnoea that physical activity brings. Changes resembling the skeletal muscle alterations occurring with deconditioning have been described in patients with COPD. These include reduction in oxidative enzyme capacity and proportion of type I fibers; and atrophy of type I fibers2. Hypophosphatemia associated with low intracellular ATP levels found in COPD may form the basis of observed muscle weakness2.
Though most of the studies have been done in lower limb skeletal muscles in COPD, probably the similar factors are responsible for the reduced muscle strength and endurance in upper limb. Several studies have shown that upper limb muscle strength influences walking distance132223. It has been shown that for a 10 kg increment in grip strength, people could walk an average of 14 m farther23. Hence measuring upper limb strength; which is a very easy and quick method compared to 6-minute walk test can give a clue about walking strength of the person. Probably by improving the pulmonary function parameters, upper limb muscle strength and the walking distance of the COPD patient can be improved.
Management of COPD should not be limited to symptomatic relief of respiratory symptoms. Pulmonary rehabilitation done in COPD patients should include effective exercise training for increasing the muscle strength and endurance; as studies have shown that strength training in patients with COPD can produce increase in muscle strength of upper and lower limbs24. Identifying those patients who have greater reduction in strength and endurance will allow early interventions targeted at increasing strength such as diet, hormonal supplementation and strength training. Evidence based clinical practice guidelines25 recommend the inclusion of exercise training targeted at the muscles of upper limbs in the physical therapy programs specific to COPD patients.
In conclusion, our study showed a significant association between handgrip muscle strength and FVC and FEV1 in COPD patients. A study with larger sample size along with varying severity of disease would be a better indicator of this relationship. Further studies will be required to evaluate the influence of improving the pulmonary function parameters on the skeletal muscle exercise capacity and indirectly the walking distance of the patient.
Acknowledgment
Authors acknowledge the Chest Research Foundation, Pune for technical support.
References
- Systemic effects of chronic obstructive pulmonary disease. Eur Respir J. 2003;21:347-60.
- [Google Scholar]
- Skeletal Muscle Dysfunction in Chronic Obstructive Pulmonary Disease - A Statement of the American Thoracic Society and European Respiratory Society. Am J Respir Crit Care Med. 1999;159:S1-S40.
- [Google Scholar]
- Systemic response to ambient particulate matter relevance to chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2:61-7.
- [Google Scholar]
- Exercise capacity and ventilatory, circulatory and symptom limitation in patients with chronic airflow limitation. Am Rev Respir Dis. 1992;146:935-40.
- [Google Scholar]
- Muscle strength, symptom intensity and exercise capacity in patients with cardiorespiratory disorders. Am J Respir Crit Care Med. 1995;152:2021-31.
- [Google Scholar]
- Skeletal muscle strength and endurance in patients with mild COPD and the effects of weight training. Eur Respir J. 2000;15:92-7.
- [Google Scholar]
- Impaired skeletal muscle endurance related to physical inactivity and altered lung function in COPD patients. Chest. 1998;113:900-5.
- [Google Scholar]
- Peripheral muscle weakness in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;158:629-34.
- [Google Scholar]
- Nutritional depletion in relation to respiratory and peripheral skeletal muscle function in out-patients with COPD. Eur Respir J. 1994;7:1793-7.
- [Google Scholar]
- Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532-55.
- [Google Scholar]
- Effect of six weeks yoga training on weight loss following step test, respiratory pressures, handgrip strength and handgrip endurance in young healthy subjects. Indian J Physiol Pharmacol. 2008;52:164-70.
- [Google Scholar]
- Peripheral muscle weakness contributes to exercise limitation in COPD. Am J Respir Crit Care Med. 1996;153:976-80.
- [Google Scholar]
- Maximal force and endurance to fatigue of respiratory and skeletal muscles in chronic hypoxemic patients: The effects of oxygen breathing. Muscle Nerve. 1995;18:495-502.
- [Google Scholar]
- Inspiratory and skeletal muscle strength and endurance and diaphragmatic activation in patients with chronic airflow limitation. Thorax. 1989;44:903-12.
- [Google Scholar]
- Muscle strength and exercise kinetics in COPD patients with a normal fat-free mass index are comparable to control subjects. Chest. 2003;124:75-82.
- [Google Scholar]
- Neurophysiological assessment of skeletal muscles in patients with chronic obstructive pulmonary disease. Egypt J Neurol Psychait Neurosurg. 2007;44:675-82.
- [Google Scholar]
- Skeletal muscle metabolites and fiber types in patients with advanced chronic obstructive pulmonary disease (COPD), with and without chronic respiratory failure. Eur Respir J. 1990;3:192-6.
- [Google Scholar]
- Fibre types in skeletal muscles of chronic obstructive pulmonary disease patients related to respiratory function and exercise tolerance. Eur Respir J. 1997;10:2853-60.
- [Google Scholar]
- Oxidative capacity of the skeletal muscle and lactic acid kinetics during exercise in normal subjects and in patients with COPD. Am J Respir Crit Care Med. 1996;153:288-93.
- [Google Scholar]
- Relationship of upper limb and thoracic muscle strength in 6 minute walk distance in COPD patients. Chest. 2006;129:551-7.
- [Google Scholar]
- The 6-min Walk Test: A quick measure of functional status in elderly adults. Chest. 2003;123:387-98.
- [Google Scholar]
- Peripheral muscle strength training in COPD - A systematic review. Chest. 2004;126:903-14.
- [Google Scholar]
- Pulmonary Rehabilitation: Joint ACCP/AACVPR Evidence-based clinical practice guidelines. Chest. 2007;131:4S-42S.
- [Google Scholar]






