Translate this page into:
Unveiling the burden of scrub typhus in acute febrile illness cases across India: A systematic review & meta-analysis
For correspondence: Dr Ravindra Kumar, ICMR-National Institute of Research in Tribal Health, Jabalpur, Madhya Pradesh 482 003, India e-mail: ravindra.kum@icmr.gov.in
-
Received: ,
Abstract
Background & objectives
Scrub typhus is an emerging mite-borne zoonotic infection that has been overlooked, despite being one of the most widespread severe vector-borne diseases. With an estimated one billion people at risk worldwide and one million annual cases, it poses a significant public health concern. While various studies have investigated the prevalence of scrub typhus in different regions of India, a comprehensive regional systematic review and meta-analysis on the seropositivity of scrub typhus among acute febrile cases has been lacking. To address this gap, we conducted a systematic review and meta-analysis to compile information on the current seroprevalence of scrub typhus in acute febrile illness cases in India.
Methods
A literature search of multiple databases on prevalence of scrub typhus in acute febrile illness in India, 60 eligible studies out of 573 studies. The prevalence of individual studies was double arcsine transformed, and the pooled prevalence was calculated using inverse variance method.
Results
In total, these studies encompassed 34,492 febrile cases. The overall seroprevalence of scrub typhus among acute febrile illness cases in India was found to be 26.41 per cent [95% confidence interval (CI): 22.03-31.03]. Additionally, the pooled case fatality rate (based on data from six studies) among scrub typhus-positive cases yielded a case fatality rate of 7.69 per cent (95% CI: 4.37-11.72).
Interpretation & conclusions
This meta-analysis shows that scrub typhus is a significant health threat in India. Preventive measures to control scrub typhus need to be given priority.
Keywords
India
meta-analysis
orientia tsutsugamushi
prevalence
scrub typhus
Scrub typhus is an acute febrile bacterial disease that is caused by an intracellular pathogen Orientia tsutsugamushi belonging to the genus Orientia transmitted by the bite of mite larvae, is widespread in the western Pacific area also called tsutsugamushi disease1. Recently, the discovery of scrub typhus caused by newly identified Orientia species, such as Candidatus Orientia chuto2, has triggered a major concern about the worldwide. Despite, scrub typhus disease’s century-long existence, it remains highly neglected and has emerged as a significant public health problem in India. Originally, scrub typhus was confined to an area of more than 8 million km2 named as the ‘tsutsugamushi triangle’, an area bounded to the north by northern Japan and the far east Russia, to the south by Australia, to the east by Japan and to the west by Pakistan, Afghanistan and India3. But, recent reports from South America and Middle East region have surfaced, making the scrub typhus a major public health concern, globally4. The pathogen is transmitted to humans and rodents through the bite of infected chiggers or mite larvae belonging to the genus Leptotrombium, with both humans and rodents serving as incidental hosts5,6. Chigger mites inhabit diverse vegetation areas, including scrub and primary forests, tall grasslands, plantations, agricultural fields and even beaches7,8. In India about 57 per cent of land is covered by crop cultivation. Farmers and agricultural workers are at highest risk of mite bite. Presently, an alarming one billion individuals in endemic areas are at risk of contracting scrub typhus, with approximately one million new infections occurring annually3,9. In Southeast Asia, scrub typhus accounts for up to 28 per cent of non-malarial fevers, and thus is a leading cause of death from any communicable disease3,10.
The disease manifests itself six to 18 days after the bite of infected mite larvae (chiggers). These trombiculid mites carry the bacteria in their salivary glands and transmit it to the host during feeding. Unfortunately, the mite bite is painless, often going unnoticed as it causes intense itching after a few hours in individuals and mites are very small, and barely visible to the naked eye10,11. The infection presents with a sudden onset of fever, headache, and myalgia11,12. Within two to three days, a maculopapular rash typically appears, accompanied by an eschar at the bite site and enlargement of local lymph nodes. As the illness progresses, interstitial pneumonitis, generalised lymphadenopathy, and splenomegaly may arise. However, due to the delayed presentation of the characteristic eschar (pathognomic lesion) in most cases and the initial flu-like symptoms that are easily disregarded, severe complications and fatalities can occur among patients with scrub typhus13. Untreated cases can lead to multi-organ failure and death, underscoring the importance of early detection and prompt treatment for improved outcomes14.Environmental factors significantly influence the occurrence of scrub typhus, with practices like proximity to water bodies, outdoor cooking, pet ownership, and vegetation increasing the risk manifold15,16.
In India, a rise in scrub typhus cases is observed during the rainy, post-monsoon and winter seasons, particularly in cooler months17-20. The disease is commonly seen in the rainy season due to increased exposure to trombiculid mites during harvesting and contact with newly growing vegetation21,22. Orientia tsutsugamushi, the bacterium responsible for scrub typhus, is transmitted through two main mechanisms: transovarial transmission (from infected female mites to their offspring via eggs) and transstadial transmission (passage from larval mite to nymph to adult). These modes of transmission fall under vertical transmission. No evidence has been reported thus far supporting horizontal transmission, where mites acquire Orientia from infected hosts and subsequently infect other host23-25. Scrub typhus is rapidly re-emerging in several regions of Micronesia, Maldives, and India, where it had previously been significantly neglected. Currently, multiple epidemics and sudden outbreaks of scrub typhus have been documented across various parts of India26. Limited diagnostic facilities, underreporting, inadequate case management, and insufficient vector control exacerbate the situation. In India, the management of scrub typhus lacks systematic case detection and appropriate measures for vector control. The combination of climate change and human expansion into previously uninhabited areas has increased the incidence and re-emergence of scrub typhus. Despite a growing awareness and the availability of reported articles, there remains a dearth of comprehensive, evidence-based data on the disease burden, prevalence, incidence, and geographic distribution. Such data are crucial for making informed decisions to implement effective prevention and control strategies. To address this knowledge gap, we conducted a systematic review and meta-analysis to estimate the burden of scrub typhus in India.
Material & Methods
Search strategy and selection criteria
The systematic review and meta-analysis followed JBI Evidence Synthesis Manual27 and the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)28. Various databases including Medline (PubMed), National Library of Medicine, Science Direct, Web of Science, and Google Scholar were searched for articles published until January 14, 2023. The search was conducted using keywords such as scrub typhus” OR “Orientia tsutsugamushi” OR “O. tsutsugamushi”) AND (India) AND (“prevalence” OR “Incidence” OR “epidemiology” OR “survey” OR “distribution”). The Clarivate Analytics EndNote web version ( https://access.clarivate.com/login?app=endnote) was used to manage duplicate records. Furthermore, a manual search of the references cited in the publications was performed. The complete search strategy is described in detail has shown in Figure 1.
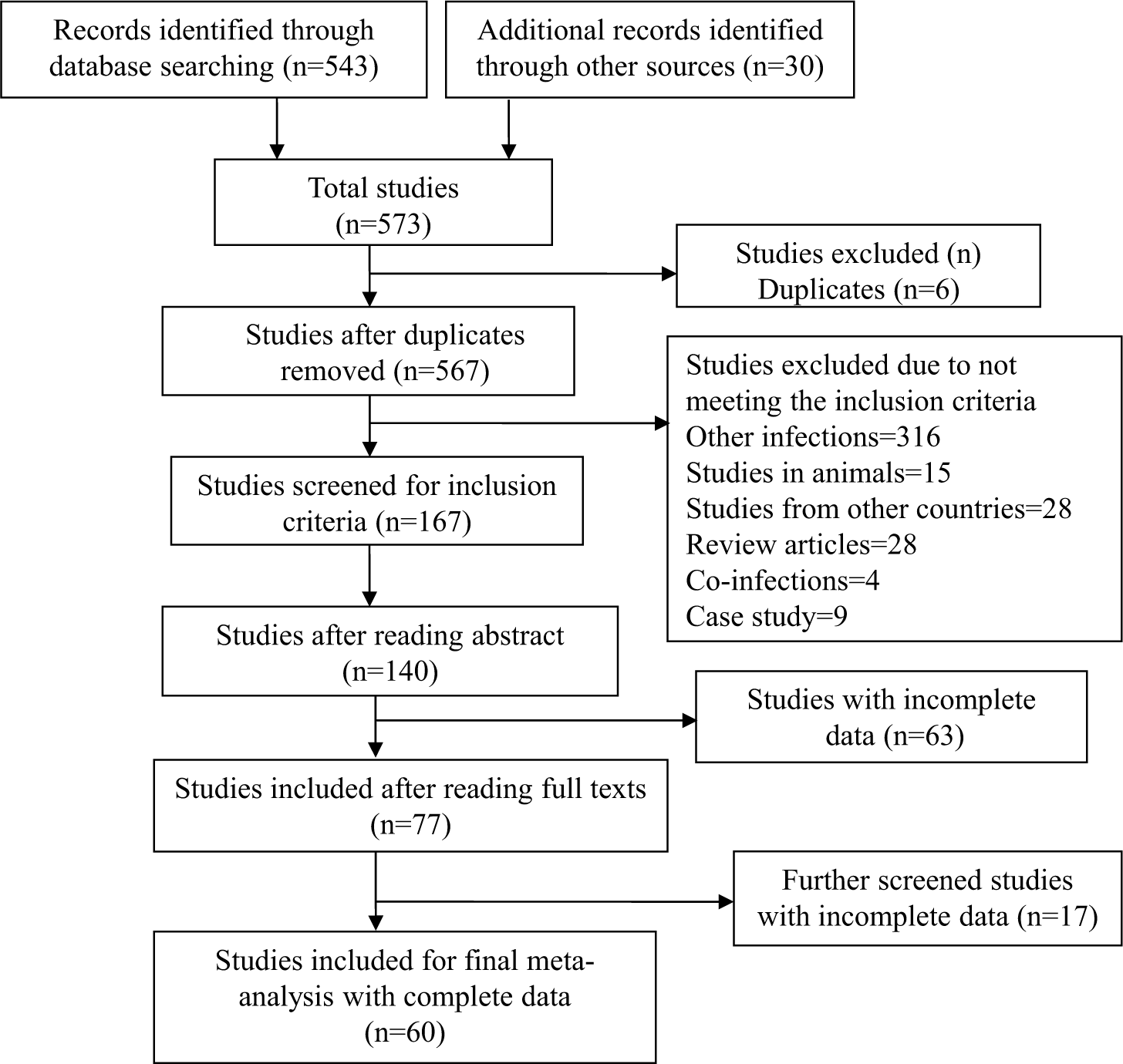
- PRISMA flowchart of studies selection. PRISMA indicates preferred reporting items for systematic reviews and meta-analyses. PRISMA, preferred reporting items for systematic reviews and meta-analysis.
Inclusion and exclusion criteria
The eligibility criteria for search strategy and studies selection were defined based on the condition, context and population framework as follows:
Population
Patients with acute undifferentiated febrile illness, pyrexia of unknown origin and acute febrile cases with fever <14 days with unknown aetiology were selected. Undifferentiated febrile illness was defined as fever of <14 days duration without any evidence of organ or system specific aetiology. Studies that focused on diseases other than undifferentiated febrile illness were excluded from this analysis.
Condition
Hospital based cross-sectional and epidemiological survey studies were included. Patients of all age groups and gender, admitted to the hospitals from different geographical regions of India were included. Outbreak studies and studies done on animals (chiggers and rodents) were excluded. Outbreak studies were not included as they can potentially lead to misinterpretation of prevalence data.
Context
Studies in which cases of scrub typhus were confirmed either by enzyme-linked immunosorbent assay (ELISA) or immunofluorescence assay (IFA) or PCR were included in this meta-analysis and those reporting scrub typhus positivity using alternative laboratory methods such as immune chromatography or Weil-Felix OX-K agglutination reaction were excluded. Weil-Felix test was initially used for the rapid sero-diagnosis of rickettsial infection in developing countries. It detects host immune response against different Proteus antigens such as OX19, OXK, and OX that cross-respond with rickettsiae. Due to cross-reactivity of the antigens there is very high chance of getting false results due to the use of non-rickettsial antigen resulted low sensitivity and specificity of the test. Currently, it has mostly been replaced by newer sensitive diagnosis tests. However, it is still in common use in resource limited settings for the primary screening of the scrub typhus that need further confirmation by the sensitive and specific tests. So, inclusion of those studies that used this test may not indicate the true positive cases of scrub typhus and may results the false prevalence of the disease. Studies describing the scrub typhus only on the basis of eschar sign were also excluded.
Initially, the selected studies underwent full abstract screening, and subsequently, the full texts of eligible studies were reviewed. To be considered eligible, the abstracts of the studies had to report the prevalence, incidence, number of reported cases, mortality, or burden of scrub typhus in any region of India. Duplicate articles, case control study, co-infection studies, animal and vector-based studies, review articles, studies involving other diseases, and studies carried out in other countries were excluded. All studies, showing the positivity and prevalence of scrub typhus among febrile patients with fever cases were selected for meta-analysis.
Data extraction
Three independent reviewers (GS, HVM and RK) used a pre-designed data extraction form to extract relevant information from the selected studies. The extracted data, including author names, publication year, study location, diagnostic test used, criteria for positivity, sample size (total number of acute febrile illness cases), fever onset, mortality, duration of sample collection, overall prevalence, gender-wise prevalence, and age groups, were recorded in a pre-designed template. The data were organised in Table I. In cases where there were discrepancies between the two reviewers, a fourth author (PS) was consulted to reach a consensus. Fever was found to be the most common symptom among scrub typhus patients. The primary outcomes of interest in this study were as follows: (a) the prevalence (proportion) of laboratory-confirmed scrub typhus infection among undifferentiated fever cases, (b) the case fatality ratio among laboratory-confirmed scrub typhus patients. The diagnosis of scrub typhus among undifferentiated fever cases and clinically suspected patients was based on commercially available serological test (IFA, IgM ELISA with a more than fourfold increase in antibody titers) or molecular laboratory assays like PCR targeting different genomic markers (56kDa, 47kDa, groEL genes). In studies where two or more tests were used for diagnosis or comparison of test efficacy, the prevalence was calculated based on the detection of Immunoglobulin M (IgM) antibodies against O. tsutsugamushi to ensure consistency. To calculate the case fatality ratio, the numerator included the reported number of deaths due to scrub typhus, while the denominator comprised laboratory-confirmed scrub typhus patients.
| Author | Year | Area | City/location | Total cases | Positive cases | Male | Female | Age (in yr) | Sample collection duration | Diagnosis test used | Fever |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abhilash et al31 | 2016 | South India | Andhra Pradesh, Kerala, Tamil Nadu | 1258 | 452 | 186 | 266 | ELISA | |||
| Ahmad et al32 | 2016 | North India | Uttarakhand | 233 | 65 | 30 | 35 | 18-65 | Dec 2012-Dec 2013 | ELISA | < 2 wk |
| Anitharaj et al33 | 2018 | South India | Puducherry | 220 | 134 | ELISA | |||||
| Bahera et al34 | 2020 | East India | Odisha | 432 | 114 | < 18 | Jan- Dec 2017 | ELISA | < 2 wk | ||
| Bal et al35 | 2019 | East India | Odisha | 413 | 201 | 124 | 77 | < 15 | June- Nov | ELISA | < 15 d |
| Bal et al36 | 2021 | East India | Odisha | 140 | 45 | ELISA | |||||
| Bithu et al37 | 2014 | North India | Rajasthan | 271 | 133 | 80 | 53 | 40-46 | Sep-Dec 2012 | ELISA | |
| Chunchanur et al38 | 2019 | South India |
Bengaluru, Karnataka |
100 | 22 | ELISA | |||||
| Dave et al39 | 2022 | West India | Udaipur, Rajasthan | 3814 | 1340 | Jan -Dec 2019 | ELISA | ||||
| Giri et al40 | 2018 | East India | Kolkata | 861 | 97 | March 2012- Dec 2015 | ELISA | ||||
| Hussain et al41 | 2022 | North India | Uttar Pradesh | 1743 | 361 | ELISA | |||||
| Jain et al42 | 2019 | North India | Haryana | 230 | 39 | ELISA | |||||
| Kalal et al43 | 2016 | South India | Bengaluru | 103 | 53 | < 18 | Jan 2010- October 2012 | ELISA | 11 d | ||
| Karthikeyan et al44 | 2019 | South India | Puducherry | 50 | 37 | ELISA | |||||
| Kavirayani et al45 | 2021 | South India | 214 | 32 | < 18 | Jan 2018 - June 2018 | ELISA | ||||
| Khan et al46 | 2012 | Northeast India | Assam, Nagaland | 314 | 108 | ||||||
| Khan et al47 | 2015 | North India | Dehradun | 3540 | 412 | ICT, ELISA | |||||
| Kolarul et al48 | 2018 | South India | Manipal | 1036 | 319 | 179 | 140 | ≥18 | ELISA, IFA | ||
| Kumar et al49 | 2014 | North India | Chandigarh | 201 | 49 | Sep 2011 - Nov 2012 | nPCR | ||||
| Lalrinkima et al50 | 2017 | Northeast India | Mizoram | 4081 | 283 | 21-30 | ICT RDT kit, ELISA | ||||
| Mahajan et al51 | 2021 | North India | Chandigarh | 224 | 77 | 2 months - 14 yr | June 2013 - Dec 2017 | ELISA | |||
| Manjunathachar et al52 | 2021 | Central India | Madhya Pradesh | 144 | 48 | ELISA, nPCR | |||||
| Mittal et al53 | 2021 | North India | Eastern Uttar Pradesh | 125 | 25 | Aug 2018- July 2019 | ELISA | ||||
| Morch et al54 | 2017 | Northeast India | Tezpur, Assam | 295 | 75 | ≥5 - 60 | April 2011-Nov 2012 | ELISA | 2-14 d | ||
| Morch et al54 | 2017 | South India | Oddanchatram, Tamil Nadu | 314 | 7 | ≥5 - 60 | April 2011-Nov 2012 | ELISA | 2-14 d | ||
| Morch et al54 | 2017 | South India | Ambur, Tamil Nadu | 302 | 35 | ≥5 - 60 | April 2011-Nov 2012 | ELISA | 2-14 d | ||
| Morch et al54 | 2017 | Central India | Mungeli, Chhattisgarh | 33 | 1 | ≥5 - 60 | April 2011-Nov 2012 | ELISA | 2-14 d | ||
| Morch et al54 | 2017 | South India | Anantpur, Andhra Pradesh | 152 | 5 | ≥5 - 60 | April 2011-Nov 2012 | ELISA | 2-14 d | ||
| Morch et al54 | 2017 | West India | Ratnagiri, Maharashtra | 237 | 20 | ≥5 - 60 | April 2011-Nov 2012 | ELISA | 2-14 d | ||
| Morch et al54 | 2017 | North India | Raxaul, Bihar | 107 | 16 | ≥5 - 60 | April 2011-Nov 2012 | ELISA | 2-14 d | ||
| Murhekar et al55 | 2016 | North India | Uttar Pradesh | 109 | 59 | Sep-Oct 2015 | ELISA, PCR | ||||
| Narvencar et al56 | 2012 | West India | Goa | 44 | 15 | June 2009-Oct 2010 | ELISA | 5 d | |||
| Oberoi et al57 | 2014 | North India | Ludhiyana, Punjab | 772 | 98 | 60 | 37 | ELISA | |||
| Panigrahi et al58 | 2022 | East India | Southern-Odisha | 170 | 74 | ELISA | |||||
| Parai et al59 | 2023 | South India | Odisha | 1840 | 523 | 18-45 | ELISA | ||||
| Paulraj et al60 | 2021 | South India | Nilgiri, Tamil Nadu | 214 | 13 | 3 | 10 | Oct 2014 - March 2016 | ELISA | ||
| Prakash et al61 | 2011 | South India | Vellore, Tamil Nadu | 87 | 51 | WF, ELISA, nPCR | |||||
| Prakash et al62 | 2022 | Central India | Chhattisgarh | 221 | 83 | ELISA, PCR | |||||
| Premraj et al63 | 2018 | South India | Kanchipuram, Tamil Nadu | 558 | 50 | 19 | 31 | >60 | June 2015 - May 2016 | ELISA | |
| Raina et al64 | 2018 | North India | Himachal Pradesh | 1164 | 262 | ELISA | |||||
| Ramyasree et al65 | 2015 | South India | Andhra Pradesh | 100 | 39 | ELISA | |||||
| Rao et al66 | 2019 | East India | Rourkela, Odisha | 287 | 10 | <15 | ELISA | ||||
| Rathi et al67 | 2011 | Central India | Akola, Maharashtra | 161 | 23 | ||||||
| Rauf et al68 | 2018 | North India | Chandigarh | 217 | 23 | 3-5 | |||||
| Rawat et al69 | 2017 | North India | Uttarakhand | 281 | 158 | 62 | 106 | Feb - Dec 2015 | IFA, ELISA, RT PCR | ||
| Rizvi et al70 | 2018 | North India | Aligarh, Uttar Pradesh | 357 | 91 | RDT, ELISA | < 5 d | ||||
| Roy et al71 | 2021 | Central India | Wardha, Maharashtra | 274 | 37 | PCR,LAMP, ELISA | 5 d | ||||
| Rupa et al72 | 2015 | South India | Puducherry | 482 | 109 | 10 months - 80 yr | Jan 2012 - June 2015 | WF, ELISA | |||
| Sahu et al73 | 2015 | East India | Eastern Odisha | 150 | 50 | 33 | 17 | April 2011 - Oct 2013 | ELISA | ||
| Sankhyan et al74 | 2014 | North India | Chandigarh, Haryana, Himachal Pradesh, Punjab, Uttar Pradesh | 35 | 15 | ELISA | |||||
| Sarangi et al75 | 2016 | East India | Odisha | 71 | 26 | <14 | July 2015 - Dec 2015. | ELISA | |||
| Shelke et al76 | 2017 | Central India | Wardha, Maharashtra | 270 | 127 | Jan 2015 - Nov 2016 | RDT, ELISA | ||||
| Shrinivasan et al77 | 2019 | South India | Vellore, Tamil Nadu | 103 | 70 | 30 | 40 | PCR | |||
| Tarai et al78 | 2022 | North India | New Delhi | 473 | 56 | ELISA, PCR | |||||
| Thangraj et al79 | 2018 | North India | Deoria and Gorakhpur, Uttar Pradesh | 819 | 155 | ELISA | |||||
| Thirunavukkarasu et al80 | 2021 | South India | Puducherry | 2710 | 660 | 350 | 310 | < 12 | ELISA | ≥7 d | |
| Usha et al81 | 2014 | South India | Tirupati, Andhra Pradesh | 280 | 158 | 25-65 | April 2011 - Dec 2012 | WF, ELISA | |||
| Usha et al82 | 2016 | South India | Andhra Pradesh | 663 | 258 | ELISA, nPCR | |||||
| Vikram et al83 | 2020 | Central India | Chhattisgarh | 169 | 35 | 16-30 | ELISA | ||||
| Vivian et al84 | 2017 | North India | Gorakhpur, Uttar Pradesh | 224 | 40 | ELISA |
ELISA, enzyme-linked immunosorbent assay; RT-PCR, reverse transcription polymerase chain reaction; nPCR-nested polymerase chain reaction; WF, Weil-Felix test, RDT, rapid diagnostic test; IFT, immunofluorescence test; LAMP, loop-mediated isothermal amplification test; d, day(s); wk, week(s).
Statistical analysis
All statistical analyses were conducted using R Studio software (version 1.2.5042, “R core team 2017) with the “meta” package29, following the guidelines of JBI guidelines for reporting systematic reviews of prevalence and incidence27. Individual studies prevalence was Freeman-Tukey Double Arcsine transformed, and Clopper-Pearson confidence interval method was used to calculate the confidence interval of individual study findings. Inverse variance method was used to calculate the pooled prevalence. Heterogeneity was tested using maximum likelihood ratio test. Baujat plot was plotted to detect the sources of heterogeneity30. Random effect model was used when there was high heterogeneity (I2>50) in the studies.
Forest plots were constructed for display of overall prevalence and prevalence in different regions across India. Funnel plot with double arcsine transformed proportion on x-axis and standard errors on y-axis was plotted to see the symmetry of publications and the Egger’s test using mixed-effects meta-regression model was used to evaluate publication bias. P<0.05 was considered statistically significant.
Results
Study characteristics
Among all databases screened, 573 studies were identified after the literature search. From PubMed, National Library of Medicine, Science Direct, and Google Scholar, 543 studies were identified, and an additional 30 records were added from other sources and cross references. After removing six duplicate records, 567 studies were found eligible for title and abstract screening. Out of these 567 studies, 400 were excluded as they didn’t satisfy study inclusion criteria. These excluded studies were review articles, book chapters, case reports, or studies performed on animals and vectors (mite) and co-infection studies, as mentioned above. All 167 studies fulfilling the inclusion criteria were reviewed from their abstract and then from their full text, which left us with 77 studies. Complete data was mentioned in only 60 studies (Fig. 1) which were found eligible for meta-analysis. The characteristics of studies included in the meta-analysis are depicted in Table I31-84.
Characteristics of included studies
Sixty studies that reported scrub typhus positivity among acute undifferentiated febrile illness cases were analysed for estimating the prevalence. All the studies were published between the year 2006 and 2023. Most studies were conducted in south India (n=20, 33.3% of included studies) followed by the north India (n=19, 31.6%), eastern part of India (n=8, 13.3%), central India (n=7, 11.6%), north eastern region (n=3, 5%) and from west India (n=3, 5%). There was one study in which, samples were collected from south, north, north-east, west and central part of India. In total there were 34,492 cases from 60 studies which were analyzed; 10,786 cases were reported from the 20 studies conducted in southern region and 11,125 cases from 19 studies from the northern region, 4,690 cases from four studies from north-eastern region, 4,095 cases from three studies from western part, 2,524 cases from eastern part, and 1272 cases were from central part of India (Table I)31-84.
Primary and secondary outcomes
Sero-prevalence of scrub typhus: The meta-analysis of 34,492 cases from 60 studied revealed the overall sero-prevalence of scrub typhus among patients with undifferentiated fever cases (with unknown aetiology or undifferentiated febrile illness) as 26.41 per cent (95% CI: 22.03-31.03) in the random effect model (Fig. 2). The sub group analysis was performed for identifying the pooled prevalence in different regions across India. The highest prevalence of scrub typhus among febrile cases was found in the south India (30.23%, 95% CI: 20.56–40.86) followed by the east India (27.49%, 95% CI:16.82-39.63), whereas the lowest prevalence was reported from north-east India (20.62%, 95% CI: 8.55-36.22). However, there was no significant difference in sero-positivity across the regions [χ25 = 1.68, df = 5, (P= 0.89)].

- Forest plot showing overall pooled prevalence and region-wise prevalence. The plot was generated using R software. CI, confidence interval.
Test of heterogeneity and sensitivity analysis
A significant degree of heterogeneity was observed in overall pooled data (I2=98%, ζ2=0.0389). To identify the source of heterogeneity, Baujat plot was plotted. As shown in Figure 3, the studies performed by Dave et al39, 2022; Khan et al47, 2015; and Lalrinkima et al50, 2017 contribute significantly to the heterogeneity.

- Baujat Plot showing studies causing heterogeneity. The numbers in the plot corresponds to the study serial number as indicated in Table I. The plot was generated using R software.
To see the impact of each study on the strength of the analysis method used in the study and recheck the conclusions for the Freeman-Tukey double arcsine transformation, sensitivity analysis was performed by omitting the individual study and pooling the results of the other studies. As shown in Figure 4, study inference is not affected by omitting individual studies.

- Sensitivity analysis plot for prevalence and respective 95 % CI by omitting one study using R software.
Publication bias
Egger’s test (t=2.05, P=0.045) shows significant publication bias with an intercept of linear regression=3.65 (95% CI: 0.15-7.14). The publication bias is also evident from the funnel plot (Fig. 5). Regression test for funnel plot asymmetry using mixed-effect regression model also shows asymmetry [z=2.0713, P=0.0383, b=0.436 (95% CI: 0.326-0.547)] in the funnel plot. The publication bias may be due to variation in sample size in the included studies.

- Funnel plot showing publication bias using R software.
Critical appraisal/ quality assessment
A standardized JBI critical appraisal tool was used to evaluate the quality of the literature for selected studies in three categories- Yes (1), No (0) and Unclear (U). The JBI critical quality assessment was done by the authors (GS and MHV) and whenever there was confusion or discrepancy in decision making, the third author (RK) verified the decision. The methodological quality assessment of each study and the risk of bias for each aspect were reported in Table II. Out of 60 studies, three were found to be of low quality as the JBI quality score for those studies was less than six from the maximum score of nine.
| Author, yr | Was the sample frame appropriate to address the target population? | Were study participants sampled in an appropriate way? | Was the sample size adequate? | Were the study subjects & the setting described in detail? | Was the data analysis conducted with sufficient coverage of the identified sample | Were valid methods used for the identification of the condition? | Was the condition measured in a standard, reliable way for all participants | Was there appropriate statistical analysis? | Was the response rate adequate, & if not, was the low response rate managed appropriately | JBI Quality score |
|---|---|---|---|---|---|---|---|---|---|---|
| Abhilash et al31 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Ahmad et al32 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Anitharaj et al33 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 7 |
| Bahera et al34 | 1 | 1 | 1 | 1 | U | 1 | 1 | 1 | 1 | 8 |
| Bal et al35 | 0 | 1 | 1 | 1 | U | 1 | 1 | 1 | 1 | 7 |
| Bal et al36 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Bithu et al37 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Chunchanur et al38 | U | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 5 |
| Dave et al39 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | U | 1 | 8 |
| Giri et al40 | 0 | 1 | 1 | 1 | U | 1 | 1 | 1 | 1 | 7 |
| Hussain et al41 | U | 1 | 1 | 0 | 1 | 1 | U | 0 | 1 | 5 |
| Jain et al42 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Kalal et al43 | 0 | 1 | 1 | 1 | U | 1 | 1 | 1 | 1 | 7 |
| Karthikeyan et al44 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | U | 1 | 7 |
| Kavirayani et al45 | 0 | 1 | 1 | 1 | U | 1 | 1 | 1 | 1 | 7 |
| Khan et al46 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 8 |
| Khan et al47 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | U | 1 | 8 |
| Kolarul et al48 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Kumar et al49 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Lalrinkima et al50 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 8 |
| Mahajan et al51 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Manjunathachar et al52 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Mittal et al53 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Morch et al54 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Morch et al54 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Morch et al54 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Morch et al54 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Morch et al54 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Morch et al54 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Morch et al54 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Murhekar et al55 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | U | 8 |
| Narvencar et al56 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Oberoi et al57 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 8 |
| Panigrahi et al58 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 8 |
| Parai et al59 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Paulraj et al60 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Prakash et al61 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 8 |
| Prakash et al62 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Premraj et al63 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Raina et al64 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Ramyasree et al65 | 1 | 1 | 0 | 1 | U | 1 | 1 | 1 | U | 6 |
| Rao et al66 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Rathi et al67 | 0 | 1 | 1 | 1 | U | 1 | 1 | 1 | 1 | 7 |
| Rauf et al68 | 0 | 1 | 1 | 1 | U | 1 | 1 | 1 | 1 | 7 |
| Rawat et al69 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Rizvi et al70 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Roy et al71 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Rupa et al72 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 8 |
| Sahu et al73 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 8 |
| Sankhyan et al74 | 0 | U | 0 | 1 | U | 1 | 1 | 1 | 1 | 5 |
| Sarangi et al75 | 0 | 1 | 1 | 1 | U | 1 | 1 | 0 | 1 | 6 |
| Shelke et al76 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Shrinivasan et al77 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 8 |
| Tarai et al78 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 8 |
| Thangraj et al79 | 0 | 1 | 1 | 1 | U | 1 | 1 | 1 | U | 6 |
| Thirunavukkarasu et al80 | 0 | 1 | 1 | 1 | U | 1 | 1 | 1 | 1 | 7 |
| Usha et al81 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 8 |
| Usha et al82 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Vikram et al83 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Vivian et al84 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
Case fatality
Of the 60 studies, six studies had been identified in which authors have reported the mortality due to scrub typhus in undifferentiated febrile illness. Among these six studies two were from south India, one from north India, one from west India, one from north-central India and one from north-western India. A total of 743 cases were estimated from those six studies and mortality was reported of 51 cases. The pooled mortality rate in scrub typhus positive cases was estimated to be 7.69 (95% CI: 4.37-11.72) (Table III85). There was a low heterogeneity in the case fatality rate across the studies (I2=67%, P<0.01) (Fig. 6).
| Author | Region | Total positive cases | Case fatality % |
|---|---|---|---|
| Ahmad et al32 | North India | 65 | 6 (9.2) |
| Bithu et al37 | North western India | 133 | 13 (9.7) |
| Chunchanur et al38 | South India | 22 | 1(4.5) |
| Narvencar et al85 | West India | 15 | 5 (33) |
| Tarai et al78 | North-central India | 56 | 5 (8.9) |
| Abhilash et al31 | South-India | 452 | 21(4.6) |

- Forest plot showing overall pooled prevalence of case fatality rate. The plot was generated using R software.
The most common complication reported in these studies were acute respiratory distress syndrome. Multi-organ distress syndrome associated with acute renal failure, meningoencephalitis, and liver dysfunction were reported among the cases who died. The highest case fatality was the outcome of severe complication due to delayed diagnosis and treatment. The results reflect the fact that the number of scrub typhus cases that actually occur are probably significantly higher than the numbers diagnosed and reported in this region. Yet the causes of this variation are unclear and can vary on host, region, and strain variables.
Discussion
Scrub typhus, a febrile illness, has experienced a surge in the number of cases in India, making it an escalating for public health. Nonetheless, the actual magnitude of the disease remains elusive due to under-diagnosis and under-reporting86. Henceforth, a systematic review and meta-analysis was undertaken to gather and analyse the published data from the past decade pertaining to the sero-prevalence of scrub typhus among patients with acute undifferentiated febrile illness, regional cut-off values or changes, and mortality rates across India. Our objective was to comprehensively understand the true prevalence of scrub typhus in the country. Following our set exclusion criteria, only hospital-based studies were included, and the estimated discovered overall sero-prevalence of scrub typhus among patients with acute undifferentiated febrile illness in India was 26.41 per cent. The study’s findings highlighted the substantial differences in scrub typhus mortality. The findings of this systematic analysis show that the southern Indian states exhibit high prevalence (30.23%) and north-eastern part of the country has relatively lower prevalence (20.62%) of scrub typhus among undifferentiated fever cases. Furthermore, despite the low prevalence of the disease in the western part of India, it had the highest case fatality rate (33%) followed by north-west India (9.7%).
The burden of scrub typhus has increased due to excessive use of broad-spectrum antibiotics for other febrile infections, particularly as a response to associated complications and mortality. Tetracycline and chloramphenicol, once popular treatments for acute febrile illness caused by scrub typhus, have become less favored due to the emergence of antimicrobial resistance. This has led to an increase in the prevalence of scrub typhus and is likely to escalate the associated morbidity and mortality, as previously mentioned. Furthermore, in tropical regions, clinical signs of scrub typhus mimic those of other acute febrile illnesses such as leptospirosis, malaria, acute hepatitis and often results in inaccurate diagnosis or misdiagnosed as viral fever and left untreated relying on self-cure87,88. All these factors may have influenced the rise of scrub typhus in India over the past two decades.
Our findings indicate that Orientia tsutsugamushi infection is widespread throughout the country. Notably, most of the studies reported were from the southern part of India due to increased awareness about the disease, heightened clinical suspicion among physicians following previously reported cases, and improved accessibility to diagnostic facilities in this region. These factors likely contributed to a greater number of studies being conducted in the southern part of the country.
A large heterogeneity among included studies was observed in present meta-analysis, which could be due to differences in time of sample collection (seasonal variation in positivity rate) or due to the use of different kits having different specificity and sensitivity. Since most of the studies had not reported the sensitivity and specificity of the method used for the diagnosis of the scrub typhus, the results of one study could not be compared with that of another. This is one the limitations of the analysis of the present investigation. Further, there were significant biases in included studies due to non-uniformity in sample size and effect size of included studies.
To effectively control and prevent the spread of scrub typhus, it is crucial to establish a robust surveillance system and continuously monitor cases in endemic regions using sensitive and early diagnostic assays. The utilization of sensitive diagnostic tests, such as scrub typhus IgM ELISA and Real time-PCR (RT-PCR) assays, has facilitated the detection of previously hidden or unidentified cases, resulting in an upsurge in reported numbers. Serological assays are primarily employed for the definitive diagnosis of scrub typhus in the absence of an eschar89. Consequently, the availability of improved tests requiring conventional and standardized laboratory facilities will likely result in a higher number of diagnosed cases. Therefore, the accessibility of sensitive and affordable point-of-care tests (POCTs) will be particularly beneficial in resource-limited settings, facilitating early disease detection and prompt treatment for scrub typhus.
In conclusion, to reduce the burden of scrub typhus, it is imperative to raise physician awareness about the disease from primary health care to tertiary health setup, implement point-of-care diagnosis, and ensure appropriate treatment with effective antibiotics. These essential measures, coupled with ongoing surveillance and enhanced diagnostic capabilities under one health aspect, will play a pivotal role in controlling the spread of scrub typhus and mitigating the impact of outbreaks of this neglected disease. In order to reduce the burden of the disease in the future, it is crucial to raise awareness about scrub typhus, implement point-of-care diagnosis, and ensure appropriate treatment.
Implication for practice
Scrub typhus is a seriously overlooked tropical disease, impacting mostly rural populations and increasing in urban regions. Our finding indicates the high burden of scrub typhus in India. When fever is the primary symptom of the illness, diagnosis becomes more difficult as other infections appear with same symptoms. This shows that a greater degree of clinical suspicion of scrub typhus should be applied to feverish patients and underlines the need for ensuring accessible and affordable diagnostic facilities in peripheral settings. Case fatality rates varied between geographical regions and states, mainly due to complication. Effective control and prevention of the spread and outbreaks of this neglected illness necessitate the implementation of early diagnostic procedures in endemic locations and a well-established surveillance system. These measures, combined with continuous surveillance and improved diagnostic capabilities, will play a vital role in controlling the spread of scrub typhus and mitigating the impact of outbreaks of this neglected disease.
Financial support & sponsorship
The study received financial support from Indian Council of Medical Research (ICMR) under extramural Project (Ref. No.2020-3473).
Conflicts of Interest
None.
Use of Artificial Intelligence (AI)-Assisted Technology for manuscript preparation
The authors confirm that there was no use of AI-assisted technology for assisting in the writing of the manuscript and no images were manipulated using AI.
References
- Orientia tsutsugamushi: A neglected but fascinating obligate intracellular bacterial pathogen. PLoS pathogens. 2017;13:e1006657.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Molecular description of a novel orientia species causing scrub typhus in Chile. Emerg Infect Dis. 2020;26:2148-56.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A review of the global epidemiology of scrub typhus. PLoS Negl Trop Dis. 2017;11:e0006062.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Detection of orientiasp. DNA in rodents from Asia, West Africa and Europe. Parasit Vectors. 2015;8:1-4.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Abundance & distribution of trombiculid mites & orientia tsutsugamushi, the vectors & pathogen of scrub typhus in rodents & shrews collected from Puducherry & Tamil Nadu, India. Indian J Med Res. 2016;144:893-900.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Evidence of natural infection of orientia tsutsugamushi in vectors and animal hosts - Risk of scrub typhus transmission to humans in Puducherry, South India. Indian J Public Health. 2020;64:27-31.
- [CrossRef] [PubMed] [Google Scholar]
- A study on ectoparasites with special reference to chigger mites on rodents/shrews in scrub typhus endemic areas of Kerala, India. Entomon. 2020;45:285-94.
- [Google Scholar]
- Scrub typhus—scientific neglect, ever-widening impact. N Engl J Med. 2016;375:913-5.
- [CrossRef] [PubMed] [Google Scholar]
- Estimating the burden of scrub typhus: A systematic review. PLoS Negl Trop Dis. 2017;11:e0005838.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Severe scrub typhus infection: Clinical features, diagnostic challenges and management. World J Crit Care Med. 2015;4:244-50.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinical manifestations of scrub typhus. Trans R Soc Trop Med Hyg. 2017;111:43-54.
- [CrossRef] [PubMed] [Google Scholar]
- Early clinical suspicion and early use of doxycycline reduces scrub typhus associated complications. J Assoc Physicians India. 2019;67:26-7.
- [PubMed] [Google Scholar]
- A systematic review of mortality from untreated scrub typhus (orientia tsutsugamushi) PLoS Negl Trop Dis. 2015;9:e0003971.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Risk factors for acquisition of scrub typhus in children admitted to a tertiary centre and its surrounding districts in South India: A case control study. BMC Infect Dis. 2019;19:665.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Epidemiology & risk factors of scrub typhus in south India. Indian J Med Res. 2016;144:76-81.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Outbreak of scrub typhus in southern India during the cooler months. Ann N Y Acad Sci. 2003;990:359-64.
- [CrossRef] [PubMed] [Google Scholar]
- A comparative study of clinical presentation of scrub typhus patients between pre and post monsoon clusters in a tertiary care hospital in Kolkata. J Indian Med Assoc. 2022;120:40-6.
- [Google Scholar]
- Scrub typhus associated acute kidney injury: An emerging health problem in Odisha, India. J Vector Borne Dis. 2021;58:359-67.
- [CrossRef] [PubMed] [Google Scholar]
- Emergence of “urban scrub typhus” during Monsoon season in an urban pocket and biodiversity hotspot of New Delhi, India. J Marine Med Soc. 2022;24:124-30.
- [Google Scholar]
- Geographical distribution, effect of season & life cycle of scrub typhus. Jk Science. 2010;12:63.
- [Google Scholar]
- Recent outbreak of scrub typhus in North Western part of India. Indian J Med Microbiol. 2014;32:247-50.
- [CrossRef] [PubMed] [Google Scholar]
- Scrub typhus ecology: A systematic review of Orientia in vectors and hosts. Parasit Vectors. 2019;12:513.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Transmission of orientia tsutsugamushi, the aetiological agent for scrub typhus, to co-feeding mites. Parasitology. 2000;120:601-7.
- [CrossRef] [PubMed] [Google Scholar]
- Transstadial and transovarial transmission of orientia tsutsugamushi in leptotrombidium imphalum and leptotrombidium chiangraiensis (Acari: Trombiculidae) J Med Entomol. 2009;46:1442-5.
- [CrossRef] [PubMed] [Google Scholar]
- The burden of scrub typhus in India: A systematic review. PLoS Negl Trop Dis. 2021;15:e0009619.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. 2015;13:147-53.
- [CrossRef] [PubMed] [Google Scholar]
- The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- How to perform a meta-analysis with R: A practical tutorial. Evid Based Ment Health. 2019;22:153-60.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A graphical method for exploring heterogeneity in meta-analyses: Application to a meta-analysis of 65 trials. Stat Med. 2002;21:2641-52.
- [CrossRef] [PubMed] [Google Scholar]
- Acute undifferentiated febrile illness in patients presenting to a tertiary care hospital in South India: clinical spectrum and outcome. J Glob Infect Dis. 2016;8:147-54.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A comparative hospital-based observational study of mono- and co-infections of malaria, dengue virus and scrub typhus causing acute undifferentiated fever. Eur J Clin Microbiol Infect Dis. 2016;35:705-11.
- [CrossRef] [PubMed] [Google Scholar]
- Serological diagnosis of acute scrub typhus in southern india: evaluation of inbios scrub typhus detect IgM rapid test and comparison with other serological tests. J Clin Diagn Res. 2016;10:DC07-DC10.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinico-epidemiological analysis of scrub typhus in hospitalised patients presenting with acute undifferentiated febrile illness: A hospital-based study from Eastern India. Indian J Med Microbiol. 2019;37:278-80.
- [CrossRef] [PubMed] [Google Scholar]
- Profile of pediatric scrub typhus in Odisha, India. Indian Pediatr. 2019;56:304-6.
- [PubMed] [Google Scholar]
- Scrub typhus associated acute kidney injury: An emerging health problem in Odisha, India. J Vector Borne Dis. 2021;58:359-67.
- [CrossRef] [PubMed] [Google Scholar]
- Possibility of scrub typhus in fever of unknown origin (FUO) cases: An experience from Rajasthan. Indian J Med Microbiol. 2014;32:387-90.
- [CrossRef] [PubMed] [Google Scholar]
- Phylogenetic diversity of orientia tsutsugamushi isolates in patients with scrub typhus in Bengaluru, India. Indian J Med Microbiol. 2019;37:438-41.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology, clinical presentation, lab diagnosis and outcome of scrub typhus outbreak in a tertiary care centerin Southern Rajasthan. J Assoc Physicians India. 2022;70:11-2.
- [PubMed] [Google Scholar]
- Scrub typhus - A major cause of pediatric intensive care admission and multiple organ dysfunction syndrome: A single-center experience from India. Indian J Crit Care Med. 2018;22:107-10.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Scrub typhus and its co-infection with leptospirosis at a tertiary care hospital in Uttar Pradesh. Trop Doct. 2022;52:302-3.
- [CrossRef] [PubMed] [Google Scholar]
- Scrub typhus infection, not a benign disease: An experience from a tertiary care center in Northern India. Med Pharm Rep. 2019;92:36-42.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Scrub typhus and spotted fever among hospitalised children in South India: Clinical profile and serological epidemiology. Indian J Med Microbiol. 2016;34:293-8.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of loop-mediated isothermal amplification assay for detection of scrub typhus in patients with acute febrile illness presenting to a tertiary care centerin Puducherry, India. J Lab Physicians. 2019;11:82-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinical profile and role of serology in pediatric acute febrile illness: Experience from a tertiary care hospital in South India. Clin Epidemiol Glob Health. 2021;12:100898.
- [Google Scholar]
- Re-emergence of scrub typhus in northeast India. Int J Infect Dis. 2012;16:e889-e90.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of scrub typhus-A cause of concern in Uttarakhand Region, India. Int J Curr Microbiol App Sci. 2015;1:101-9.
- [Google Scholar]
- Scrub typhus diagnosis on acute specimens using serological and molecular assays—a 3-year prospective study. Diagn Microbiol Infect Dis. 2018;91:112-7.
- [CrossRef] [PubMed] [Google Scholar]
- Scrub typhus is an under-recognized cause of acute febrile illness with acute kidney injury in India. PLoS Negl Trop Dis. 2014;8:e2605.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Scrub typhus in mizoram, India. J Vector Borne Dis. 2017;54:369-71.
- [CrossRef] [PubMed] [Google Scholar]
- Spectrum of multiorgan dysfunction in scrub typhus infection. J Trop Pediatr. 2021;67:fmab074.
- [CrossRef] [PubMed] [Google Scholar]
- Determination of cut-off of diagnostic ELISA for Scrub typhus in endemic setup: Central India. J Vector Borne Dis. 2021;58:90-3.
- [CrossRef] [PubMed] [Google Scholar]
- Scrub typhus: An under-reported and emerging threat-hospital based study from central and eastern Uttar Pradesh, India. J Vector Borne Dis. 2021;58:323-8.
- [CrossRef] [PubMed] [Google Scholar]
- Acute undifferentiated fever in India: A multicentre study of aetiology and diagnostic accuracy. BMC Infect Dis. 2017;17:665.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Acute encephalitis syndrome in Gorakhpur, Uttar Pradesh, India–Role of scrub typhus. J Infect. 2016;73:623-6.
- [CrossRef] [PubMed] [Google Scholar]
- Scrub typhus in patients reporting with acute febrile illness at a tertiary health care institution in Goa. Indian J Med Res. 2012;136:1020-4.
- [PubMed] [PubMed Central] [Google Scholar]
- Scrub typhus-an emerging entity: A study from a tertiary care hospital in North India. Indian J Public Health. 2014;58:281-3.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology of scrub typhus in a tertiary care hospital of Southern Odisha: A cross sectional study. Indian J Med Microbiol. 2023;42:92-6.
- [CrossRef] [PubMed] [Google Scholar]
- Scrub typhus seroprevalence from an eastern state of India: Findings from the state-wide serosurvey. Trans R Soc Trop Med Hyg. 2023;117:22-7.
- [CrossRef] [PubMed] [Google Scholar]
- First seroprevalence report of scrub typhus from the tribal belts of the Nilgiris district, Tamil Nadu, India. Indian J Med Res. 2021;153:503-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Nested polymerase chain reaction on blood clots for gene encoding 56kDa antigen and serology for the diagnosis of scrub typhus. Indian J Med Microbiol. 2011;29:47-50.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of an in-house PCR for diagnosing scrub typhus along with a preliminary study of genotypic characterization of Orientia tsutshugamushi circulating in Chhattisgarh: A prospective clinico-microbiological study. Indian J Med Microbiol. 2022;40:510-5.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical profile and risk factors associated with severe scrub typhus infection among non-ICU patients in semi-urban south India. J Vector Borne Dis. 2018;55:47-51.
- [CrossRef] [PubMed] [Google Scholar]
- Coinfections as an aetiology of acute undifferentiated febrile illness among adult patients in the sub-Himalayan region of north India. J Vector Borne Dis. 2018;55:130-6.
- [CrossRef] [PubMed] [Google Scholar]
- Seroprevalence of scrub typhus at a tertiary care hospital in Andhra Pradesh. Indian J Med Microbiol. 2015;33:68-72.
- [CrossRef] [PubMed] [Google Scholar]
- Dengue, chikungunya, and scrub typhus are important etiologies of non-malarial febrile illness in Rourkela, Odisha, India. BMC Infect Dis. 2019;19:572.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Rickettsial diseases in central India: proposed clinical scoring system for early detection of spotted fever. Indian Pediatr. 2011;48:867-72.
- [CrossRef] [PubMed] [Google Scholar]
- Non-respiratory and non-diarrheal causes of acute febrile illnesses in children requiring hospitalization in a tertiary care hospital in North India: a prospective study. Am J Trop Med Hyg. 2018;99:783-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Epidemiological, clinical and laboratory profile of scrub typhus cases detected by serology and RT-PCR in Kumaon, Uttarakhand: a hospital-based study. Trop Doct. 2018;48:103-6.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of scrub typhus in pyrexia of unknown origin and assessment of interleukin-8, tumor necrosis factor-alpha, and interferon-gamma levels in scrub typhus-positive patients. Indian J Pathol Microbiol. 2018;61:76-80.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of nested PCR and loop mediated isothermal amplification assay (LAMP) targeting 47 kDa gene of Orientia tsutsugamushi for diagnosis of scrub typhus. Indian J Med Microbiol. 2021;39:475-8.
- [CrossRef] [PubMed] [Google Scholar]
- Serodiagnosis of scrub typhus at a tertiary care hospital from Southern India. J Clin Diagn Res. 2015;9:DC05-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Scrub typhus in a tertiary care hospital in the eastern part of Odisha. Apollo Medicine. 2015;12:2-6.
- [Google Scholar]
- Clinical profile of scrub typhus in children and its association with hemophagocytic lymphohistiocytosis. Indian Pediatr. 2014;51:651-3.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical profile of scrub typhus in children treated in a tertiary care hospital in eastern India. Pediatria Polska. 2016;91:308-11.
- [Google Scholar]
- Spectrum of infections in acute febrile illness in central India. Indian J Med Microbiol. 2017;35:480-4.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of nested polymerase chain reaction and real-time polymerase chain reaction targeting 47kda gene for the diagnosis of scrub typhus. Indian J Med Microbiol. 2019;37:50-3.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiological, clinical and genetic characterization of scrub typhus in patients presenting with acute febrile illness in New Delhi. Indian J Med Microbiol. 2022;40:552-6.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors for acquiring scrub typhus among children in Deoria and Gorakhpur districts, Uttar Pradesh, India, 2017. Emerg Infect Dis. 2018;24:2364-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prospective study to assess the response to therapy and its predictors in children with scrub typhus. J Trop Pediatr. 2021;67:fmab087.
- [CrossRef] [PubMed] [Google Scholar]
- Seroprevalence of scrub typhus among febrile patients–a preliminary study. Asian J Pharm Clin Res. 2014;7:19-21.
- [Google Scholar]
- Molecular characterization of orientia tsutsugamushi serotypes causing scrub typhus outbreak in southern region of Andhra Pradesh, India. Indian J Med Res. 2016;144:597-603.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Scrub typhus and leptospirosis in rural and urban settings of central India: A preliminary evaluation. Trop Doc. 2020;50:111-5.
- [CrossRef] [PubMed] [Google Scholar]
- Scrub typhus as an etiology of acute febrile illness in Gorakhpur, Uttar Pradesh, India, 2016. Am J Trop Med Hyg. 2017;97:1313-5.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Scrub typhus in patients reporting with acute febrile illness at a tertiary health care institution in Goa. Indian J Med Res. 2012;136:1020-4.
- [PubMed] [PubMed Central] [Google Scholar]
- Revisiting scrub typhus: A neglected tropical disease. Comp Immunol Microbiol Infect Dis. 2022;90-91:101888.
- [CrossRef] [PubMed] [Google Scholar]
- Leptospirosis in central India: A retrospective study to explore burden of tropical illness. Ind J Med Microbiol. 2024;1:51.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of torch infections and its associated poor outcome in high-risk pregnant women of Central India: Time to think for prevention strategies. Indian J Med Microbiol. 2020;38:379-384.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis of scrub typhus: recent advancements and challenges. 3 Biotech. 2020;10:396.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]






