Translate this page into:
Unreliability of three commercial Coxiella burnetii phase II IgM ELISA kits for the seroscreening of acute Q fever in human cases
Reprint requests: Dr Selvaraj Stephen, Department of Microbiology, Mahatma Gandhi Medical College & Research Institute, Pondy-Cuddalore Main Road, Pillaiyarkuppam, Puducherry 607 402, India e-mail: stephens4950@gmail.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Seroprevalence of Q fever (QF) caused by Coxiella burnetii has been reported from different parts of India. Usually serological/molecular tests are employed for detection of infection. The present study was undertaken to verify the validity of three different QF phase II IgM ELISA kits for acute QF diagnosis by comparing with the gold standard indirect fluorescent antibody assay (IFA).
Methods:
Fifty eight serum samples collected from 42 patients (26 patients provided acute sample only and 16 both acute and convalescent samples) which were examined by all three commercial kits, were cross-checked with QF Phase II IgM IFA for confirmation.
Results:
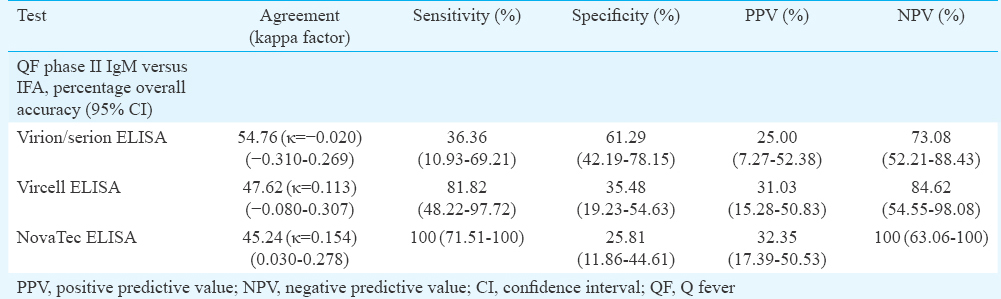
Eleven patients were positive for C. burnetii antibodies by IFA in acute and/or convalescent serum samples. Taking IFA as a reference, percentages of sensitivity, specificity, positive predictive value and negative predictive value for Virion-Serion/Vircell/NovaTec were 36.36, 61.29, 25.00, 73.08; 81.82, 35.48, 31.03, 84.62 and 100, 25.81, 32.35, 100 per cent, respectively.
Interpretation & conclusions:
The three different ELISA kits exhibited poor agreement amongst them and unacceptable level of false positivity. IFA remains to be the only option for diagnosing acute QF. Discrepancy between the clinical findings and IFA/ELISA results needs confirmation by C. burnetii DNA detection in real-time polymerase chain reaction.
Keywords
Coxiella burnetii
ELISA false positivity
indirect fluorescent antibody assay
Q fever
Q fever (QF) in humans and animals caused by Coxiella burnetii has been reported throughout the world1. Reports of coxiellosis in different countries have raised the awareness level of Q fever234. Slaughterhouse workers are at high risk and pregnant women are at low risk to contract QF56. During 1979-1986, country-wide serological surveys established the prevalence of QF in India78910, with cases of human abortions, endocarditis and neonatal septicaemia reported subsequently111213.
C. burnetii occurs in nature in phase I in animals and arthropods. In vitro passage in yolk sac or transmission to humans leads to conversion to phase II. Phase II IgM/IgG antibodies are most prevalent during acute infection and phase I IgG antibodies are indicative of chronic infection to C. burnetii23. In its acute form, QF manifests itself as subclinical infection, febrile illness resembling flu, atypical pneumonia, pneumonitis, hepatitis, meningitis and infrequently as chronic fatigue syndrome with prolonged fatigue, arthralgia, myalgia, muscle fasciculation, blurred vision, sweats and enlarged painful lymph nodes2. Endocarditis is the most common manifestation of chronic QF12312. The causative organism C. burnetii, an intracellular Gram-negative bacterium does not grow on cell-free medium and can only be cultivated in the laboratory animals (guinea pigs)/yolk sacs of developing chick embryos/tissue cultures. This being a Category B/Bio-safety level 3 pathogen with potential for bioterrorism11, isolation attempts are made only in rickettsial research/reference laboratories. Hence, culture is not an option in infected cases. Therefore, serological/molecular tests are employed for diagnosing the infection. Conventionally, serological tests such as Luoto's capillary agglutination test14, Fiset's micro-agglutination test15, complement fixation test2 and immunoperoxidase test1 were used. However, only ELISA and indirect fluorescent antibody assay (IFA) are used in practice in several countries123456161718192021. Serological diagnostic kits such as IFA are expensive. For detection of C. burnetii, DNA, polymerase chain reaction (PCR), quantitative PCR (qPCR)222324, and a new test, loop-mediated isothermal amplification (LAMP)25 have been employed. LAMP/PCR/qPCR kits need to be imported and standardized. Running real-time PCR is not feasible in many laboratories due to the exorbitant cost of the machine. Therefore, the objective of this study was to verify the reliability of three commercial Coxiella burnetii Phase II IgM ELISA kits available in India for acute QF diagnosis, by comparing with phase II IgM IFA.
Material & Methods
This study was conducted during April 2013-January 2015 in the department of Microbiology and Paediatrics, Mahatma Gandhi Medical College and Research Institute (MGMC and RI), and departments of General Medicine and Microbiology, Indira Gandhi Government General Hospital and Post-Graduate Institute, Puducherry, India. Majority of the patients were from rural areas of Puducherry and surrounding Cuddalore, Neyveli, Virudhachalam and Villupuram districts of Tamil Nadu. The Institutional Human Ethical Committee of MGMC and RI approved this research project. Informed written consent was obtained from adult patients and parents/guardians of children, before collection of blood samples. Inclusion criteria were high-grade fever with or without chills and rigour; fever with either pneumonia/pneumonitis, or with rash/hepatosplenomegaly/jaundice/lymphadenopathy/thrombocytopaenia, or with constitutional symptoms such as malaise, myalgia, nausea and vomiting. Exclusion criteria were fever due to urinary tract infection/malaria/enteric fever; culture-positive bacterial pneumonia; patients with other bloodstream infections; bleeding disorders and fever of more than four weeks duration (pulmonary tuberculosis).
Sample size calculation was made considering the national average prevalence 16 per cent for human QF during the past six decades789. The power of the study was 74 per cent. Of the 470 patients registered in the study, only 310 provided both acute and convalescent blood samples. The remaining 160 patients did not turn up for the convalescent sample collection. Of the 310 patients, after excluding 35 lipaemic and haemolyzed samples, 275 samples were processed. Paired blood samples (5 ml) in sterile plastic plain tubes without anti-coagulants were collected from these 275 patients at 2-3 wk intervals, over a period of 22 months. All 275 patients could not be screened by QF Phase II IgM ELISA because of the unreliability of all three kits. Only 42 patients were finally tested by all three ELISA kits with the positivity in one/two/three kits. Because of discrepancy amongst these three kits, confirmation by phase II IFA IgM was carried out. Sixteen patients belonged to the first group (paired samples) and the remaining 26 patients in the second group (acute samples only) (total 58 serum samples).
ELISA for Coxiella burnetii antibody testing: Serum was separated, aliquoted and stored at −20°C till the time of testing. Three different ELISA kits were evaluated: (i) C. burnetii (QF) phase II IgM - ELISA, NovaTec, Immundiagnostica GmbH, Dietzenbach, Germany; (ii) C. burnetii phase II IgM - Virion/Serion, Immundiagnostica GmbH, Wurzburg, Germany; and (iii) C. burnetii ELISA phase II IgM - Vircell, Granada, Spain. Samples positive in one or more ELISA kits were cross-checked for confirmation by IFA.
The following biological positive controls collected from MGMC and RI were included: typhoid (Widal positive) (2), falciparum malaria (1), dengue (3) and rheumatoid arthritis (2). Biological negative controls include typhoid (5), leptospirosis (2), vivax malaria (1) and dengue (3). The tests were carried out strictly adhering to the technical instructions provided by the manufacturers of these three kits and as performed by earlier researchers19212627. All three kits were coated with killed phase II C. burnetii antigen. Procedure and interpretation of the test results were more or less common for all three kits. Briefly, serum samples was diluted 1:100 for NovaTec and Virion/Serion but 1:20 for Vircell. Plates were incubated for 1 h±5 min at 37°C±1°C, followed by 3-5 washes with wash buffer and then aspiration. C. burnetii anti-IgM conjugate (100 μl) was added and incubated for 30-60 min at room temperature. After three washes and aspiration, 100 μl 3,3’,5,5’-tetramethylbenzidine (TMB) substrate solution was dispensed into all wells and incubated for 15 min at room temperature in the dark. Stop solution (50-100 μl) was added to all wells and the plates were read within half an hour. Optical density (OD) readings were taken with the wavelength of 450/620 nm for NovaTec and Vircell but 405/620 nm for Virion/Serion using iMark Microplate Reader (Bio-Rad Laboratories Inc, Shinagawa-Ku, Tokyo, Japan).
Cut-off value calculation was similar for both NovaTec and Vircell kits. Antibody index=(Sample OD/Cut-off OD)×10. The samples with OD values above the cut-off 11 nephelometric turbidity unit were considered positive and those below the cut-off 9 were taken as negative. Borderline samples with cut-off 9-11 were tested in triplicate, while other samples were run in duplicate. For Virion/Serion, 10 per cent and above the cut-off value was taken as positive, whereas 10 per cent and below the cut-off was considered as negative.
Indirect fluorescent antibody assay (IFA): This test was carried out with QF IFA IgM Antibody kit (Fuller Laboratories, Fullerton, California, USA) which identified both phase I and phase II IgM antibodies against C. burnetii. Fifty eight serum samples from 42 patients which were screened by all three commercial ELISA kits, were taken up for this purpose. IFA was performed by adhering to the manufacturer's instructions and as reported by Hackert et al21. Patients’ serum samples were initially diluted to 1:16 in IgM sample dilution buffer provided with the kit. After the final wash, the slides were dried, mounted with the mounting medium and read with ×400 magnification, at 390 nm using Primo Star iLED Fluorescent microscope (Carl Zeiss MicroImaging GmbH, Göttingen, Germany).
Statistical analysis: Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and their respective 95 per cent confidence intervals (CIs) were calculated considering QF IFA IgM as gold standard. For other parameters (Spearman's correlation and Kappa), statistical analysis was performed using IBM 2008 SPSS Statistics version 17.0 for Windows (SPSS Inc., Chicago, IL, USA). Chi-square test with Yates correction (Fisher's test) for small number of samples was performed for categorical data.
Results
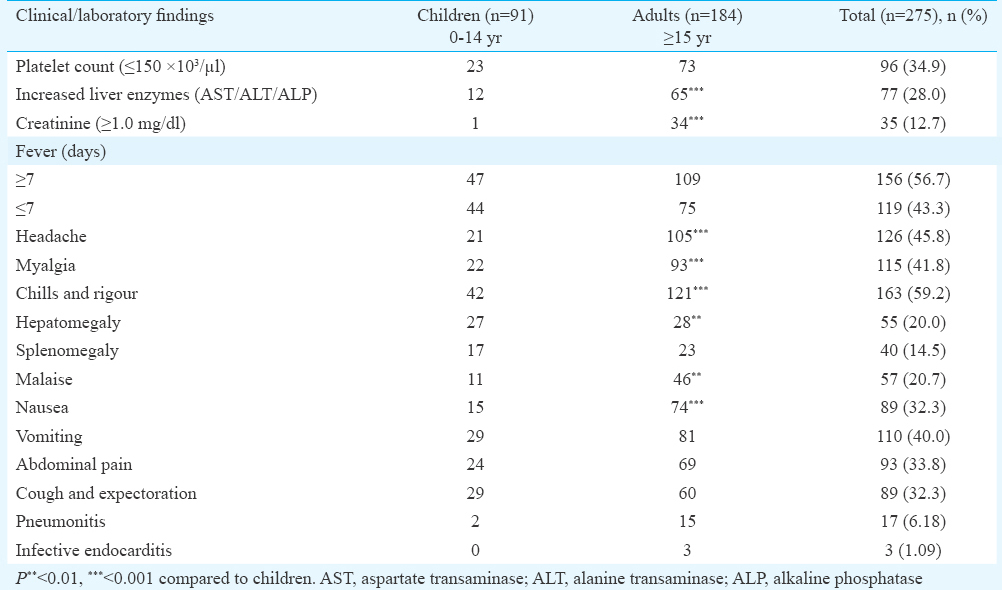
Clinical and laboratory findings of 275 patients recruited in the study are presented in Table I. Of the 42 patients screened by all four serological tests for QF, 11 IFA-positive patients could be considered as presumptive QF cases. This included six children in the age group of 0-14 yr and five adults aged 15 yr and above (range 2 to 60 yr). Mean age was 20.19 ±17.9 and male/female ratio was 4:7. The highest percentage of seropositivity of 80.95 per cent (34/42) was shown by NovaTec ELISA kit followed by Vircell with 69.04 per cent (29/42) and Virion/Serion with 38.09 per cent (16/42). While comparing the results of QF phase II IgM ELISA and IFA, it was observed that of the 42 patients positive in one or more ELISA tests, only 11 were positive for phase II IgM in IFA (Table II). QF phase II IgM IFA titres ranged from 1:16 to 1:512 and QF phase I IgM IFA titres ranged from 1:16 to 1:32. Phase I and phase II IgM antibodies were seen in four patients. Sensitivity, specificity, PPV and NPV of the kits are presented in Table III. Among the eight biological positive controls, one patient whose serum was positive in Widal test, was positive for both phase I and phase II QF IgM IFA with a titre of 1:32 each. The patient's serum was also positive for QF phase II IgM by all three ELISA kits. Eleven biological negative controls were negative in all four serological tests for QF.

Discussion
A significant difference was observed between children and adults regarding certain parameters; more adult patients had low platelet counts, increased liver enzymes and creatinine, headache, myalgia, chills and rigour, malaise and nausea. Hepatomegaly was seen in more number of children than adults. However, analysis of clinical findings and laboratory parameters of 11 QF IFA phase II IgM-positive cases did not reveal any significant difference between adults and children.
It is generally accepted that IFA is the gold standard for the serological diagnosis and confirmation of QF and other rickettsial diseases12317192021. ELISA has also been considered sensitive, specific and useful in serological surveys124521. According to Kantsø et al26, Vircell QF phase II IgM ELISA kit had a sensitivity of 15 per cent and specificity of 94 per cent. As per our data, the percentage sensitivity and specificity for Vircell were 81.82 and 35.48 per cent, respectively. Meekelenkamp et al20 compared the sensitivity and specificity of Focus IFA and Virion/Serion ELISA. The sensitivity of the IFA and ELISA tests was 100 and 85.7 per cent, with a specificity of 95.3 and 97.6 per cent, respectively. According to Herremans et al18, the Focus IFA, ELISA (Virion/Serion) and Complement Fixation Assay (CFA, Virion/Serion) were all suitable serodiagnostic assays to diagnose acute QF, but the IFA remained an important tool in the follow up of patients and in identifying patients at risk for developing chronic QF. In their study, sensitivity and specificity of Virion/Serion Phase II IgM ELISA in diagnosing acute QF were found to be 60 and 100 per cent, respectively. As per our findings, the percentage sensitivity and specificity of Virion/Serion kit were 36.36 and 61.29, respectively. Medić et al27 reported that of the 43 notified cases of acute QF, 37 were laboratory confirmed by NovaLisa Phase II IgM and/or IgG. The sensitivity and specificity of NovaTec ELISA kit were reported to be 100 and 88.4 per cent, respectively, in comparison to IFA in another study28. In our study, 100 per cent sensitivity but the lowest specificity of 25.81 per cent were found for NovaTec ELISA. Raven et al29 drew the attention regarding the unpredictability of phase II IgM antibody positivity as a sole proof of acute QF and recommended to look for seroconversion of IgG, together with the clinical suspicion. A new algorithm for acute QF diagnosis182122232430 emphasized the need to perform qPCR for confirmation. In resource-poor settings, the diagnosis may be limited to ELISA, and where facilities exist, IFA is carried out. In the initial phase of our research, a poor agreement in the seropositivity was observed amongst all three kits. Kappa values were not significant. Hence, the gold standard IFA (Phase II IgM) was the only option. As per the instructions provided by the manufacturers, IgM phase II IFA titres of ≥1:16 were taken as positive. The 11 patients had phase II IgM IFA titres ranging from 1:16 to 1:512. C. burnetii phase I IgM was seen together with phase II IgM in four patients.
The most commonly used means of confirming the diagnosis of acute QF is demonstration of a four-fold rise in phase II IgG by IFA between serum samples from the acute and convalescent phases taken 3-6 wk apart. Of the 16 patients for whom sufficient quantity of acute and convalescent serum samples were available for all the four tests, four-fold or more increase in titres in paired serum samples in QF phase II IgM IFA was demonstrated in only one patient, while seroconversion was observed in three cases. Six patients with only acute samples were positive for C. burnetii antibody in phase II IgM IFA. Eleven of the 42 patients (26.2%) could be identified as probable cases of acute QF, on the strength of positive QF phase II IgM in IFA.
This study had some limitations. QF PCR could not be performed since these kits were not available in India and any imported PCR kit needs to be first validated and standardized. QF phase II IgG IFA could not be carried out.
To conclude, disagreement in results of the three commercial C. burnetii phase II IgM ELISA kits was observed. Hence, their continued use for screening/serodiagnosis of acute QF needs re-thinking. QF IFA phase II IgG and IgM are to be carried out for paired serum samples, and in case of discrepancy, qPCR must be performed for confirmation.
Acknowledgment
Funding by Indian Council of Medical Research (ICMR), New Delhi, for this research project is acknowledged. Authors acknowledge the Chairman, Vice-Chancellor and Dean (Research, Post Graduate studies and Allied Health Sciences) of Mahatma Gandhi Medical College and Research Institute, Puducherry, for providing the facilities.
Conflicts of Interest: None.
References
- Q fever in humans and animals in the United States. Vector Borne Zoonotic Dis. 2002;2:179-91.
- [Google Scholar]
- Serological and molecular evidence of Coxiella burnetii in samples from humans and animals in China. Ann Agric Environ Med. 2016;23:87-91.
- [Google Scholar]
- Seroprevalence of brucellosis, leptospirosis, and Q fever among butchers and slaughterhouse workers in South-Eastern Iran. PLoS One. 2016;11:e0144953.
- [Google Scholar]
- Adverse pregnancy outcomes and Coxiella burnetii antibodies in pregnant women, Denmark. Emerg Infect Dis. 2014;20:925-31.
- [Google Scholar]
- Prevalence of Coxiella burnetii infection among humans and domestic animals of Rajasthan State, India. J Hyg Epidemiol Microbiol Immunol. 1979;23:67-73.
- [Google Scholar]
- Sero-epidemiological studies on coxiellosis in animals and man in the state of Uttar Pradesh and Delhi (India) Int J Zoonoses. 1979;6:67-74.
- [Google Scholar]
- Comparison of PCR, IF, pathology and isolation for Q fever in humans with spontaneous abortion. J Clin Microbiol. 2008;46:2038-44.
- [Google Scholar]
- Bartonella quintana and Coxiella burnetii as causes of endocarditis, India. Emerg Infect Dis. 2008;14:1168-9.
- [Google Scholar]
- First case series of emerging rickettsial neonatal sepsis identified by polymerase chain reaction-based deoxyribonucleic acid sequencing. Indian J Med Microbiol. 2013;31:343-8.
- [Google Scholar]
- A microagglutination technique for detection and measurement of rickettsial antibodies. Acta Virol. 1969;13:60-6.
- [Google Scholar]
- Laboratory diagnosis of rickettsioses: Current approaches to diagnosis of old and new rickettsial diseases. J Clin Microbiol. 1997;35:2715-27.
- [Google Scholar]
- Evaluation of a diagnostic algorithm for acute Q fever in an outbreak setting. Clin Vaccine Immunol. 2011;18:963-8.
- [Google Scholar]
- Comparison of the performance of IFA, CFA, and ELISA assays for the serodiagnosis of acute Q fever by quality assessment. Diagn Microbiol Infect Dis. 2013;75:16-21.
- [Google Scholar]
- Efficiency of various serological techniques for diagnosing Coxiella burnetii infection. Acta Virol. 2005;49:123-7.
- [Google Scholar]
- Comparison of ELISA and indirect immunofluorescent antibody assay detecting Coxiella burnetii IgM phase II for the diagnosis of acute Q fever. Eur J Clin Microbiol Infect Dis. 2012;31:1267-70.
- [Google Scholar]
- Q fever: Single-point source outbreak with high attack rates and massive numbers of undetected infections across an entire region. Clin Infect Dis. 2012;55:1591-9.
- [Google Scholar]
- Real-time PCR with serum samples is indispensable for early diagnosis of acute Q fever. J Clin Microbiol. 2010;26:1978-82.
- [Google Scholar]
- Clinical evaluation of a new PCR assay for detection of Coxiella burnetii in human serum samples. J Clin Microbiol. 1998;36:77-80.
- [Google Scholar]
- Comparison of PCR and serology assays for early diagnosis of acute Q fever. J Clin Microbiol. 2003;41:5094-8.
- [Google Scholar]
- Molecular detection of Coxiella burnetii using an alternative Loop-mediated isothermal amplification assay (LAMP) Vet Ital. 2015;51:73-8.
- [Google Scholar]
- Comparison of two commercially available ELISA antibody test kits for detection of human antibodies against Coxiella burnetii. Scand J Infect Dis. 2012;44:489-94.
- [Google Scholar]
- Q fever outbreak in the village of Noćaj, Srem county, Vojvodina Province, Serbia, January to February 2012. Euro Surveill. 2012;17:pii: 20143.
- [Google Scholar]
- Prevalence of Coxiella burnetii infection in humans occupationally exposed to animals in Poland. Vector Borne Zoonotic Dis. 2015;15:261-7.
- [Google Scholar]
- Solitary IgM phase II response has a limited predictive value in the diagnosis of acute Q fever. Epidemiol Infect. 2012;140:1950-4.
- [Google Scholar]
- Diagnosis and management of Q fever - United States, 2013: Recommendations from CDC and the Fever Working Group. MMWR Recomm Rep. 2013;62(RR-03):1-30.
- [Google Scholar]






