Translate this page into:
Understanding the pro- & anti-inflammatory cytokine profile in Japanese encephalitis & their implications in survival outcome
For correspondence: Dr Ajanta Sharma, Department of Microbiology, Gauhati Medical College, Guwahati 781 032, Assam, India e-mail: ajantasharma2002@yahoo.com
-
Received: ,
Accepted: ,
Abstract
Background & objectives
We aimed to conduct a comprehensive analysis of the cytokine profile in Japanese encephalitis (JE) patients and healthy individuals. Additionally, the correlation between the cytokines and the disease outcome in terms of survival or non-survival was also studied.
Methods
The study included 72 laboratory-confirmed JE cases and 50 healthy controls. Plasma levels of cytokines viz., GM-CSF, IFN-γ, IL-2, IL-4, IL-5, IL-10, IL-12, IL-13, and TNF-α were analysed using Bio-plex200 (Bio-Rad) following manufacturer’s guidelines and compared between JE patients and healthy control group. Additionally, quantitative real-time PCR (qRT-PCR) was done for the quantification of expression of the above-mentioned cytokine genes.
Results
Except IL-4 and IL-13, the levels of GM-CSF, IFN-γ, IL-2, IL-5, IL-10, IL-12, and TNF-α were significantly higher in JE patients in comparison to healthy controls. Significantly upregulated expression of IL-12, IL-10, and TNF-α was observed in the JE group as compared to that in healthy controls. Additionally, significantly downregulated expression of IL-4and IL-13 was observed in the JE group compared to the control group.
Interpretation & conclusions
A higher level of several pro-inflammatory cytokines and downregulation of a few anti-inflammatory cytokines were observed in JE patients compared to the healthy controls indicating co-association of inflammation with disease severity. Hence, a regulator of these pro and anti-inflammatory cytokines may stand out as a potential candidate for therapy in JE.
Keywords
Blood brain barrier
cytokine
Japanese encephalitis
neuroinflammation
TNF-α
IL
Japanese encephalitis (JE) contributes to the majority of the viral encephalitis cases in Asia, with an annual estimate of 68,000 clinically diagnosed cases and globally 13,600 to 20,400 deaths1. A significant proportion of surviving patients experience critical neurological and psychological consequences1,2. The inflammatory response triggered by the Japanese encephalitis virus (JEV) worsens the disease by causing neuronal apoptosis, disrupting the blood-brain barrier (BBB), and restricting neural progenitor cell development3-7. JEV-induced inflammation affects cytokine and chemokine production, with the inflammatory response and subsequent neuronal damage influencing clinical manifestations and histopathological characteristics in viral encephalitis3,8,9. Pro-inflammatory cytokines and chemokines act together to cause neuronal injury, while chemotactic cytokines secreted by activated microglia and astrocytes attract inflammatory cells10. The cytokine levels indirectly measure the inflammatory cascade in the brain. Studies on viral encephalitis patients have documented elevated levels of TNF-α and IL-6 in CSF and serum11-13.
Only a few studies have linked IFN-α, TNF-α, and IL-8 to poor prognosis in JE, but our understanding of the early innate and cellular immune responses during JE remains limited12,14,15. Understanding the excessive peripheral inflammatory response in JE is crucial for identifying emerging therapeutic targets that can prevent or reduce neuroinvasion. This study aimed to understand the mechanisms behind the excessive systemic inflammatory response in JE to identify potential therapeutic targets. These targets could help prevent neuro-invasion by restoring BBB integrity and reducing neuroinflammation. Additionally, there is limited information on the expression profiles of pro- and anti-inflammatory cytokines during JEV infection and their influence on survival and recovery outcomes.
The objectives of this study were to; (i) determine the levels of nine proinflammatory and anti-inflammatory cytokines in JE patients and healthy controls, (ii) evaluate the quantification of gene expression of these cytokines in JEV infection and explore their potential therapeutic role, and (iii) analyse the relationship of different cytokines with the outcome (survivor and non-survivor).
Materials & Methods
This cross-sectional study was conducted at the department of Microbiology, Gauhati Medical College, Guwahati, Assam, after obtaining the ethical clearance from the Institutional Ethics Committee.
Study design
A descriptive study was carried out in a hospital setting from January 2022 to August 2023. The research work was carried out at the State Level Virus Research and Diagnosis Laboratory (SVRDL) and Multidisciplinary Research Unit (MRU) of the institute.
Sample size
The calculated sample size was 122, based on an estimated true proportion rate of 0.39 at a 95% confidence level, assuming a desired precision rate of nine per cent. This calculation utilised the formula n=(Z2 × P × (1 - P))/e2, where the Z-score, which is the critical value from the standard normal distribution for the desired confidence level [e.g., 1.96 for a 95% confidence level (CI)], was used alongside the population proportion (P) and the margin of error (e) to define the precision of the estimate16.
Criteria for case enrolment
As per the National Vector Born Disease Control Programme (NVBDCP) and the World Health Organisation (WHO) case definition, acute encephalitis syndrome (AES) was defined as congregation in a person of any age, at any time of year the symptoms of acute onset of fever (>38°C) in the last seven days with one or more of the following: a change in mental status (including symptoms such as confusion, disorientation, coma or inability to talk), and/or new onset of seizures (excluding simple febrile seizures)17. The samples were collected from the participants before the initiation of the treatment protocol on the day of admission. The participants testing positive for pyogenic meningitis or other bacterial infections were excluded from the study.
The cerebrospinal fluid (CSF) and serum samples from 58 symptomatic participants, as well as CSF samples from 14 symptomatic participants, were collected to confirm of JE. Whole blood samples were collected from all 72 laboratory-confirmed JE cases and from 50 healthy controls. For analysis of the cytokine gene expression, whole blood from 50 laboratory-confirmed cases of JE and 50 age and sex-matched healthy controls were considered.
Detection of Japanese Encephalitis Virus (JEV)
For laboratory confirmation of JE, IgM antibodies specific to JEV were identified using IgM antibody capture ELISA kits sourced from the National Institute of Virology (NIV) in Pune, India, followed by Real-Time PCR for JEV according to established protocols as per NVBDCP guideline (2009), which states that the presence of JE virus-specific IgM antibody in a single sample of serum and/or cerebrospinal fluid (CSF), as detected by an IgM capture ELISA specifically for JE virus or nucleic acid detection by PCR is a case of laboratory-confirmed JE17.The serum and/or CSF samples positive for JEV IgM ELISA were further subjected to JEV PCR. The Viral nucleic acid was extracted using QIAamp Viral RNA Mini kit (Qiagen, Hilden, Germany). cDNA was prepared using Verso cDNA synthesis kit (Thermo Scientific, Waltham, MA, USA). Samples were further screened by SYBR Green real-time PCR (Bio-Rad Laboratories Inc., 2000 Alfred Nobel Drive, Hercules, CA, USA) for JEV. The primers were designed at the Indian Institute of Technology, Guwahati, Assam, targeting the E gene and NS1 gene of JEV. The following two sets of primers were used qJEV E Forward primer (GCCCCAACTTGCGCTGAATA), qJEV E Reverse primer (GGGAAGGGAAGCATTGACAC), qJEV NS1 Forward primer (GCTGGTACGGAATGGAAATC), and qJEV NS1 Reverse primer (CTCGCCAAATC AGTGTAAG).
Cross-reactivity of the samples was excluded by subjecting the samples to Dengue NS1 antigen ELISA (InBios), Dengue IgM ELISA (NIV Pune), Chikungunya IgM ELISA (InBios) and IgM ELISA for West Nile virus (InBios). The patients who were negative for Dengue virus, Chikungunya virus, and West Nile virus were included in the study for cytokine analysis. The control samples were also pre-screened for the presence of IgM antibodies against flaviviruses such as dengue, chikungunya, and West Nile virus.
Estimation of pro-inflammatory and anti-inflammatory cytokine levels in JE patients
The pro-inflammatory cytokines GM-CSF, IFN-γ, TNF-α, IL-2, IL-12 (P70) and anti-inflammatory cytokines IL-4, IL-5, IL-10, IL-13 were analysed in the Multiplex Bead Based Cytokine Platform Assay (Bio-Plex 200, Bio-Rad, USA) using the Bio-Plex Pro Human Cytokine Th1/Th2 Panel 9-Plex KIT (Bio- Rad, USA) following manufacturer’s guidelines. The limit of detection for various cytokines (Assay sensitivity) was IL-2: 0.75 pg/ml, IL-4: 0.09 pg/ml, IL-5: 0.86 pg/ml, IL-10: 0.69 pg/ml, IL-12 (p70): 0.78 pg/ml, IL-13: 0.22 pg/ml, GM-CSF: 0.19 pg/ml, IFN-γ: 1.05 pg/ml, TNF-α: 1.13 pg/ml.
Analysis of cytokine gene expression in JE cases
Total RNA extraction
Total RNA was extracted using QAamp RNA blood mini kit (Qiagen, Germantown, Maryland) using the manufacturer’s guidelines, followed by conversion to cDNA.
Quantification of gene expression of pro-inflammatory and anti-inflammatory cytokine pathway-related genes by SYBR green assay
Quantification of the expressions of nine cytokine genes GM-CSF, IFN-γ, Il-2, IL-4, IL-5, IL-10, IL-12, IL-13, and TNF-α was performed by using SYBR green chemistry in a QuantStudio 5 platform (Applied Biosystem, MA 01915, USA). The primer sequences of GM-CSF, IFN-γ, Il-2, IL-4, IL-5, IL-10, IL-12, IL-13, and TNF-α were taken from published literature given in supplementary table and obtained from (Integrated DNA Technologies)18-24. The PCR amplification of all the genes was normalised to endogenous control18S rRNA. Each target gene was analysed in triplicate. The quantification of gene expression protocol was adapted from Livak et al25.
The PCR cycling conditions for all target genes included an initial denaturation step at 95°C for 15 sec, followed by 40 cycles of denaturation at 95°C for 5 sec and annealing/extension at 60°C for 30 sec. Reactions were conducted in a final volume of 10 µl, containing SYBR Green PCR master mix (iTaq Universal SYBR® Green Supermix, Bio-Rad Laboratories, USA), specific primers for each gene, ROX dye, and 1 µl of cDNA. Each amplification batch included a no-template control (NTC), and cDNA from a healthy individual (not part of the study) served as a calibrator (reference sample) to evaluate intra- and inter-assay variability, which was less than 0.5 Ct. The relative quantification was performed using the 2−ΔΔ Ct method25.
Using 18S rRNA as the endogenous control, ΔCt values were determined by subtracting the Ct values of 18S rRNA from the Ct values of each specific target cytokine gene for each sample. Subsequently, ΔΔCt values were calculated by subtracting the average ΔCt of the calibrator from the ΔCt of the normalised target values. The results are expressed as the fold change in target gene expression relative to a reference sample, normalised to the endogenous control (18S rRNA).
Statistical analysis
The statistical analysis was done by using the statistical software GraphPad PRISM version 8.0.1.
Results
The age of the study participants ranged from 4 to 65 yr, with a median age (± standard deviation) of 25±24.9 yr. Of the 72 study participants, 45 (62.5%) were male, and 27 (37.5%) were female, with a male-to-female ratio of 1.6:1. The average duration of the illness till sample collection was 5±2.23 days. The predominant clinical symptoms included fever (100%), headache (48.6%), change in mental status (61.1%), neck rigidity (54.1%), unconsciousness (20.8%), paralysis (5.6%), and altered sensorium (1.4%). We performed correlation analyses between the cytokine profile and sex, as well as different age groups. However, there was no significant association observed between these factors and cytokine profile. As all the cases presented with severe forms of disease as per WHO criteria, further subgrouping was not done according to the severity of the disease26.
Pro-inflammatory and anti-inflammatory cytokine levels in JE patients
The result of the comparative analysis of cytokine level in JE positive and healthy control samples were analysed by Mann-Whitney U test and are presented in figure. The current study revealed that the levels of cytokines in individuals with JE were significantly higher compared to that in healthy controls: GM-CSF (73.03 vs. 44.90; P<0.0001), IFN-γ (69.28 vs. 32.32; P<0.0001), IL-2 (74.42 vs. 42.90; P<0.0001), IL-5 (69.54 vs. 47.84; P=0.0007), IL-10 (66.67 vs. 41.23; P<0.0001), IL-12(64.30 vs. 42.31; P=0.0003) and TNF-α (78.49 vs. 32.77; P<0.0001). However, IL-4 level (46.17 vs. 87.42; P<0.0001) was found to be significantly lower in JE group as compared to that in healthy control. On the contrary, the lower level of IL-13 (55.81 vs. 61.53; P=0.3521) in the JE group compared to healthy control was statistically insignificant.
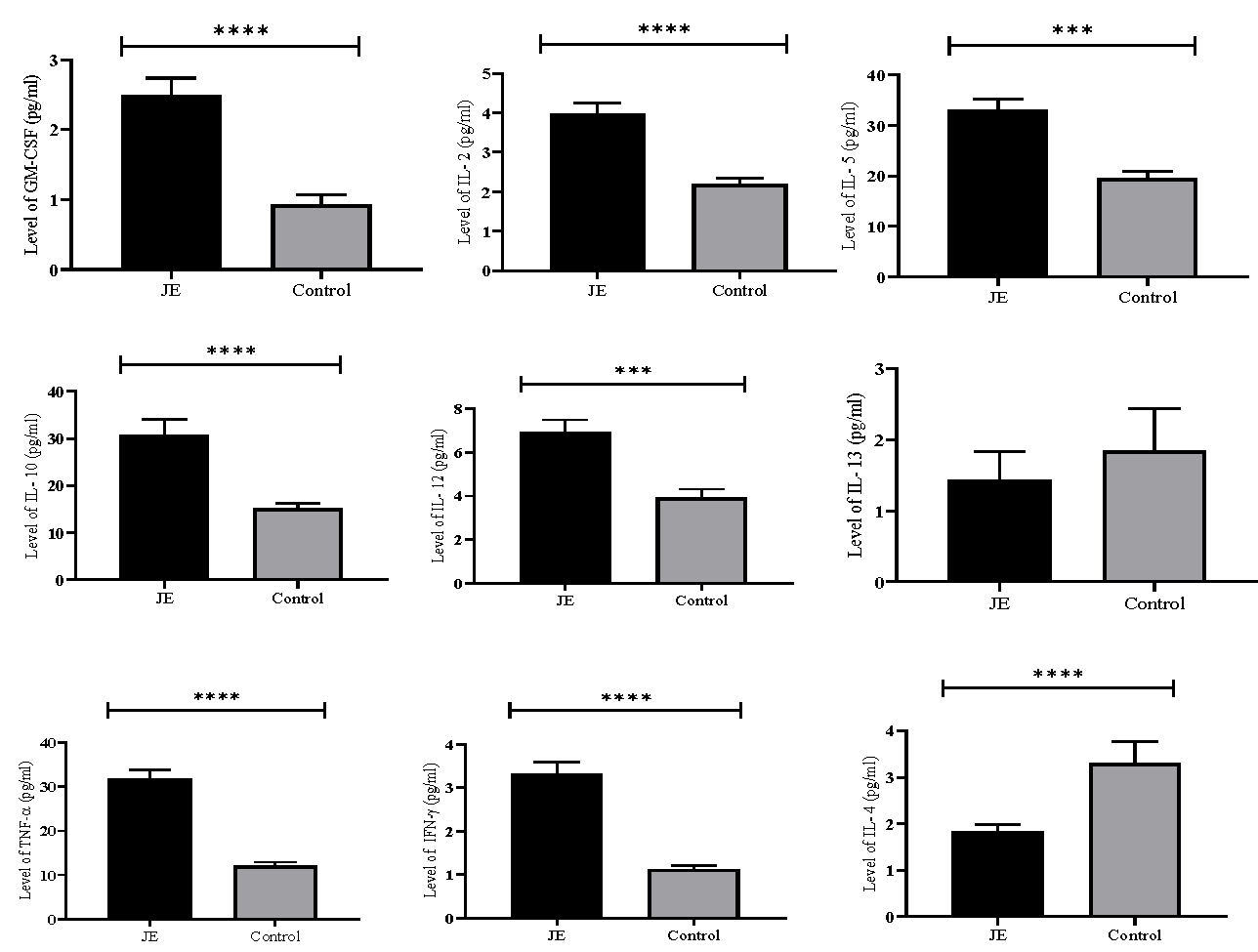
- Comparative analysis of cytokine level in JE positive and healthy control samples. The plasma concentrations GM-CSF, IL-2, IL-5, IL-10, IL-12, IL-13, TNF-α, IFN-γ and IL-4 in JE and healthy control. Results are presented as mean±standard error of mean (SEM). JE vs. healthy control: P***<0.001, ****<0.0001.
Quantification of cytokine gene expression by qRT-PCR in JE cases compared to healthy controls
The data of gene expression levels of GM-CSF, IFN-γ, Il-2, IL-4, IL-5, IL-10, IL-12, IL-13 and TNF-α in the JE and healthy control group were analysed by Mann-Whitney U test and are presented in table. Significantly upregulated expression of IL-12 (P<0.0001), IL-10 (P=0.0085), and TNF-α (P=0.005), were observed in the JE group compared to healthy control. Additionally, significantly downregulated expression of IL-4 (P=0.001) and IL-13 (P=0.009) were observed in the JE group as compared to the healthy control group. However, there was no significant difference in the expression level of GM-CSF, IFN- γ, IL-2, and IL-5 between the JE and healthy control group.
| Analyte | JE positive (Mean rank) | Heathy control (Mean rank) | P value |
|---|---|---|---|
| GM-CSF | 41.68 | 36.25 | 0.2899 |
| IFN γ | 44.44 | 36.56 | 0.1307 |
| IL2 | 42.46 | 38.54 | 0.4535 |
| IL4 | 32.09 | 48.91 | 0.001* |
| IL5 | 43.94 | 36.16 | 0.1334 |
| IL10 | 47.29 | 33.71 | 0.0085* |
| IL12 | 50.6 | 30.4 | <0.0001* |
| IL13 | 33.73 | 47.28 | 0.0087* |
| TNF α | 47.76 | 33.24 | 0.0048* |
Relationship of different cytokines with outcome (survivor and non-survivor)
The association between the cytokine levels and the outcome, i.e., survival or non-survival, of JE patients was analysed using Pearson’s correlation. We observed a negative correlation between the peripheral level of GM-CSF and survival [(r=-0.32), (95% CI: -0.53 to -0.09)], as well as TNF-α level and survival (r=-0.26; 95% CI: -0.47 to -0.03)]. This suggests that survival is low in the presence of higher levels of pro-inflammatory cytokines such as TNF-α and GM-CSF.
Discussion
In the current study, in JE patients, pro-inflammatory cytokines such as GM-CSF, IFN-γ, IL-2, IL-12, and TNF-α were significantly elevated compared to healthy controls. Conversely, anti-inflammatory cytokines IL-5 and IL-10 were also higher in JE patients, while IL-4 levels were notably lower. The excessive production of these cytokines have been recorded to be linked to poorer outcomes in JE cases14. Interestingly, cytokine inhibitors that target interferon regulators and TNF-α receptors are potential candidates for adjunctive therapy in JE animal model27,28. Preclinical studies documented increased levels of IFN-γ, TNF-α, IL-6, IL-1β, IL-18, and IL-12 in response to JEV infection4,6,29-33. Following JEV infection, host cells release type-1 IFN, TNF-α, and IFN-γ, which trigger inflammatory responses that inhibit viral replication. Furthermore, upon adhering to natural killer (NK) cells, IFN-α and IFN-β initiate the lytic activity that eliminates the JEV-infected cells. Notably, IL-12 is released early during infection to initiate antiviral activity, followed by secretion of IFN-γ, which stimulates MHC-II molecule-expressing brain macrophages, which aids in the generation of additional cytokines that prevent viral replication34. However, prolonged production of IFN-γ can exacerbate neuroinflammation and disruption of BBB and lead to tissue damage, potentially resulting in neurologic complications such as seizures and cognitive impairment35.
The current study reported a high concentration of IL-12 in JE as compared to healthy controls. IL-12 is being explored as a therapeutic target for managing the neuroinflammatory response in JE due to its promising role in enhancing protective immune responses against viral infections. It promotes Th1 responses, activates NK cells, and boosts cytotoxic T-cell activity, which is crucial for an effective immune response against JEV. Targeting IL-12 signalling pathways may offer promising strategies for controlling neuroinflammation and improving outcomes in JE36.
While IL-2 is crucial for coordinating the immune response to JEV, its excessive production can lead to neuroinflammation and CNS damage. Modulating IL-2 activity or blocking its signalling pathways may help reduce neuroinflammation associated with JEV infection35. We report here elevated levels of IL-2 in JE patients compared to healthy controls. Additionally, increased levels of IL-5 were found in JE patients in our study. IL-5, typically associated with eosinophil activation and allergic reactions, may also be involved in neuroinflammation by impacting the recruitment and activation of immune cells within the CNS36.
GM-CSF activates and recruits CNS immune cells, specifically microglia, as well as infiltrating monocytes and macrophages37, and a higher level of it was identified in this study in the JE group compared to the healthy control. GM-CSF is also known to promote microglia and macrophages to produce pro-inflammatory cytokines and chemokines, facilitating the activation and recruitment of additional immune cells alongside the secretion of TNF-α, IL-1β, and IL-638.
In the current study, IL-13 level was found to be lower in JE patients than in the control. While its role in neuroinflammation related to JE is not well-studied, some research suggests that IL-13 may enhance BBB permeability, allowing for increased immune cell infiltration into the central nervous system (CNS). Conversely, other studies indicate that IL-13 can protect the BBB by reducing endothelial cell activation and maintaining its function39. Following JE infection, IL-13 may contribute to neurodegeneration but can also limit neuronal damage in the CNS, suggesting that elevated IL-13 levels may reflect a protective response to JE infection.
The present study found that expression of IL-12, IL-10, and TNF-α was upregulated, while IL-4 and IL-13 were downregulated in JE patients compared to healthy controls. There is limited data on the implications of cytokines in JE, and the mechanisms of cytokine production remain unclear. The decreased expression of IL-4 may be linked to the recovery phase of JE, as IL-4 plays a significant role in preventing neuronal damage by inhibiting activated microglia from producing TNF-α and nitric oxide40,41.
The naïve CD4+ T cells develop into Th2 cells in response to IL-4, which again releases cytokines with anti-inflammatory properties such as IL-4, IL-5, and IL-1042. As stated by Saxena et al43, these cytokines aid in controlling immune responses and reducing inflammation IL-4 may prevent immune cells and inflammatory mediators from infiltrating the CNS during neuroinflammatory diseases by maintaining the BBB integrity44,45.
The present study documented an upregulated expression of the anti-inflammatory cytokine IL-10, potentially indicating increased activity of anti-inflammatory Treg cells and highlighting IL-10’s protective role after immune insult. A recent study emphasised the protective role of IL-10 in tick-borne encephalitis, noting that IL-10 knockout mice exhibited higher mortality rates after infection with the Oshima strain46,47.
Comparable cytokine levels were previously reported in flavivirus infections like Dengue. Puc et al48 (2021) noted elevated levels of GM-CSF, IL-1β, IL-2, IL-6, IL-8, IL-10, IL-12p70, IL-17A, IFN-γ, and TNF, alongside decreased IL-4 levels. In contrast, our study found increased GM-CSF, IFN-γ, IL-2, IL-5, IL-10, IL-12, and TNF-α in JE, with lower IL-4 and IL-13 levels. Bhatt et al49 examined cytokine profiles in Dengue but did not observe significant changes in IL-5 levels.
In this study, we observed significantly upregulated expression of IL-12, IL-10, and TNF-α in the JE group compared to healthy controls, alongside significantly downregulated expression of IL-4 and IL-13 levels. Similar findings were reported in Dengue infections50-52.
In conclusion, the differential expression of cytokine regulators in JE infection indicates potential areas for further research in the future. An in-depth understanding of the dysregulated immune responses underlying the pathogenesis of JE disease may help identify therapeutic windows. Given the considerable morbidity and mortality recorded annually in the country, as well as the low uptake of JE vaccines in many communities, continued research efforts in this direction are necessary.
Financial support & sponsorship
The study received funding from the Department of Health Research, Ministry of Health and Family Welfare, Government of India-Indian Council of Medical Research, New Delhi (NER/71/2020-ECD-I).
Conflicts of Interest
None.
Use of Artificial Intelligence (AI)-Assisted Technology for manuscript preparation
The authors confirm that there was no use of AI-assisted technology for assisting in the writing of the manuscript and no images were manipulated using AI.
References
- Estimated global incidence of Japanese encephalitis: a systematic review. Bull World Health Org. 2011;89:766-74.
- [Google Scholar]
- Japanese encephalitis prevention and control: advances, challenges, and new initiatives. Emerging Infections. 2008;8:93-124.
- [Google Scholar]
- Glial activation involvement in neuronal death by Japanese encephalitis virus infection. J Gen Virol. 2010;91:1028-37.
- [Google Scholar]
- Japanese encephalitis virus infection induces IL-18 and IL-1beta in microglia and astrocytes: Correlation with in vitro cytokine responsiveness of glial cells and subsequent neuronal death. J Neuroimmunol. 2008;195:60-72.
- [Google Scholar]
- Abrogated inflammatory response promotes neurogenesis in a murine model of Japanese encephalitis. PLoS One. 2011;6:e17225.
- [Google Scholar]
- Viral infection of the central nervous system and neuroinflammation precede blood-brain barrier disruption during Japanese encephalitis virus infection. J Virol. 2015;89:5602-14.
- [Google Scholar]
- Breakdown of blood-brain barrier by virus-induced cytokine during Japanese encephalitis virus infection. Int J Exp Pathol. 1992;73:603-11.
- [Google Scholar]
- TNF-α and IL-1β mediate Japanese encephalitis virus-induced RANTES gene expression in astrocytes. Neurochem Int. 2011;58:234-42.
- [Google Scholar]
- CCL2, but not its receptor, is essential to restrict immune privileged central nervous system-invasion of Japanese encephalitis virus via regulating accumulation of CD11b(+) Ly-6C(hi) monocytes. Immunology. 2016;149:186-203.
- [Google Scholar]
- Susceptibility of human embryonic stem cell-derived neural cells to Japanese encephalitis virus infection. PLoS One. 2014;9:e114990.
- [Google Scholar]
- Japanese encephalitis virus infects neural progenitor cells and decreases their proliferation. J Neurochem. 2008;106:1624-36.
- [Google Scholar]
- Levels of interferon in the plasma and cerebrospinal fluid of patients with acute Japanese encephalitis. J Infect Dis. 1987;155:797-9.
- [Google Scholar]
- Venous thrombosis-associated inflammation and attenuation with neutralizing antibodies to cytokines and adhesion molecules. Arterioscler Thromb Vasc Biol. 1995;15:258-68.
- [Google Scholar]
- Correlation of tumor necrosis factor levels in the serum and cerebrospinal fluid with clinical outcome in Japanese encephalitis patients. J Med Virol. 1997;51:132-6.
- [Google Scholar]
- Secretion of the chemokine interleukin-8 during Japanese encephalitis virus infection. J Med Microbiol. 2000;49:607-12.
- [Google Scholar]
- Incidence rate of acute encephalitis syndrome without specific treatment in India and Nepal. Indian J Community Med. 2012;37:240-51.
- [Google Scholar]
- Guidelines for surveillance of acute encephalitis syndrome (with special reference to Japanese Encephalitis). Available from: http://nvbdcp.gov.in/WriteReadData/l892s/AES_guidelines.pdf, accessed on September 10, 2024.
- Granulocyte-macrophage colony-stimulating factor modulates myeloid-derived suppressor cells and treg activity in decompensated cirrhotic patients with sepsis. Front Immunol. 2022;13:828949.
- [Google Scholar]
- Adipose-derived mesenchymal stem cells exert antiinflammatory effects on chondrocytes and synoviocytes from osteoarthritis patients through prostaglandin E2. Arthritis Rheum. 2013;65:1271-81.
- [Google Scholar]
- Particulate matter exposure and the changes in immune biomarkers: Effects of biyeom-go on the nasal mucosa of patients with allergic rhinitis and a particulate matter-treated mouse model. Evid Based Complement Alternat Med. 2022;2022:4259669.
- [Google Scholar]
- SJSZ glycoprotein (38kDa) prevents thymus atrophy and enhances expression of IL-2 and IL-12 in diethylnitrosamine-induced hepatocarcinogenesis. Int Immunopharmacol. 2012;13:362-9.
- [Google Scholar]
- Use of quantitative real-time PCR to determine immune cell density and cytokine gene profile in the tumor microenvironment. J Immunol Methods. 2003;280:1-11.
- [Google Scholar]
- CyProQuant-PCR: A real time RT-PCR technique for profiling human cytokines, based on external RNA standards, readily automatable for clinical use. BMC Immunol. 2005;6:5.
- [Google Scholar]
- 5-Aza-2’-deoxycytidine enhances lipopolysaccharide-induced inflammatory cytokine expression in human dental pulp cells by regulating TRAF6 methylation. Bioengineered. 2019;10:197-206.
- [Google Scholar]
- Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta c(T)) method. Methods. 2001;25:402-8.
- [Google Scholar]
- Available from: https://www.who.int/news-room/fact-sheets/detail/japanese-encephalitis#, accessed on May 9, 2024.
- Pre-treatment with scopolamine naturally suppresses Japanese encephalitis viral load in embryonated chick through regulation of multiple signaling pathways. Appl Biochem Biotechnol. 2021;193:1654-7.
- [Google Scholar]
- Platelet factor 4 promotes rapid replication and propagation of dengue and Japanese encephalitis viruses. Ebio Medicine. 2019;39:332-47.
- [Google Scholar]
- Proinflammatory cytokines and chemokines in humans with Japanese encephalitis. J Infect Dis. 2004;190:1618-26.
- [Google Scholar]
- Cytokines and chemokines in viral encephalitis: A clinicoradiological correlation. Neurosci Lett. 2010;473:48-51.
- [Google Scholar]
- Minocycline differentially modulates viral infection and persistence in an experimental model of Japanese encephalitis. J Neuroimmune Pharmacol. 2010;5:553-65.
- [Google Scholar]
- A model to study neurotropism and persistency of Japanese encephalitis virus infection in human neuroblastoma cells and leukocytes. J Gen Virol. 2004;85:635-42.
- [Google Scholar]
- Cytolytic effector pathways and IFN-γ help protect against Japanese encephalitis. Eur J Immunol. 2013;43:1789-98.
- [Google Scholar]
- Type I interferon receptor signaling of neurons and astrocytes regulates microglia activation during viral encephalitis. Cell Rep. 2018;25:118-29.
- [Google Scholar]
- Astrocyte-targeted gene delivery of interleukin 2 specifically increases brain-resident regulatory T cell numbers and protects against pathological neuroinflammation. Nat Immunol. 2022;23:878-91.
- [Google Scholar]
- Modulation of the immune-related gene responses to protect mice against Japanese encephalitis virus using the antimicrobial peptide, tilapia hepcidin 1-5. Biomaterials. 2011;32:6804-14.
- [Google Scholar]
- Intracerebral GM-CSF contributes to transendothelial monocyte migration in APP/PS1 Alzheimer’s disease mice. J Cereb Blood Flow Metab. 2016;36:1978-91.
- [Google Scholar]
- A preliminary neuropathological study of Japanese encephalitis in humans and a mouse model. Trans R Soc Trop Med Hyg. 2006;100:1135-45.
- [Google Scholar]
- Neuroprotective role of IL-4 against activated microglia. J Immunol. 1993;151:1473-81.
- [Google Scholar]
- Antiinflammatory properties of a peptide derived from interleukin-4. Cytokine. 2013;64:112-21.
- [Google Scholar]
- Macrophage function in tissue repair and remodeling requires IL-4 or IL-13 with apoptotic cells. Science. 2017;356:1072-6.
- [Google Scholar]
- An insufficient anti-inflammatory cytokine response in mouse brain is associated with increased tissue pathology and viral load during Japanese encephalitis virus infection. Arch Virol. 2008;153:283-92.
- [Google Scholar]
- MHCII-independent CD4+ T cells protect injured CNS neurons via IL-4. J Clin Invest. 2015;125:699-714.
- [Google Scholar]
- Tumor necrosis factor receptor-1-induced neuronal death by TRADD contributes to the pathogenesis of Japanese encephalitis. J Neurochem. 2007;103:771-83.
- [Google Scholar]
- Protective role of TNF-α, IL-10 and IL-2 in mice infected with the oshima strain of tick-borne encephalitis virus. Sci Rep. 2014;4:5344.
- [Google Scholar]
- Cytokine signature of dengue patients at different severity of the disease. Int J Mol Sci. 2021;22:2879.
- [Google Scholar]
- Temporal cytokine storm dynamics in dengue infection predicts severity. Virus Res. 2024;341:199306.
- [Google Scholar]
- Altered T helper 1 reaction but not increase of virus load in patients with dengue hemorrhagic fever. FEMS Immunol Med Microbiol. 2005;44:43-50.
- [Google Scholar]
- Kinetics of dengue virus-specific serum immunoglobulin classes and subclasses correlate with clinical outcome of infection. J Clin Microbiol. 2001;39:4332-8.
- [Google Scholar]
- Immune response to dengue virus infection in pediatric patients in new delhi, India--association of viremia, inflammatory mediators and monocytes with disease severity. PLoS Negl Trop Dis. 2016;10:e0004497.
- [Google Scholar]






