Translate this page into:
Typical & atypical enteropathogenic Escherichia coli in diarrhoea & their role as carrier in children under five
Reprint requests: Dr. Shukla Das, Department of Microbiology, University College of Medical Sciences & Guru Teg Bahadur Hospital, Dilshad Garden, Delhi 110 095, India e-mail: shukladas_123@yahoo.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Multidrug-resistant enteropathogenic Escherichia coli (EPEC) is responsible for a large number of cases of infantile diarrhoea in developing countries, causing failure in treatment with consequent health burden and resulting in a large number of deaths every year. This study was undertaken to determine the proportion of typical and atypical EPEC in under five children with diarrhoea and controls, their function as a carriage and to identify virulent genes associated with them.
Methods:
During the study period, 120 stool samples including 80 from controls children were collected and analyzed for the presence of EPEC using standard bacteriological methods. Isolates were subjected to antimicrobial testing by disc diffusion method. Isolates confirmed as E. coli by phenotypic method were further tested for the presence of attaching and effacing (eae) and bundle-forming pilus (bfpA) genes by real-time SYBR Green-based polymerase chain reaction.
Results:
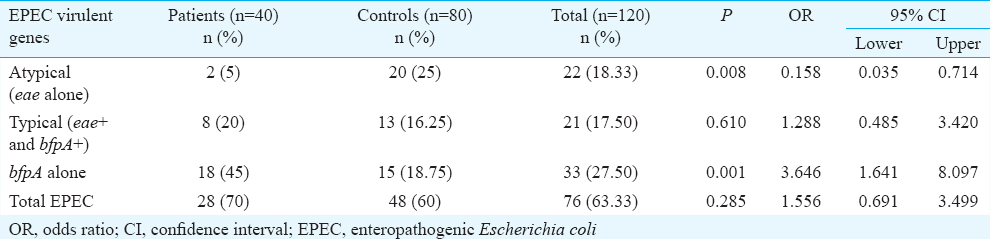
All isolates were tested for the presence of EPEC. The frequency of typical EPEC was 20 and 16.25 per cent whereas the frequency of atypical EPEC strains was 5 and 23.75 per cent in patients and controls, respectively (P< 0.05) and bfpA was seen in 45 and 18.75 per cent isolates of diarrhoeal patients and controls, respectively.
Interpretation & conclusions:
Our results showed that typical EPEC was a common cause of diarrhoea, but at the same time, atypical EPEC was emerging as colonizers in the intestine of children with and without diarrhoea in and around Delhi. Children can be considered asymptomatic carriers of these pathogens and can transmit them to other susceptible children. Adequate steps need to be taken to stop these strains from developing and spreading further.
Keywords
Atypical enteropathogenic Escherichia coli
diarrhoea
EPEC
real-time polymerase chain reaction
typical enteropathogenic Escherichia coli
Enteropathogenic Escherichia coli (EPEC) is the main cause of childhood diarrhoea in developing countries1; the frequency of its occurrence is very low in developed countries because of better hygienic conditions. Mechanism and aetiology of EPEC causing diarrhoea is different from other virulent categories of E. coli. The frequency of EPEC contamination is highest in first six months following birth2. The main phenomenon of EPEC pathogenesis involves an attaching and effacing lesion, followed by a series of physiological changes in the intestinal cells3. The eae gene, which is located in the locus of enterocyte effacement4 pathogenicity island, and the bundle-forming pilus (bfpA) gene, which is located on a plasmid called the EPEC adherence factor, have been used for identification of EPEC and for the subdivision of this group of E. coli into typical and atypical strains56. Studies from India and other countries have also shown the occurrence of increased frequency of atypical EPEC from children without diarrhoea478. There are many factors responsible for high prevalence of diarrhoeal infection in developing countries including illiteracy and unhygienic environment9. In India, there are a few reports of acute diarrhoea due to EPEC and are poorly documented due to the self-limiting nature of the clinical illness101112. The presence of EPEC as a colonizer in the gut of healthy children raises the concern of potential carriers amongst young children serving as diarrhoeal burden in the population713. Hence, this study was carried out to compare the proportion of typical and atypical E. coli from children under five suffering from acute diarrhoea with that of healthy children and their drug resistance patterns.
Material & Methods
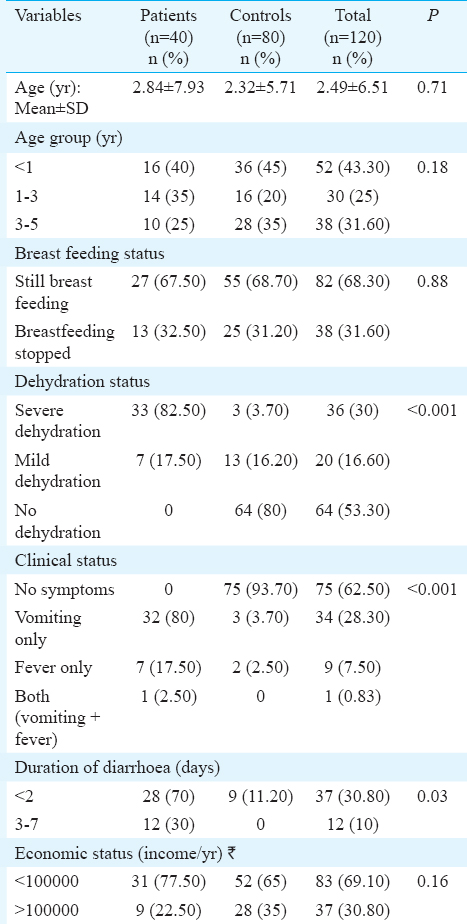
The study population comprised two groups. Group 1 included 40 children below 5 yr of age suffering from acute diarrhoea (<72 h duration) and attending the Paediatrics outpatient department (OPD) of Guru Teg Bahadur hospital, University College of Medical Sciences, Delhi, India, during July 2012 and July 2013. Group 2 included 80 healthy children below five years of age, who were not suffering from diarrhoea or any other disease14. The control samples were collected from siblings of children who came with the patient as well as from the children who came for vaccination in the paediatric OPD. Demographic information including age, breastfeeding status, dehydration status and clinical status was obtained for all cases and controls.
Considering the prevalence of E. coli isolated from our hospital in the past two years as 37.50 per cent (35-40%) and to study a difference of 15 per cent, sample of 40 isolates was required in each group15. Informed written consent was obtained from the parents of the participants. The study protocol was approved by the Institutional Ethical Committee.
Fresh stool samples were collected into a clean, dry disposable container with tight lid (in case of small children, rectal swab or stool from diapers were collected) from all the children and inoculated on medium as per standard laboratory methods, and E. coli was identified phenotypically based on conventional biochemical reactions16.
The specimens were processed according to the guidelines provided for the laboratory diagnosis of enteric pathogens17. Specimens were inoculated onto MacConkey agar plates and incubated aerobically at 37°C for 24 h. Two or three lactose fermenting colonies previously identified as E. coli were inoculated on Mueller-Hinton agar for antibiotic susceptibility testing and DNA extraction.
Antimicrobial susceptibility testing: Antimicrobial susceptibility testing was performed on Mueller-Hinton agar plates by disc diffusion method as per Clinical & Laboratory Standards Institute (CLSI) guidelines18. The E. coli American Type Culture Collection (ATCC) strain 25922 was included as a susceptible control in all antimicrobial resistance screening tests.
DNA extraction: Lactose-fermenting colonies on MacConkey agar (4-5 in no.) were selected for DNA extraction using a commercial kit (Real Biotech Corporation, Taiwan). Primer sequences1920 and their melting temperature (Tm) are shown in Table I.

Standard bacterial control strains for EPEC were obtained from the National Institute of Cholera and Enteric Diseases (Kolkata, India). Non-pathogenic E. coli strain ATCC 25922, which is devoid of virulence genes of diarrhoeagenic E. coli, was used as a negative control.
A 96 multiwell white opaque plate (Roche, Germany) was used to perform real-time polymerase chain reaction (PCR), with each well containing 10 μl SYBR Green I master mix (2×), 5 μl of the extracted DNA, 1 μl of each primer (10 μM for each primer forward and reverse) and water to make up the final volume to 20 μl in each well.
DNA amplification was carried out in a Roche Light Cycler 480-II (Germany) using a pre-incubation step at 95°C for 10 min21, followed by 30 cycles of amplification with denaturation at 95°C for 20 sec, annealing at 50°C for 30 sec and extension at 72°C for 20 sec, then single cycle of melting curve step followed by cooling. Melting peak for each gene was shown and average Tm was calculated by the inbuilt software. Amplified PCR products were analyzed by electrophoresis on 1.5 per cent agarose gel stained with ethidium bromide at 125 volts for 45 min in a 13-well apparatus to observe any non-specific amplification. A molecular marker of 100 bp was used to determine the size of the amplicons22.
The criteria23 for determination of typical and a typical EPEC were defined as follows: the presence of eae and bfpA for typical EPEC and presence of eae only depicts atypical E. coli. Glyceraldehyde 3-phosphate dehydrogenase gene with amplicon size 170 bp was used as internal quality control.
Statistical analysis: Statistical Package for the Social Sciences (SPSS; Version 20.0, Armonk, NY, USA) was used for data analysis. Chi-square test was used to test the significance of association between categorical variables. Student's t test was used for comparing means. Fisher's exact test was applied where more than or equal to 20 per cent of the cells had an expected value of less than or equal to 5. In Table II, P values are based on Chi-square statistics, whereas the odds ratios (ORs) and its exact confidence limits have been calculated using an online statistical software (www.OpenEpi.com) which uses a programme for calculating ORs and its exact confidence limits, developed by Martin and Austin24. Cases and controls are the dependent or regressed (y) variables whereas the strains are the independent (x) variables. In Table III, the results of multiple logistic regression are displayed. The y variable is diarrhoea whereas the variables mentioned as predictors such as age, gender, presence of typical EPEC, atypical EPEC and bfpA are the independent (x) variables in the multiple logistic regression models. In multiple logistic regressions, adjusted ORs are obtained by adjusting for the other confounding variables present in the model. Thus, an adjusted OR is a measure of an independent effect of the regressor variable on the regressed variable.
Results
A total of 120 stool specimens (40 from children with diarrhoea, 80 from healthy control children) were collected. There was a male preponderance of 60.83 per cent (73) versus 39.16 per cent females (47) with age of 23.65±19.97 months in the study population. Representative genes of EPEC as analyzed by real-time PCR are shown in the Figure.
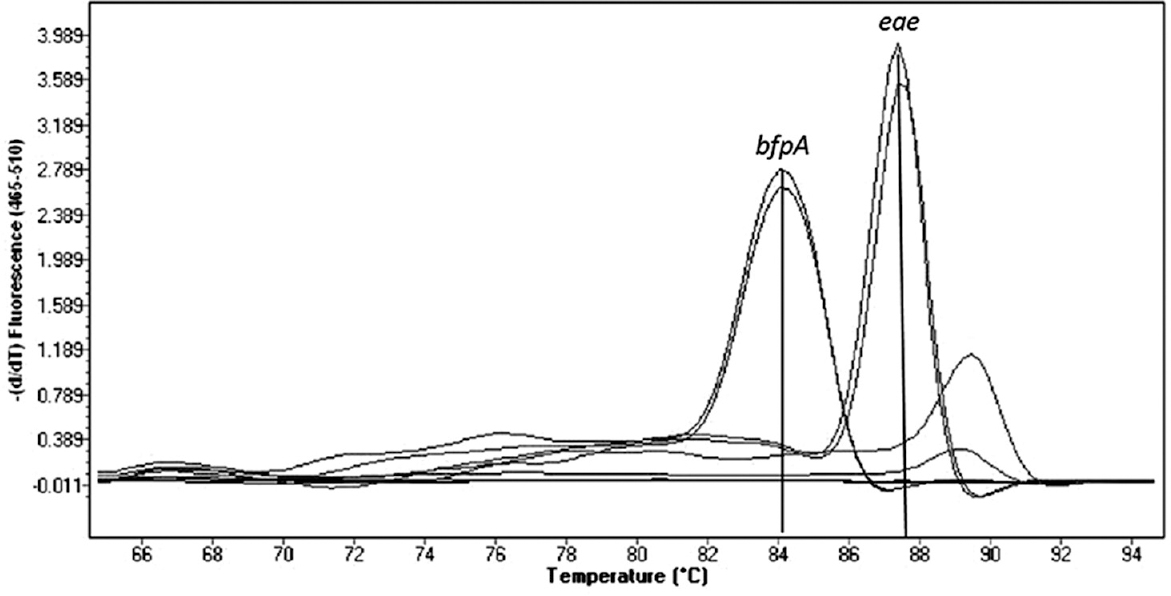
- Real-time polymerase chain reaction assay showing amplified peaks of enteropathogenic Escherichia coli genes. X-axis represents melting temperature and Y-axis represents the rate of change of fluorescence over time.
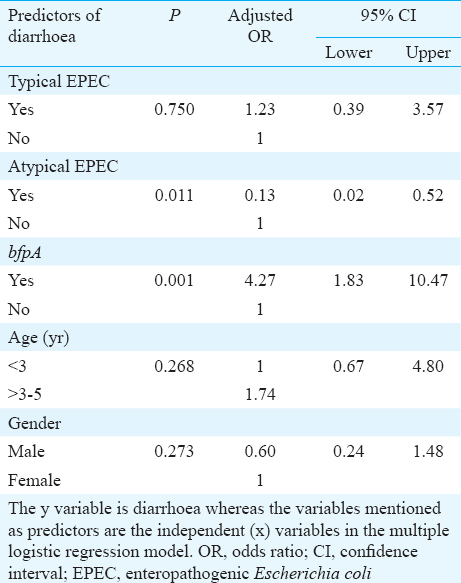
A summary of the pathotypes is shown in Table II. Typical EPEC (eae+ & bfp+) was not significantly different in eight (20%) patients than in 13 (16.25%) healthy children. However, atypical EPEC (eae+ & bfp-) strains which were significantly (P=0.008) higher in controls 20 (23.75%) than two diarrhoeal patients (5%). Eighteen (45%) isolates from children with diarrhoea and 15 (18.75%) isolates from controls were found to be possessing bfpA gene alone and this difference was significant (P< 0.001). Atypical EPEC was a protective factor whereas bfpA was a risk factor for diarrhoea and these were significant by logistic regression model. Age group and sex were not found to be significant independent predictors of EPEC infection (Table III). Dehydration status, clinical status, and duration of diarrhoea were significantly different between patients and controls (Table IV). Other demographic variables were similar in patients and control groups.
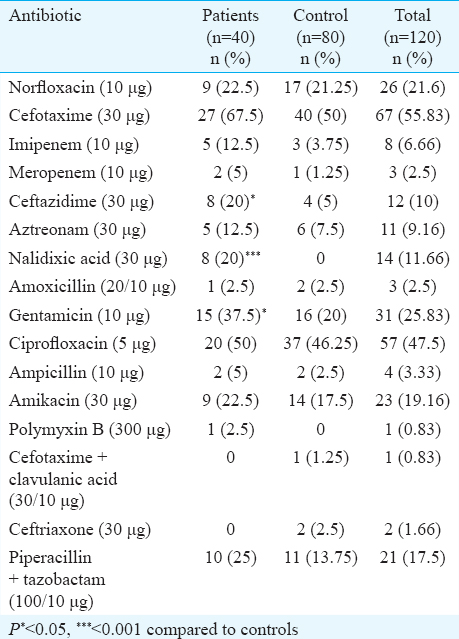
Analysis of antimicrobial resistance patterns was performed for all typical and atypical EPEC isolates from diarrhoeal patients and healthy children. Resistance to all tested antimicrobial agents was higher in isolates from patients than in isolates from healthy participants. Most isolates were sensitive to polymyxin B, ceftriaxone and cefotaxime + clavulanic acid (Table V). Among typical EPEC, cefotaxime resistance was observed in 67.50 per cent (n=27) of patients as compared to 50 per cent (40) in healthy controls. Nalidixic acid, ceftazidime and gentamicin resistance was significantly different in patients and controls. Atypical EPEC isolates showed less resistance to all antimicrobial drugs in comparison to typical EPEC. All atypical EPEC isolates were sensitive to imipenem and gentamicin unlike typical EPEC. In controls, resistance showed by ciprofloxacin and norfloxacin were 46.25 and 21.25 per cent, respectively. It was noted that 5.20 per cent isolates from healthy participants and 15.78 per cent diarrhoeal isolates (typical + atypical) were resistant to more than five antimicrobials.
Discussion
EPEC has been identified as an important cause of infantile diarrhoea in all developing countries. Demographic, clinical and nutritional factors have been evaluated as possible risk factors for EPEC causing diarrhoea25. Transmission of infection due to enteric pathogens is usually common among children with no evident signs and symptoms of gastroenteritis, and many of these children serve as a source of exposure to their families26. Hence, such asymptomatic children may contribute to the transmission of disease to other children. Diarrhoea caused by EPEC is usually self-limited and rehydration is the most effective treatment. The use of antibiotics, in general, is of minor importance and has been criticized on the grounds of drug toxicity and the risk of increasing antimicrobial resistance27. Despite being a self-contained disease, plasmid-mediated antibiotic resistance is common in E. coli due to indiscriminate antibiotic usage28.
In our study, the frequency of EPEC was higher than documented by other Indian studies122829, which was evident in the age group of 3-5 yr in the healthy group. This finding was in accordance with another study from Gaza30. In India, one study31 showed the presence of only diarrhoeagenic E. coli (typical EPEC) in non-diarrhoeal stool samples. It has been well documented that typical and atypical EPEC was different in all aspects, namely, antibiotic resistance and mechanism, phenotypic and genotypic characters32. The isolation of bfp gene alone in our study was similar to studies from South Africa and Iran333435. This finding needs to be further confirmed as bfpA alone (eae absence is not well documented) may not be truly pathogenic and responsible for diarrhoea. The rate at which E. coli is acquiring mutation is much higher than the estimated quantity36. In developing countries, multidrug resistance was observed because these drugs are widely used as the first choice of treatment37.
There were some limitations in our study. The number of investigated isolates was low. For a more representative result, examination of a larger number of isolates should be taken into consideration.
To conclude, EPEC isolates that possessed the eae gene were a common cause of diarrhoea in children. Atypical EPEC is emerging as colonizers of the intestine of children.
Acknowledgment
Authors thank to all children (and their parents) who participated in the study. Authors also acknowledge the assistance rendered by laboratory staff who assisted in sample collection, and Council of Scientific and Industrial Research (CSIR), New Delhi, India, for financial support.
Conflicts of Interest: None.
References
- Enteropathogenic Escherichia coli infection: History and clinical aspects. Br J Biomed Sci. 2002;59:123-7.
- [Google Scholar]
- Aetiology of diarrhoea in a birth cohort of children aged 0-2 year(s) in rural Mirzapur, Bangladesh. J Health Popul Nutr. 2006;24:25-35.
- [Google Scholar]
- Molecular pathogenesis, epidemiology and diagnosis of enteropathogenic Escherichia coli. Salud Publica Mex. 2007;49:376-86.
- [Google Scholar]
- New insights into the epidemiology of enteropathogenic Escherichia coli infection. Trans R Soc Trop Med Hyg. 2008;102:852-6.
- [Google Scholar]
- Identification of two new intimin types in atypical enteropathogenic Escherichia coli. Int Microbiol. 2006;9:103-10.
- [Google Scholar]
- Detection of diarrheagenic Escherichia coli from children with and without diarrhea in Salvador, Bahia, Brazil. Mem Inst Oswaldo Cruz. 2007;102:839-44.
- [Google Scholar]
- Investigation of diarrhoeic faecal samples for enterotoxigenic, Shiga toxin-producing and typical or atypical enteropathogenic Escherichia coli in Kashmir, India. FEMS Microbiol Lett. 2006;261:238-44.
- [Google Scholar]
- The epidemiology of diarrhoeal diseases in early childhood. A review of community studies in Guinea-Bissau. Dan Med Bull. 2000;47:340-58.
- [Google Scholar]
- Heterogenic virulence in a diarrheagenic Escherichia coli: Evidence for an EPEC expressing heat-labile toxin of ETEC. Int J Med Microbiol. 2015;305:47-54.
- [Google Scholar]
- Trends in the prevalence of diarrheagenic Escherichia coli among hospitalized diarrheal patients in Kolkata, India. PLoS One. 2013;8:e56068.
- [Google Scholar]
- Prevalence and characterization of diarrheagenic Escherichia coli isolated from adults and children in Mangalore, India. J Lab Physicians. 2012;4:24-9.
- [Google Scholar]
- Frequency and characteristics of diarrhoeagenic Escherichia coli strains isolated from children with and without diarrhoea in Rio de Janeiro, Brazil. J Infect. 2004;48:161-7.
- [Google Scholar]
- The Global Enteric Multicenter Study (GEMS) of diarrheal disease in infants and young children in developing countries: Epidemiologic and clinical methods of the case/control study. Clin Infect Dis. 2012;55(Suppl 4):S232-45.
- [Google Scholar]
- Practicals issues in calculating the samples size for previous study. Arch Orofac Sci. 2006;1:9-14.
- [Google Scholar]
- Mycology. In: Color atlas and textbook of diagnostic microbiology (6th ed). Philadelphia, PA: Lippincott Williams & Wilkins; 2006. p. :983-1057.
- [Google Scholar]
- Biochemical tests for identification of medical bacteria (3rd ed). Philadelphia: Lippincott Williams and Williams; 2000.
- Clinical & Laboratory Standards Institute. Performance standards for antimicrobial disc susceptibility tests; approved standard. Document M02-A11 (11th ed). Wayne, PA: CLSI; 2012.
- Cloning and characterization of the eae gene of enterohaemorrhagic Escherichia coli O157:H7. Mol Microbiol. 1992;6:411-7.
- [Google Scholar]
- Identification of enteropathogenic Escherichia coli by PCR-based detection of the bundle-forming pilus gene. J Clin Microbiol. 1995;33:1375-7.
- [Google Scholar]
- Antibodies as thermolabile switches: High temperature triggering for the polymerase chain reaction. Biotechnology (N Y). 1994;12:506-9.
- [Google Scholar]
- Single multiplex PCR assay to identify simultaneously the six categories of diarrheagenic Escherichia coli associated with enteric infections. J Clin Microbiol. 2005;43:5362-5.
- [Google Scholar]
- Typical and atypical enteropathogenic Escherichia coli. Emerg Infect Dis. 2002;8:508-13.
- [Google Scholar]
- An efficient program for computing conditional maximum likelihood estimates and exact confidence limits for a common odds ratio. Epidemiology. 1991;2:359-62.
- [Google Scholar]
- Country characteristics and acute diarrhea in children from developing nations: A multilevel study. BMC Public Health. 2015;15:811.
- [Google Scholar]
- Infectious diseases and daycare and preschool education. J Pediatr (Rio J). 2007;83:299-312.
- [Google Scholar]
- Antibiotic prescribing and associated diarrhoea: A prospective cohort study of care home residents. Age Ageing. 2015;44:853-60.
- [Google Scholar]
- Role of enteropathogenic Escherichia coli in paediatric diarrhoeas in South India. Mater Sociomed. 2012;24:178-81.
- [Google Scholar]
- Emerging trends in the etiology of enteric pathogens as evidenced from an active surveillance of hospitalized diarrhoeal patients in Kolkata, India. Gut Pathog. 2010;2:4.
- [Google Scholar]
- Prevalence of enteric pathogen-associated community gastroenteritis among kindergarten children in Gaza. J Biomed Res. 2015;29:61-8.
- [Google Scholar]
- Virulence characteristics and molecular epidemiology of diarrheagenic Escherichia coli (DEC) associated with sporadic cases in a tertiary care hospital in Manipal-Southern India. World J Med Sci. 2007;2:63-4.
- [Google Scholar]
- Genetic elements associated with antimicrobial resistance in enteropathogenic Escherichia coli (EPEC) from Brazil. BMC Microbiol. 2010;10:25.
- [Google Scholar]
- Typical and atypical enteropathogenic Escherichia coli. Emerg Infect Dis. 2002;8:508-13.
- [Google Scholar]
- Characterization of diarrheagenic antimicrobial resistant Escherichia coli isolated from pediatric patients in Tehran, Iran. Iran Red Crescent Med J. 2014;16:e12329.
- [Google Scholar]
- Gene encoding virulence markers among Escherichia coli isolates from diarrhoeic stool samples and river sources in rural Venda communities of South Africa. Water. 2004;30:37-42.
- [Google Scholar]
- The Escherichia coli common pilus and the bundle-forming pilus act in concert during the formation of localized adherence by enteropathogenic E. coli. J Bacteriol. 2009;191:3451-61.
- [Google Scholar]
- Species-specific cell adhesion of enteropathogenic Escherichia coli is mediated by type IV bundle-forming pili. Cell Microbiol. 2002;4:29-42.
- [Google Scholar]






