Translate this page into:
Trends in dengue virus positivity & serotyping in Rajasthan
For correspondence: Dr Bharti Malhotra, Department of Microbiology, Sawai Man Singh Medical College, Jaipur 302 004, Rajasthan, India e-mail: drbhartimalhotra@gmail.com
-
Received: ,
Accepted: ,
Abstract
Background & objectives
Dengue virus causes frequent outbreaks and epidemics with high morbidity and mortality. It is important to monitor the trends of the dengue virus and its serotypes. We carried out the present work to study the prevalence of the dengue virus and its serotypes in clinically suspected cases of dengue in Rajasthan.
Methods
A total of 1,47,777 individuals reporting as pyrexia of unknown origin or clinically suspected of dengue infection were included in this study. The presence of dengue NS1 antigen and dengue IgM was tested by ELISA. Nucleic acid extraction and PCR was done for detection of dengue virus RNA. Serotyping of representative dengue PCR-positive samples was done by real time PCR.
Results
Of the 1,47,777 dengue suspected cases, 28092 (19.01%) were positive for dengue by NS1antigen or IgM ELISA. Year-wise percentage positivity from 2015 to 2022 was 30.42, 16.49, 17.81, 20.15, 20.50, 9.25, 24.55 and 15.16 per cent, respectively. Males of age >15 yr were found to be more commonly affected. The number of dengue cases was significantly higher during the post-monsoon period throughout the eight-year study period. All four dengue serotypes circulated during the study period. DENV-2 and DENV-3 were the predominant serotypes during 2015 to 2017, while DENV-1 and DENV-2 were predominant during 2018 to 2022.
Interpretation & conclusion
The findings of this study suggest that the dengue positivity in Rajasthan was the highest in post-monsoon season among adult males. The serotype prevalent varied from time to time and was helpful in understanding the changing epidemiology of DENV.
Keywords
Dengue
IgM
NS1
PCR
serotyping
virus
Dengue has, over the years, emerged as one of the most important mosquito-borne viral disease globally. Although severe dengue epidemics have been reported from only nine countries before 1970s, at present the disease is endemic in more than 100 countries1. Dengue viruses (DENV) belong to family Flaviviridae and its genome is a positive-sense single-stranded RNA that encodes three structural and seven non-structural (NS) proteins2. There are four serotypes of the virus referred to as DENV-1, DENV-2, DENV-3 and DENV-4 and based upon the nucleotide sequences of the envelope (E), E/NS1 or capsid-premembrane (CprM) gene. Furthermore, these four DENV serotypes have further been classified phylogenetically into three to five genotypes2. The severity of disease caused due to dengue ranges from a subclinical infection to a mild self-limiting disease and even more severe Dengue Haemorrhagic Fever/Dengue Shock Syndrome (DHF/ DSS). Infections by DENV-2 have been associated with severe dengue, including DHF and DSS3; moreover DENV-3 also causes severe forms of dengue, including shock and severe liver involvement4. On the other hand, less severe clinical signs have been linked to DENV-1 and DENV-4 serotypes5. The ever changing epidemiology of DENV and its serotypes has been evidenced in our country during various dengue epidemics. Outbreaks of dengue occurred in Madhya Pradesh and Vellore by DENV-3 in 1966, while DENV-4 was isolated during epidemic in Kanpur in 1968 and all four serotypes in Vellore in 19686. DENV-2 and DENV-4 were responsible for epidemic in Kanpur, while DENV-1 and DENV-3 in Rajasthan in 1969, DENV-3 in Rajasthan in 1985, DENV-2 for epidemics in Gujarat State during 1988 and 1989, Haryana and Delhi in 1996 while DENV-1 was isolated during the 1997 epidemic at Delhi7. Though similar studies have been done in Rajasthan earlier but their numbers are quite low and as the epidemiology of DENV keep changing so it is essential to carry out regular surveillance to monitor the trends of dengue virus positivity and carry out serotyping to know the serotypes responsible for outbreak. Therefore, the present work was done to study the trends of DENV infection during an eight-year time period from 2015-2022 to review the seasonal variation and prevalent dengue serotypes circulating in Rajasthan during this period.
Materials & Methods
Sampling frame and strategy
The study was undertaken on the blood samples from Pyrexia of Unknown Origin (PUO) cases clinically suspected of having dengue infection as per the National Vector Borne Disease Control Programme (NVBDCP) case definition (ncvbdc.mohfw.gov.in). These samples were either received in the laboratory for testing as per Chief Medical and Health Officer’s (CMHO) instructions from each district of Rajasthan or were collected from cases visiting medical out-patient department (OPD) or in-patient department (IPD) of Sawai Maan Singh (SMS) Hospital and attached group of hospitals, Jaipur. Clinical and epidemiological information of the affected individuals were collected on a requisition form. The study was approved by the Ethics Committee of SMS Hospital, Jaipur.
The type of test to be done was decided on the basis of clinical history and the duration of illness. Serum samples of the patients having fever less than five days were tested for DENV-non-structural protein1 antigen (NS1Ag), while those with fever of >5 days were tested for IgM antibody.
Sample collection
Peripheral blood samples (5ml) from individuals clinically suspected of dengue infection and presenting with dengue-like symptoms having pyrexia of unknown origin along with one of any signs and symptoms such as nausea or vomiting, rash, arthralgia or retro-orbital pain were collected after obtaining a written informed consent. These samples were transported to the laboratory maintaining cold chain. Blood samples from total 1,47,777 patients clinically suspected of dengue infection during the study period of eight years, i.e. from January 2015 to December 2022 were processed at the DHR State Virology Research and Diagnostic Laboratory, Jaipur. Serum was separated from blood samples by centrifugation at 3000 rpm for 5 min.
Serology
NS1Ag was tested using NS1 antigen capture ELISA kit (PanBio, Brisbane, Australia) and IgM antibody tested by Dengue IgM Antibody Capture ELISA (MAC-ELISA) kit provided by the National Institute of Virology (NIV), Pune, India according to manufacturers’ protocol.
Reverse transcriptase-Real time PCR (RT-qPCR)
Sixty to eighty per cent of dengue NS1 Ag positive samples from each geographical region having representation of various districts in Rajasthan were randomly selected for qPCR. The Rajasthan State is divided into four geographical regions, namely: (i) desert region (Barmer, Bikaner, Churu, Ganganagar, Hanumangarh, Jaisalmer, Jalore, Jodhpur, Nagaur, Sikar); (ii) Hadoti region/southeastern Rajasthan (Banswara Baran Bundi Chittorgarh Jhalawar Pratatpgarh); (iii) eastern fertile plain (Alwar, Bharatpur, Dausa, Dholpur, Jaipur, Jhunjhunu, Karauli, Kota, Sawai Madhopur, Tonk); and (iv) Aravali region/Hilly terrain (Ajmer, Bhilwara, Dungarpur, Pali, Rajsamand, Sirohi, Udaipur) and the data was analyzed accordingly. About 200µl of serum sample was used for total nucleic acid extraction by Nucleisens Easy Mag semi-automated nucleic acid extraction system (Biomerieux, Netherlands) and was eluted in a volume of 60 µl. The one-step qRT-PCR for DENV was done using AgPath one step RT PCR kit and primers and probes reported earlier7.
Dengue serotyping
Representative numbers (20-30%) of Dengue PCR positive samples having Ct value less than 30 cycles were used for Dengue Serotyping. Multiplex One step RT PCR was done to detect the circulating strains of DV 1–4 using the primers and probes described earlier8.
Statistical Analysis
Data were tabulated and the annual percentage change (APC) in positivity for each year was calculated using MS Excel Version 2007. The graphs depicting monthly trends of dengue NS1Antigen and IgM positivity were also prepared using MS Excel Version 2007. Descriptive statistics were expressed in percentage and proportions. Bivariate analysis of categorical variables was done by the chi-square test using online Medcalc software. Post-hoc test (Turkey’s HSD test) was done to determine differences in dengue prevalence in specific regions in different years. P<0.05 was considered to be statistically significant.
Results
Demographics and seasonal trends of dengue
Year-wise distribution of number of patients tested and number positive by NS1Ag ELISA or IgM ELISA for dengue from 2015-2022 is given in Table I. An increasing trend of dengue positivity was observed in 2015-2016, 2016-17, 2017-18, 2018-19 with a sharp increase in 2020-21 with an APC in positivity of 1.53, 1.80, 3.41, 0.99 and 53.14, respectively. On the other hand, a sharp decrease in positivity was observed in 2019-20 and 2021-22.
| Year | DEN NS1 Ag ELISA positive numbers (%) | DEN NS1 Ag ELISA negative numbers (%) | DEN IgM ELISA positive numbers (%) | DEN IgM ELISA negative numbers (%) | Total (NS1 + IgM) ELISA tested numbers (%) | Total (NS1 + IgM) ELISA positive numbers (%) | Annual % change (APC) |
|---|---|---|---|---|---|---|---|
| 2015 | 1411 (13.9) | 2430(5.24) | 1218(6.79) | 3582(4.89) | 2629(9.36) | 6012 (5.02) | |
| 2016 | 1789 (17.63) | 9709 (20.92) | 1121 (6.25) | 5031 (6.86) | 2910 (10.36) | 14740 (12.32) | 1.53 |
| 2017 | 1107 (10.91) | 4126 (8.89) | 2170 (12.09) | 10996 (15.01) | 3277 (11.67) | 15122 (12.64) | 1.8 |
| 2018 | 1762 (17.36) | 8123 (17.5) | 2298 (12.81) | 7967 (10.87) | 4060 (14.45) | 16090 (13.44) | 3.41 |
| 2019 | 1050 (10.34) | 4556 (9.82) | 3294 (18.36) | 12295 (16.78) | 4344 (15.46) | 16851 (14.08) | 0.99 |
| 2020 | 208 (2.05) | 3392 (7.31) | 1087 (6.06) | 9311 (12.71) | 1295 (4.61) | 12703 (10.61) | -10.03 |
| 2021 | 1190 (11.73) | 4681 (10.09) | 4922 (27.43) | 14101 (19.24) | 6112 (21.76) | 18782 (15.69) | 53.14 |
| 2022 | 1631 (16.08) | 9387 (20.23) | 1834 (10.22) | 9998 (13.64) | 3465 (12.33) | 19385 (16.2) | -6.19 |
The trends of dengue NS1 and IgM positivity in different years are given in figure 1. Highest positivity was seen in the year 2015. Positivity was 17.94 per cent by NS1Ag and 19.61 per cent by IgM ELISA. Lowest positivity was in the year 2020 with 5.78 per cent positive by NS1 ELISA and 10.45 per cent by IgM ELISA. Age and gender-wise distribution of dengue positive cases from 2015 to 2022 are given in Table II. A significant difference in the distribution of males and females across different age groups was observed in the years 2015, 2017, 2018 and 2019.
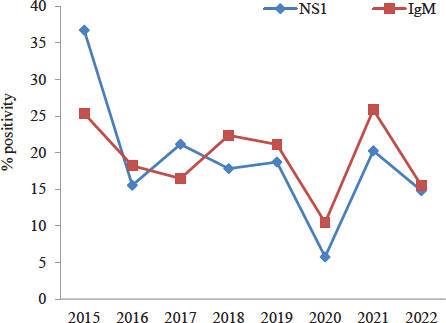
- Yearly trends of dengue NS1 and IgM positivity in different years from 2015 to 2022.
| Year | Gender | 0-15yr (%) | 16-30 yr (%) | 31-45 yr (%) | 46-60 yr (%) | >60yr (%) | Total |
|---|---|---|---|---|---|---|---|
| 2015 | Male | 779 (40.05) | 946 (48.64) | 129 (6.63) | 63 (3.24) | 28 (1.44) | 1945 |
| Female | 191 (27.92) | 397 (58.04) | 63 (9.21) | 22 (3.22) | 11 (1.61) | 684 | |
| 2016 | Male | 576 (27.12) | 1117 (52.59) | 304 (14.31) | 99 (4.66) | 28 (1.32) | 2124 |
| Female | 225 (28.63) | 378 (48.09) | 131 (16.67) | 39 (4.96) | 13 (1.65) | 786 | |
| 2017 | Male | 589 (25.39) | 1270 (54.74) | 307 (13.23) | 122 (5.26) | 32 (1.38) | 2320 |
| Female | 311 (32.5) | 457 (47.75) | 120 (12.54) | 55 (5.75) | 14 (1.46) | 957 | |
| 2018 | Male | 451 (17.42) | 1459 (56.35) | 461 (17.81) | 154 (5.95) | 64 (2.47) | 2589 |
| Female | 207 (14.07) | 937 (63.7) | 228 (15.5) | 70 (4.76) | 29 (1.97) | 1471 | |
| 2019 | Male | 249 (9.88) | 1418 (56.29) | 657 (26.08) | 154 (6.11) | 41 (1.63) | 2519 |
| Female | 224 (12.27) | 971 (53.21) | 469 (25.7) | 140 (7.67) | 21 (1.15) | 1825 | |
| 2020 | Male | 4 (0.44) | 462 (50.83) | 382 (42.02) | 52 (5.72) | 9 (0.99) | 909 |
| Female | 4 (1.04) | 175 (45.34) | 177 (45.85) | 26 (6.74) | 4 (1.04) | 386 | |
| 2021 | Male | 597 (14.58) | 2615 (63.86) | 692 (16.9) | 159 (3.88) | 32 (0.78) | 4095 |
| Female | 311 (15.42) | 1328 (65.84) | 299 (14.82) | 64 (3.17) | 15 (0.74) | 2017 | |
| 2022 | Male | 372 (15.56) | 1330 (55.65) | 488 (20.42) | 162 (6.78) | 38 (1.59) | 2390 |
| Female | 140 (13.02) | 613 (57.02) | 231 (21.49) | 73 (6.79) | 18 (1.67) | 1075 | |
| Total | Male | 3617 (19.15) | 10617 (56.2) | 3420 (18.1) | 965 (5.11) | 272 (1.44) | 18891 |
| Female | 1613 (17.53) | 5256 (57.12) | 1718 (18.67) | 489 (5.31) | 125 (1.36) | 9201 |
P*<0.05 indicate that there is a significant difference in the distribution of males and females across different age groups in the years 2015, 2017, 2018 and 2019
Among the total positive cases (28092), positivity was higher among males (18891, 67.24%) as compared to females (9201, 32.75%).
Majority (81.38%) of the infected individuals were adults aged >15 yr (Table II). Among adults, those aged between 16-30 yr had the highest positivity rates with 56.50 per cent of the total infections.
The results of post–hoc test indicated that the eastern plain region had a significantly higher prevalence compared to Aravali (P<0.001), Hadoti (P=0.012), and desert (P<0.001). Moreover, there was no significant difference in prevalence between the Aravali, Hadoti and the Thar desert regions. The details of per cent positivity of dengue for each geographical region are shown in Table III. In the eastern plain region, maximum positivity was from Jaipur district (24.23%), followed by Kota (21.82%). Seasonal variations of the disease were observed each year; majority of cases were observed during and after the monsoon season (July–December) whereas few sporadic cases were reported throughout other seasons of each year. Seasonal distribution of dengue positive cases is shown in figure 2.
| Year | Dengue per cent positivity | |||||||
|---|---|---|---|---|---|---|---|---|
| 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | |
| Aravali region (Hilly terrain) | 16.36 | 13.84 | 13.46 | 15.28 | 11.42 | 10.83 | 18.3 | 12.8 |
| Eastern plain region (fertile plain) | 26.08 | 22.08 | 18.41 | 20.2 | 21.69 | 9.32 | 22.04 | 23.42 |
| Hadoti region (Southeastern Rajasthan) | 17.6 | 18.84 | 16.91 | 18.3 | 20.65 | 12.67 | 15.82 | 17.24 |
| Desert region (Thar desert) | 13.23 | 15.73 | 15.55 | 14.5 | 18.49 | 10.09 | 14.06 | 15.96 |
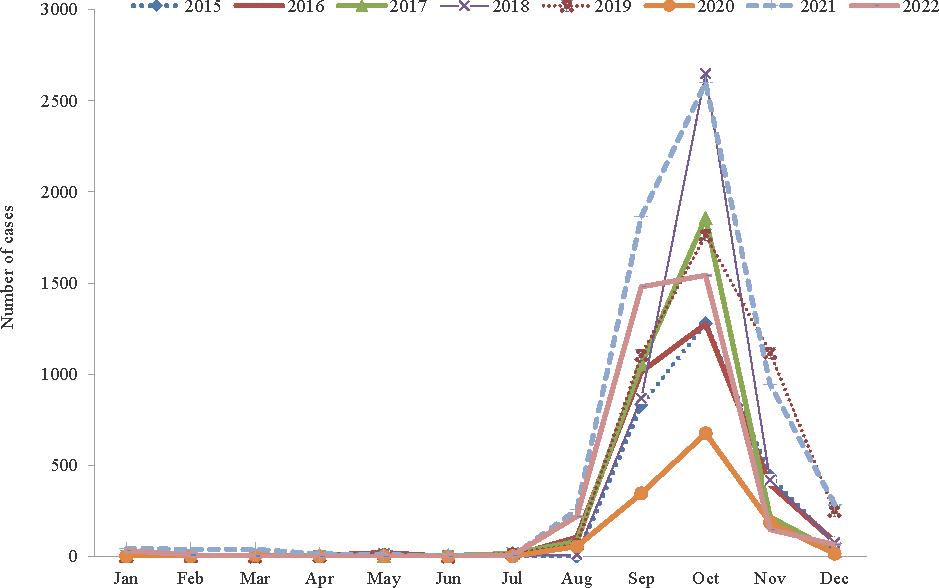
- Monthly trends showing seasonal variation of dengue in Rajasthan from year 2015 to 2022.
Dengue serotype distribution and clinical presentation
A total of 7675 DENV-NS1Ag positive samples were subjected to RT-PCR, out of which 76.69 per cent (5886/7675) were found to be positive for DENV-RNA. A total of 2339 PCR positive samples with Ct<30 cycles were processed for serotyping (Table IV). DENV-2 was the most common serotype identified in 48.23 per cent cases followed by DENV-3 (29.41%) and DENV-1 (17.79%). DENV-4 was present only in 0.34 per cent cases (Fig. 3). Mixed infection with more than one dengue serotype was found in 99 (4.23%) samples; of which 96 samples were co-infected with two dengue serotypes and three samples were infected with three serotypes. DENV-3 was the predominant serotype in 2015 and 2016 followed by DENV-2; both DENV-2 and DENV-3 in 2017 and DENV-2 in 2018. In 2019, DENV-1 and DENV-2 were the predominant serotypes. From 2020 onwards, DENV-2 became the predominant circulating serotype.
| PCR tested | PCR positive | Den serotyped | % Den serotyped | D1 | D2 | D3 | D4 | Mixed | |
|---|---|---|---|---|---|---|---|---|---|
| 2015 | 1229 | 997 | 901 | 90 | 122 (13.54) | 326 (36.18) | 396 (43.95) | 3 (0.34) | 54 (5.99) |
| 2016 | 1125 | 906 | 286 | 31.56 | 14 (4.9) | 99 (34.62) | 156 (54.54) | 1 (0.35) | 16 (5.59) |
| 2017 | 874 | 699 | 204 | 29.18 | 26 (12.74) | 89 (43.63) | 83 (40.69) | 3 (1.47) | 3 (1.47) |
| 2018 | 1247 | 934 | 295 | 31.58 | 69 (23.39) | 179 (60.68) | 37 (12.54) | 1 (0.34) | 9 (3.05) |
| 2019 | 829 | 663 | 186 | 28.05 | 91 (48.93) | 87 (46.77) | 7 (3.76) | 0 | 1 (0.54) |
| 2020 | 172 | 137 | 97 | 70.8 | 14 (14.43) | 83 (85.57) | 0 | 0 | 0 |
| 2021 | 961 | 732 | 191 | 26.09 | 49 (25.65) | 123 (64.4) | 5 (2.62) | 0 | 14 (7.33) |
| 2022 | 1238 | 918 | 179 | 19.49 | 31 (17.32) | 144 (80.45) | 4 (2.23) | 0 | 0 |
| Total | 7675 | 5886 | 2339 | 100 | 416 | 1130 | 688 | 8 | 97 |
Den, dengue
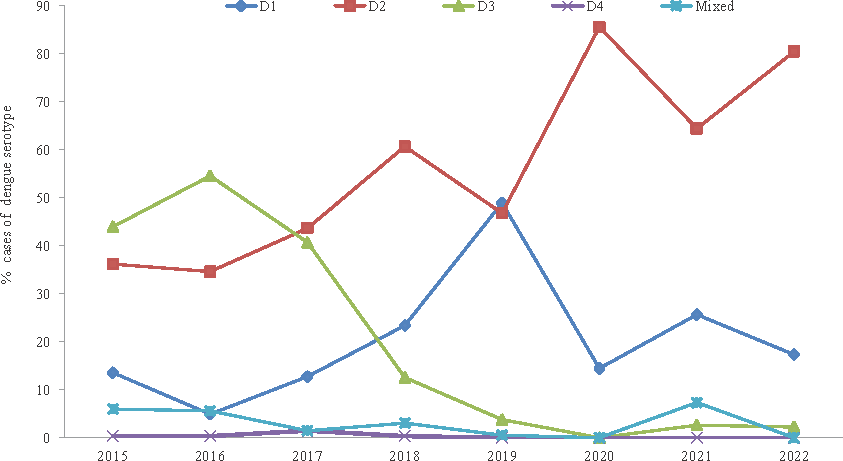
- Circulating dengue serotypes detected by PCR in Rajasthan from year 2015 to 2022.
The clinical presentation of individuals infected with different dengue serotypes is given in Table V. They were divided into three disease severity groups as per WHO 2009 dengue classification. Out of total 2339 cases in which serotyping was done, 33.99 per cent cases were dengue cases without warning signs, 42.71 per cent were dengue cases with warning signs and 23.3 per cent were severe dengue cases. A significant association was found between dengue serotypes and disease severity (P<0.05) (Table IV). Dengue infections without warning signs were observed more frequently in those who were infected with DENV-3 (347/688; 50.44%) and DENV-1 (202/416; 48.56%) as compared to other serotypes. Notably, DENV-2 infected individuals developed severe dengue more frequently (385/1130; 34.07%). No significant difference was found in disease severity when infection due to single and multiple serotypes (with more than one serotype) were compared (P>0.05).
| Disease severity | DENV-1 | DENV-2 | DENV-3 | DENV-4 | Mixed | Total |
|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Dengue without warning signs | 202 (48.56) | 204 (18.05) | 347 (50.44) | 3 (37.5) | 39 (40.21) | 795 (33.99) |
| Dengue with warning signs | 166 (39.9) | 541 (47.88) | 252 (36.62) | 3 (37.5) | 37 (38.14) | 999 (42.71) |
| Severe dengue | 48 (11.54) | 385 (34.07) | 89 (12.94) | 2 (25) | 21 (21.65) | 545 (23.3) |
| Total | 416 (17.79) | 1130 (48.31) | 688 (29.41) | 8 (0.34) | 97 (4.15) | 2339 |
P*<0.05 denotes that there is a significant association between the infecting dengue serotype and dengue disease severity
Discussion
During the last two decades, India has experienced several dengue outbreaks. India is now a dengue endemic zone with outbreaks occurring almost every year9. Regular surveillance of dengue virus infection is therefore essential for early detection of outbreak and to initiate timely preventive and control measures for management of public health in dengue endemic areas.
In India, temperature variation occurs in various climatic zones at both temporal and spatial scales, and these variations influence the Extrinsic Incubation period (EIP)10. EIP is the viral incubation period between the ingestion of the virus by a mosquito after taking a viremic blood meal and the time that the mosquito becomes infectious. After the vector ingests the virus, some time is required for the virus replication, escaping the midgut, and spreading throughout the body of the mosquito until it reaches the salivary glands from where it can be passed on to another host. The EIP plays a key role in determining the risk of occurrence and magnitude of dengue outbreaks in a particular region, as it influences the proportion of mosquitoes that survive to become infectious after exposure to dengue virus
The State of Rajasthan is located in western India and is classified as arid/semi-arid region because there is severe water scarcity and poor rainfall in this region. The mean annual temperature of Rajasthan is 16.6°C and the temperature varies considerably across different seasons, thus influencing EIP dynamics10. The EIP for dengue viruses decreases when the temperature increases from 26 to 30°C. In India, this temperature range is generally experienced during monsoon or early post-monsoon periods, as reported in an earlier study10. During the late post-monsoon or winter periods, the northwestern parts of India usually exhibit low-temperature conditions. In cold conditions, DENV cannot reproduce in mosquitoes, and thus transmission does not occur.
Rajasthan is one of the dengue endemic regions in India, despite being a dry desert State11. In this study, the dengue positivity varied across different years. The positivity showed a fluctuating trend with minimum positivity in 2020 and maximum in 2015. Low positivity in 2020 could be attributed to the COVID-19 pandemic during which most of the people stayed in their homes, thereby decreasing the possibility of being infected with the vector mosquitoes, or they were not visiting the healthcare facilities. A study from Maharashtra reported an increase in the number of dengue positive cases during the study period from January 2015 till December 201912. Another study from Odisha also reported an increase in the number of dengue positive cases from August 2016 to December 201913.
Aedes aegypti, the vector mosquito species which has been reported to be prevalent in Rajasthan14,15 prefers particular environments to breed such as areas where there is water storage and waste disposal is not adequate. Aedes mosquitoes have diurnal biting activities in both indoor and outdoor environments. Exposure to these types of environments may be linked to demographic factors such as age and gender. In this study, males were more vulnerable to infection (67.25%) compared to females (32.75%). Adult males appeared more likely to be exposed to dengue vector mosquitoes as the males spent more time outside their houses and were likely to be more easily infected during daytime hours at either workplace or during travel to and from work. On the other hand, there were relatively less chances of getting infected in house owing to the use of personal protective measures like mosquito repellents and mosquito nets during sleeping or resting. Also, there were differences among males and females in the use of health services and treatment seeking, which may have influenced the clinical outcome. Moreover, biological differences among male and female genders might have also contributed to differences in disease susceptibility. The gender differences in the number of reported incident dengue fever cases in six Asian countries have been reported earlier by Anker et al16. Another study from Asia, such as from Singapore, have also found greater prevalence among males17. Studies from India have also reported similar findings18,19. Murhekar et al20 (2019) in their study reported higher dengue positivity among males (30.0%) than in females (26.1%) (P< 0.001) and male preponderance in all the age groups. In another study from Lucknow, males were reported to have been affected twice as much as the females19. This can be attributed to gender-related differences in exposures such as time away from home.
Some studies have reported age to be a risk factor for clinical dengue infection21. In this study, although people of all age groups had dengue infection, majority of the infected patients individuals were adults in the age group of 16-30 yr (56.5%). Similarly, a study from Andhra Pradesh also reported 18-30 yr to be the commonest (54.3%) age group affected22. Other studies from India have also reported young adults to be the predominantly affected age group19-21,23,24.
In this study, most of the dengue positive cases were from eastern part of Rajasthan. It was found that the eastern plain region had a significantly higher prevalence of dengue. Jaipur district in the eastern plain region reported maximum cases followed by Kota district. A possible reason for this may be the location of the testing laboratory, which is situated in Jaipur, the capital city of Rajasthan where more patients come from nearby districts.
The peak of dengue cases occurred at the beginning and end of the rainy season which could be due to climatic change which provides favorable temperature and humidity conditions for vector mosquito breeding. An earlier study conducted at nine selected places of Jaipur city to determine the positive larval incidence rate of the mosquito vector species during August 2021 to July 2022 found an ideal circumstance for mosquito breeding with varying temperature and humidity between 13.86°C - 35.70°C and 20-86.4 mg/m3 respectively14. In this study, the maximum number of positive cases were recorded in the post-monsoon period (September–November), which could be due to stagnant water conditions in the rainy season, which favour the breeding of vector mosquito. Study from Delhi revealed that the seasonal pattern of dengue cases was similar every year (2015–2018); dengue cases increased from July to August, were maximum in September to October and declined in December. A study by Kumawat et al14 also reported a remarkable larval proliferation in containers like plastic jars, earthen pots, metal drums, broken buckets, tyres, coconut shells, cement tanks, and other water containers that were positioned inside and outside the houses in the selected regions which served as mosquito breeding habitats. Mutheneni et al10 studied the importance of climatic parameters with respect to dengue disease burden in various climatic zones of India and showed a significant association between dengue cases and annual rainfall in the States of Haryana, Punjab, Rajasthan and Kerala. A study from Lucknow also reported maximum number of cases during the post monsoon period25.
Dengue serotyping conducted in the present study demonstrated co-circulation of all the four serotypes of DENV in Rajasthan as also reported in some other Indian studies26,27. It has been reported that co-circulation of all the serotypes contributes to the severity of dengue fever. In the present study, DENV-3 was the predominant serotype followed by DENV-2 in 2015 and 2016 and both DENV-2 and DENV-3 in 2017. A study from Jodhpur, Rajasthan conducted between 2014 to 2018 also reported DENV-3 to be the most common serotype present in 55 per cent cases28. A study from Central Delhi reported DENV-3 to be the prevalent circulating dengue serotype followed by DENV-1 during 2015 outbreak29. While the study from Pune reported predominance of DENV-2 (40.6%) in 201630, the study from Assam reported DENV-1(69.15%) to be the predominant serotype during 2016-2017 followed by DENV-2 (29.90%) and DENV-3 (0.93%)31. In the present study, DENV-2 was the predominant serotype in 2018 while in 2019 both DENV-1 and DENV-2 were predominant. From 2020 onwards, DENV-2 was found to be the predominant serotype. As per Alagarasu et al32 during 2018, 35.72 per cent samples were positive for DENV-2 in India, followed by DENV-1 (25.92%), DENV-3 (25.18%) and DENV-4 (6.95%). The authors also reported DENV-2 to be the predominant serotype in the States of Rajasthan, Andhra Pradesh, Gujarat, Jharkhand, Odisha, Puducherry, Tamil Nadu, Telangana and Uttar Pradesh; DENV-1 in Assam, Himachal Pradesh, Kerala, Karnataka and Nagaland; DENV-3 in Haryana and Madhya Pradesh and DENV-4 in Andaman and Nicobar Islands. These authors also did phylogenetic characterization of dengue sequences and the predominant circulating serotype DENV-2 in Rajasthan clustered in lineage IA32. Another study conducted from Jaipur, Rajasthan during the period of January 2019 to June 2020 reported maximum cases (34%) of DENV-2, followed by DENV- 3 (26%), DENV- 1 (21%) and DENV- 4 (15%)33.
In the present study, dengue cases infected with DENV-2 serotype developed more serious disease as compared to other serotypes (385/1130; 34.07%). On similar lines, a study from Gujrat reported DENV-3 and DENV-2 to be associated with a more serious disease than other serotypes34, while studies from Malaysia and Brazil reported significant association of infection with DENV-2 serotype with disease severity35,36. These findings are in concordance with the present study. We found no significant association between co-infection with multiple dengue serotypes and disease severity despite the fact that secondary infection is associated with more sever disease as reported in previous studies. The reason may be the simultaneous transmission of multiple serotypes in infected cases as also reported in another study from Gujrat34.
Co-infection with two and three dengue serotypes was observed in 4.23 per cent cases in the present study, with the most common co-infection pattern being DENV-2 and DENV-3 serotypes. Infection with more than one dengue serotype was observed in earlier studies from Delhi37-40. Alagarasu et al32 reported multiple serotype infections in 6.22 per cent samples with DENV-2 and DENV-3 in maximum multiple serotype infections during 2018. Another study from Gujrat conducted during 2019-2020 reported mixed infection of more than one dengue serotype in eight per cent cases41, while the study from Jaipur, Rajasthan during 2019-2020 reported concurrent infections in 0.04 per cent cases33. Chances of co-infection with more than one dengue serotype are usually higher when multiple serotypes co-circulate in a population32. Co-circulation of all the four serotypes during the same period of time has also been reported in other studies2,42. As per NVBDCP data, minimum case fatality (0.07%) was reported in the year 2022 while maximum (0.35%) was reported in the year 2020.
A major limitation of this study was that serotyping was done for the representative samples. Initially maximum possible PCR positive samples were tested for serotyping but later only representative samples were tested for serotyping due to cost limitations. Adequate statistical analysis by space, time and age group was not done. Also the trend analysis taking climatic variables as covariates was not done. Another limitation was that though the samples for this study were received from all districts of Rajasthan but the number of samples received varied between districts. There may hence have been a bias in testing as only a few number of samples were received from some districts as compared to other districts. The number of samples from Jaipur district was highest due to the location of hospital and the testing laboratory in Jaipur. Therefore, it may have sample bias to analyze and compare data from different districts. Moreover, both ELISA were not done in all cases, and this may have affected the per cent positivity as many patients may not have provided a proper history of duration of illness.
Overall, dengue positivity was highest during the post monsoon season, though few cases were observed in the non-monsoon season too. Even though Rajasthan is a dessert area we found high positivity for Dengue, the D2 serotype was found to be associated with more severe diseases, and all the four serotypes were found circulating in the State.
Financial support & sponsorship
This study received financial support (Grant No. VIR/7/2011-ECD-I dated 29/08/2011 and Grant No. V 25011/210/2015-HR dated 30.07.2015) from the Indian Council of Medical Research, New Delhi.
Conflicts of Interest
None.
Use of Artificial Intelligence (AI)-Assisted Technology for manuscript preparation
The authors confirm that there was no use of AI-assisted technology for assisting in the writing of the manuscript and no images were manipulated using AI.
References
- Epidemiology of dengue: past, present and future prospects. Clin Epidemiol. 2013;5:299-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Molecular evolution and distribution of dengue viruses type 1 and 2 in nature. Virology. 1990;174:479-93.
- [CrossRef] [PubMed] [Google Scholar]
- Serotype influences on dengue severity: A cross-sectional study on 485 confirmed dengue cases in Vitória, Brazil. BMC Infect Dis. 2016;16:320.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinical differences observed in patients with dengue caused by different serotypes in the epidemic of 2001/2002, occurred in Rio de Janeiro. Rev Soc Bras Med Trop. 2004;37:293-5.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical presentation of dengue by serotype and year of epidemic in Martinique. Am J Trop Med Hyg. 2014;91:138-45.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Development of a multiplex real-time RT-PCR assay for simultaneous detection of dengue and chikungunya viruses. Arch Virol. 2015;160:323-7.
- [CrossRef] [PubMed] [Google Scholar]
- Analytical and clinical performance of the CDC real time RT-PCR assay for detection and typing of dengue virus. PLoS Negl Trop Dis. 2013;7:e2311.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Awareness of changing trends in epidemiology of dengue fever is essential for dengue surveillance. Indian J Med Microbiol. 2012;30:222-6.
- [CrossRef] [PubMed] [Google Scholar]
- Dengue burden in India: Recent trends and importance of climatic parameters. Emerg Microbes Infect. 2017;6:e70.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Outbreak investigation of Dengue in rural area of Rajasthan. Indian J Med Microbiol. 2023;45:100398.
- [CrossRef] [PubMed] [Google Scholar]
- A study of trend of incidence of dengue cases attending a tertiary care hospital in urban Maharashtra. Int J Community Med Public Health. 2020;7:3218-2.
- [CrossRef] [Google Scholar]
- Seroprevalence and changing trend of dengue in a tertiary care hospital, Bhubaneswar, Odisha: Four-year retrospective study. Indian J Microbiol Res. 2022;9:50-4.
- [CrossRef] [Google Scholar]
- Seasonal prevalence of dengue vector mosquito Aedes aegypti Linn in Jaipur city, Rajasthan, India. J Vector Borne Dis. 2023;60:421-6.
- [CrossRef] [PubMed] [Google Scholar]
- Distribution of dengue virus types in Aedes aegypti in dengue endemic districts of Rajasthan, India. Indian J Med Res. 2009;129:665-8.
- [PubMed] [Google Scholar]
- Male-female differences in the number of reported incident dengue fever cases in six Asian countries. Western Pac Surveill Response J. 2011;2:17-23.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Changing pattern of dengue transmission in Singapore. Dengue Bulletin. 2001;25:40-4.
- [Google Scholar]
- Changing trends of dengue disease: A brief report from a tertiary care hospital in New Delhi. Braz J Infect Dis. 2011;15:184-5.
- [CrossRef] [PubMed] [Google Scholar]
- Observation on dengue cases from a virus diagnostic laboratory of a tertiary care hospital in North India. Indian J Med Res. 2015;142(Suppl):S7-S11.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Epidemiology of dengue fever in India, based on laboratory surveillance data, 2014-2017. Int J Infect Dis. 2019;84S:S10-S14.
- [CrossRef] [PubMed] [Google Scholar]
- Dengue infection in North India: An experience of a tertiary care center from 2012 to 2017. Tzu Chi Med J. 2019;32:36-40.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Demographic and clinical profile of dengue fever in a tertiary care hospital of South India. J Assoc Physicians India. 2020;68:24-7.
- [PubMed] [Google Scholar]
- Awareness and outcome of changing trends in clinical profile of dengue fever: A retrospective analysis of dengue epidemic from January to December 2014 at a tertiary care hospital. J Assoc Physicians India. 2017;65:42-6.
- [PubMed] [Google Scholar]
- Prevalence and epidemiological aspect of dengue fever in western Rajasthan in year 2018. Int J Res Med Sci. 2019;7:3735-8.
- [CrossRef] [Google Scholar]
- Trend of dengue virus infection at Lucknow, north India (2008- 2010): A hospital based study. Indian J Med Res. 2012;136:862-7.
- [PubMed] [PubMed Central] [Google Scholar]
- Genetic characterization of dengue virus serotypes causing concurrent infection in an outbreak in Ernakulam, Kerala, South India. Indian J Exp Biol. 2010;48:849-57.
- [PubMed] [Google Scholar]
- Occurrence of concurrent infections with multiple serotypes of dengue viruses during 2013-2015 in northern Kerala, India. Peer J. 2017;5:e2970.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A 5-year study of dengue seropositivity among suspected cases attending a teaching hospital of North-Western region of India. J Med Virol. 2021;93:3338-43.
- [CrossRef] [PubMed] [Google Scholar]
- Circulating Dengue Serotypes in Central Delhi during 2015 Outbreak. J Adv Res Med. 2020;7:1-4.
- [CrossRef] [Google Scholar]
- Co-circulation of all the four dengue virus serotypes and detection of a novel clade of DENV-4 (genotype I) virus in Pune, India during 2016 season. PLoS One. 2018;13:e0192672.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Molecular typing of dengue viruses circulating in Assam, India during 2016-2017. J Vector Borne Dis. 2020;57:249-58.
- [CrossRef] [PubMed] [Google Scholar]
- Serotype and genotype diversity of dengue viruses circulating in India: A multi-centre retrospective study involving the virus research diagnostic laboratory network in 2018. Int J Infect Dis. 2021;111:242-52.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of dengue serotypes and its correlation with the laboratory profile at a tertiary care hospital in Northwestern India. Cureus. 2021;13:e15029.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Identification of prevalent dengue serotypes by reverse transcriptase polymerase chain reaction and correlation with severity of dengue as per the recent World Health Organization classification (2009) Indian J Med Microbiol. 2018;36:273-8.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical manifestations of dengue in relation to dengue serotype and genotype in Malaysia: A retrospective observational study. PLoS Negl Trop Dis. 2018;12:e0006817.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Serotype influences on dengue severity: A cross-sectional study on 485 confirmed dengue cases in Vitória, Brazil. BMC Infect Dis. 2016;16:320.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- An epidemiological study of dengue and its coinfections in Delhi. Int J Infect Dis. 2018;74:41-6.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular investigation of 2013 dengue fever outbreak from Delhi, India. PLoS Curr. 2014;6 ecurrents.outbreaks.0411252a8b82aa933f6540abb54a855f
- [CrossRef] [Google Scholar]
- Concurrent infections by all four dengue virus serotypes during an outbreak of dengue in 2006 in Delhi, India. Virol J. 2008;5:1.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Occurrence of co-infection with dengue viruses during 2014 in New Delhi, India. Epidemiol Infect. 2017;145:67-77.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Co-circulation of all the four serotype of dengue virus in endemic region of Saurashtra, Gujarat during 2019-2020 Season. J Clin Diagn Res. 2021;15:DC46-DC49.
- [CrossRef] [Google Scholar]
- Co-circulation of all four dengue virus serotypes: First report from Odisha. Indian J Med Microbiol. 2017;35:293-5.
- [CrossRef] [PubMed] [Google Scholar]






