Translate this page into:
The utility of immunohistochemistry for detecting mycobacterial infections in bronchoalveolar lavage & bronchial washings
For correspondence: Dr Meenakshi Swain, Department of Pathology, Apollo Hospitals, Jubilee Hills, Hyderabad 500 033, Telangana, India e-mail: meenakshiswain@gmail.com
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Tuberculosis, most commonly caused by Mycobacterium tuberculosis (MTB), is an infectious bacterial disease, with a major impact on global health. In this study, immunohistochemistry (IHC), acid-fast bacilli (AFB) culture and Ziehl-Neelsen (ZN) staining, techniques were compared on bronchoalveolar lavage (BAL) and bronchial washings (BW) with respect to sensitivity and specificity for detecting mycobacteria, taking culture as the gold standard.
Methods:
Consecutive BAL and BW specimens were included in the study, over a period of one year for which AFB cultures were available. Samples with diagnosis other than inflammatory pathology such as malignancies or inadequate samples were excluded. A total of 203 BAL and BW specimens from patients with age ranging from 14 to 86 yr were analyzed for the presence of mycobacteria. The utility and efficacy of ZN stain and IHC in detecting mycobacteria was tested using AFB culture as a gold standard.
Results:
Out of 203 cases, 10.3 per cent (n=21) were positive on AFB culture. Of these, 5.9 per cent (n=12) smears were positive for ZN stain, whereas IHC positivity was seen in 8.4 per cent (n=17) of the cases. ZN staining had a sensitivity of 57.1 per cent and a specificity of 100 per cent whereas, IHC had a sensitivity of 81 per cent and a specificity of 81.9 per cent.
Interpretation & conclusions:
Comparison with AFB culture (gold standard), IHC was found to be superior to ZN stain in terms of sensitivity, whereas ZN stain was found to be superior to IHC in terms of specificity. These findings therefore suggest that IHC may be a useful adjunct to ZN stain in the detection of mycobacteria in specimens from the respiratory tract.
Keywords
Bronchoalveolar lavage
bronchoalveolar washing
culture
immunohistochemistry
mycobacteria
Tuberculosis is an infectious and contagious bacterial disease. It continues to be one of the most prevalent infectious diseases in India, with an incidence of 1.98 million cases per year1. It is estimated that one third of the world population is infected with Mycobacterium tuberculosis (MTB)2 . As the incidence of multidrug resistant tuberculosis and HIV-associated tuberculosis, especially by atypical mycobacteria, is on the rise and majority of people with TB have latent infection, the development of new diagnostic and screening tools has become necessary in order to facilitate early diagnosis and control of the disease3.
High specificity has been observed in the detection of mycobacteria in specimens such as sputum, bronchoalveolar lavage (BAL) or induced sputum for the diagnosis of pulmonary tuberculosis3,4. In this study, three tests were compared, namely immunohistochemistry (IHC), culture of acid-fast bacilli (AFB) and Ziehl-Neelsen (ZN) staining, for the diagnosis of MTB, considering culture as the gold standard, to determine their efficacy, sensitivity as well as the ease of detection of mycobacteria.
Material & Methods
The study was carried in the department of Microbiology, Apollo Hospital, Hyderabad, Telangana, India. All consecutive BAL and bronchial washings (BW) specimen submitted to the department, over a period of one year (from May 2017 to April 2018) for which AFB cultures were available, were included in the study. Samples with a diagnosis other than inflammatory pathology like malignancies or inadequate samples were excluded. The study was approved by the Institutional Ethics Committee.
A total of 203 BAL and BW specimens were analysed from patients with age ranging from 14 to 86 yr. Giemsa, Papanicolaou and Ziehl-Neelsen (ZN) stains were performed on smears as per standard procedures5. ZN stain was done after destaining the Giemsa-stained smears in retrospective cases, while in prospective cases; it was done on fresh smears using the standard procedure. Hematoxylin and eosin (H and E) staining and IHC were done on cell blocks to determine the presence of mycobacteria.
Immunohistochemistry (IHC) was performed on cell blocks prepared from the respective BAL and BW using M. tuberculosis concentrated (catalogue no. CP 140 A; Biocare Medical, USA) and pre-diluted (1:200) rabbit polyclonal antibody (catalogue no. PP 140 AA; Biocare Medical) in an automated slide stainer, Ventana BenchMark® XT (Roche, USA) by following manufacturer’s instructions. This antibody is reactive with mycobacteria species including M. tuberculosis, M. avium, M. phlei and M. parafortuitum. BD BACTEC MGIT (mycobacteria growth indicator tube) 960 automated mycobacterial detection system (Becton Dickinson, USA) was used for AFB culture. It was used as a gold standard for comparing the efficacy of ZN and IHC stains.
Results & Discussion
To the best of our knowledge, this is one of the few studies with such a large sample size comparing the efficacy of ZN stain and IHC for detecting mycobacteria on fluids from BAL and BW. Out of the 203 samples studied, 141 (69.50%) were male and 62 (30.50%) were female. Of these, 112 were BAL samples (55.17%) and 91 were BW (44.83%).
In the present study, 175 cases were reported as smears on cytology showing an inflammatory pathology and were negative for malignancy. These showed inflammation composed of neutrophils and lymphocytes admixed with benign squamous cells and ciliated columnar cells with alveolar macrophages. Ten of the 203 specimens were reported as smears showing inflammation with tuberculous etiology based on the presence of AFB on ZN stain. Thirteen were reported as negative for malignancy; three showed inflammation with fungal hyphae and two showed inflammation along with numerous macrophages.
Out of the 203 specimens, 21 were AFB culture positive, accounting for 10.3 per cent of the total number of cases. This included both typical and atypical mycobacteria. On ZN staining, the smears showing beaded, bright pink rods were reported as AFB positive (Fig. 1). Twelve out of 203 (5.91%) cases showed positivity on ZN staining. None of the samples showed false positivity (Table I).
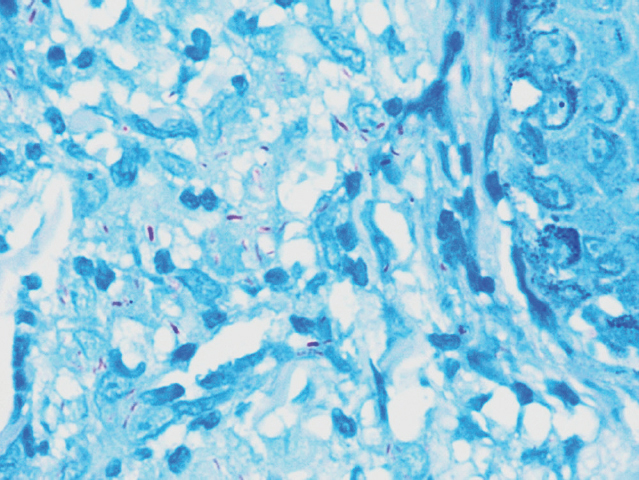
- Acid-fast bacilli (ZN stain, ×100).
| Results | IHC, n (%) | ZN, n (%) |
|---|---|---|
| True positive | 17 (8.37) | 12 (5.91) |
| False positive | 33 (16.25) | 0 (0) |
| True negative | 149 (73.39) | 182 (89.65) |
| False negative | 4 (1.97) | 9 (4.43) |
| Sensitivity of detecting mycobacteria (%) | 81 | 57.10 |
| Specificity of detecting mycobacteria (%) | 81.90 | 100 |
| Positive predictive value (%) | 34 | 100 |
| Negative predictive value (%) | 97.40 | 95.30 |
| Positive likelihood ratio | 4.48 (95% CI: 3.08-6.48) | Infinity |
| Negative likelihood ratio | 0.23 (95% CI: 0.10-0.56) | 0.43 (95% CI: 0.26-0.70) |
CI, confidence interval
The lowest ZN positivity of mycobacterial strains (0%) was reported by Radhakrishnan et al6 and by Padmavathy et al7, whereas the highest positivity of the same (50%) was reported by Purohit et al8. Typically, ZN staining is positive only when the bacillary count is more than 10,000 organisms/ml of the specimen9. Other reasons for false negativity could be technical problems leading to suboptimal staining. Antimycobacterial therapy can also alter capsule integrity to render organism non-acid-fast10; hence, partially treated smears may also be negative on ZN stain. As the AFB gets engulfed and phagocytosed by the macrophages, only fragments of bacilli are left in the lesion which are not identified by ZN stain11. Due to intense phagocytic activity of the macrophages in tuberculous granulomas, the morphological characteristics of AFB often get distorted. This may account for the low detectability on ZN staining similar to the finding in the present study.
The IHC staining of bacilli showing different staining patterns is shown in Figure 2. IHC positivity was reported as intracellular as well as extracellular fine brown granularity or as slender rods (Fig. 2C). In the present study, 50 out of 203 (24.63%) showed positive staining on IHC, 17 (8.37%) were true positive i.e. these cases were culture positive. However, 33 cases were false positive (16.25%) i.e. these cases were IHC positive but culture negative (Fig 2G and H).
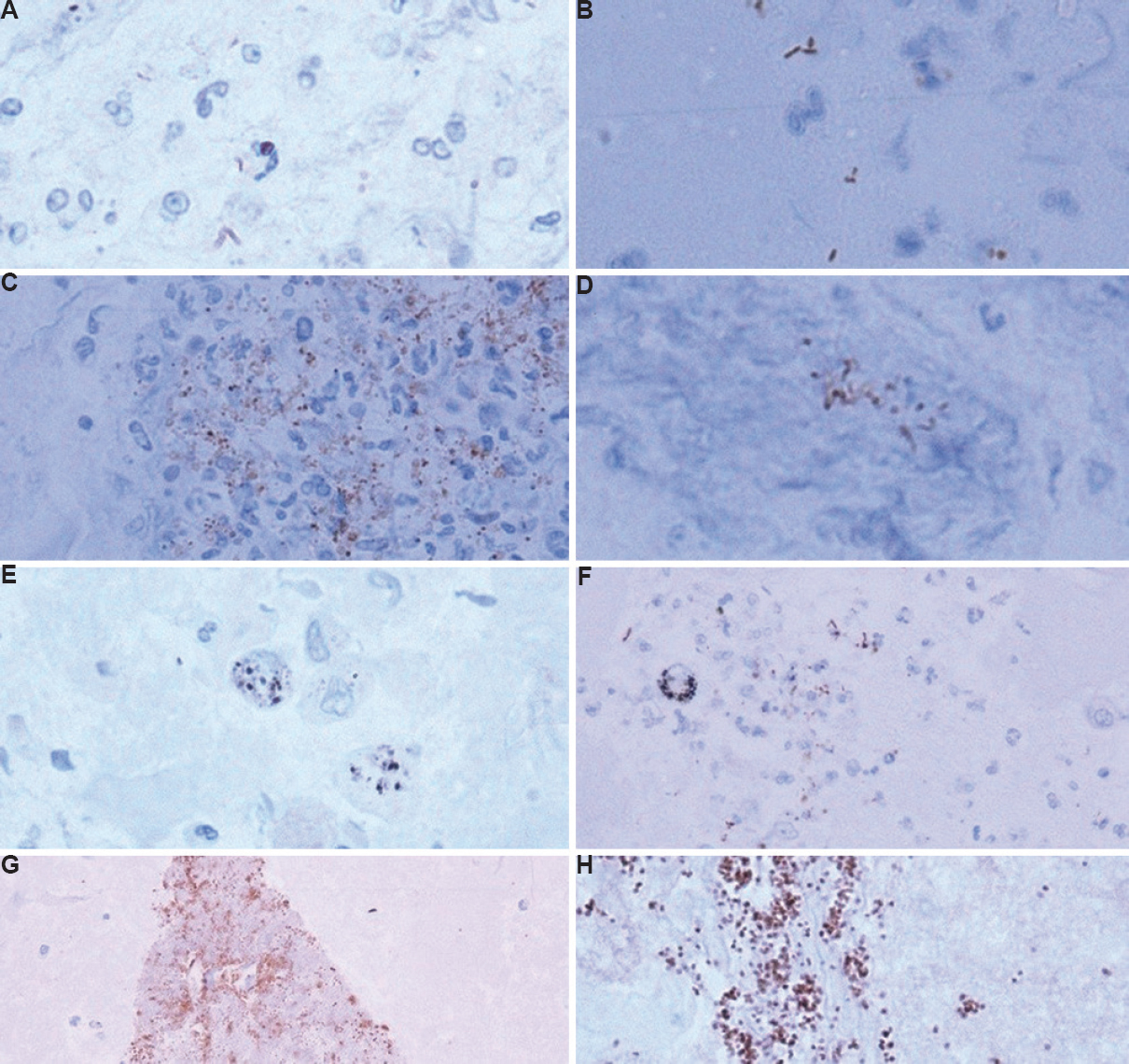
- (A) Weak IHC positivity showing bacilli in clusters (×100). (B) Extracellular, stubby, dark bacilli, possibly contaminant (×100). (C) IHC positive bacilli entrapped in cluster of histiocytes and neutrophils (×40). (D) Cluster of IHC positive bacilli (×100). (E) Macrophages showing intracellular black anthracotic pigment (IHC, ×100). (F) IHC-positive bacilli and central macrophage showing blackish intracellular anthracotic pigment (×40). (G) Contaminant, (IHC, x40) (H) Dark brown course granularity, false positive (IHC, ×40) IHC, immunohistochemistry.
The bacilli were mostly seen in clusters (Figs 2C and D), however, a few were also seen as singly scattered (Fig. 2A). Two fluids which showed histiocytes and neutrophils on smears, particularly displayed intracellular positivity in histiocytes or within aggregates of histiocytes and neutrophils (Figs 2C and D). In these smears, AFB were seen on ZN staining. The contaminants and dark brown course granularity resulting in false positive interpretation are shown in Figures 2G and H.
The lowest IHC positivity of 64 per cent was reported by Mustafa et al12, whereas the highest positivity of 100 per cent was reported by Barbolini et al13 and Goel and Budhawar14 on tissue sections. The possible reasons for high false positivity in this study could be contamination due to various reasons such as unsterile sampling technique, unsterile containers, long storage time of sample or contamination during processing. For example, the use of egg albumin stored for a long time for preparing cell blocks is a good medium for the growth of contaminants. The contaminants were recognized as short, stubby rods (Fig. 2B) rather than slender beaded rods or fine granularity. Usually, these were seen in groups or big clusters, often extracellular.
The other reason for false positivity could be the use of the polyclonal antibody, which might have resulted in the uptake of the stain by mycobacteria other than MTB. Morphologically, typical and atypical mycobacteria cannot be distinguished on immunohistochemical stain15. This indicates that the use of polyclonal antibody for IHC may increase the chances of false positivity. IHC stains the dead as well as fragmented bacilli. It has the capacity to stain antigenic dust as well, which is not stained by ZN stain16. This may be the cause of the higher sensitivity of IHC.
In this study, differentiating IHC stain from intracellular anthracotic pigment (Fig. 2E) and haemosiderin pigment (Fig. 2F) in the macrophages was a challenge. It was found that the anthracotic pigment appeared darker and blackish as compared to the positive IHCstain which is brown, and haemosiderin pigment showed coarse staining pattern only within macrophages.
Three cases were positive for AFB culture as well as ZN stain; however, these did not show IHC positivity. The reason for this false negativity cannot really be ascertained but could possibly be due to technical issues.
The result of IHC staining (81% true positivity) in the current study is similar to the studies by Baba et al17, and Purohit et al8. The results of studies using monoclonal antibodies however, appear to be superior, with 100 per cent IHC positivity6,13. As reported by Purohit et al8, the overall sensitivity and specificity of IHC with monoclonal antibodies such as anti-MPT64 were 92 and 97 per cent, respectively, while the corresponding values for anti-BCG were 88 and 85 per cent. Comparison with various studies on IHC for MTB is shown in Table II.
| Study | Number of cases | Type of antibody used | ZN positivity (%) | IHC positivity (%) | ZN sensitivity (%) | ZN specificity (%) | IHC sensitivity (%) | IHC specificity (%) |
|---|---|---|---|---|---|---|---|---|
| Radhakrishnan et al6, 1991 | 10 | IgG anti-mycobacterial Ab | 0 | 100 | NA | NA | NA | NA |
| Mukherjee et al19, 2002 | 50 | Polyclonal anti-BCG | 44 | 87 | NA | NA | NA | NA |
| Mustafa et al12, 2006 | 55 | Polyclonal anti-BCG | 0 | 64 | NA | NA | 90 | 83 |
| Baba et al17, 2008 | 25 | Ani-MPT and anti-BCG | NA | 80 | NA | NA | 81 | 100 |
| Kundu et al18, 2014 | 100 | Polyclonal anti-BCG | 23 | 72 | 31 | 96 | 95 | 35 |
| Barbolini et al13, 1989 | 23 | Monoclonal antibodies, 60.15, 61.3 | 15 | 100 | NA | NA | NA | NA |
| Luo20, 1990 | 137 | Polyclonal anti-BCG | 34 | 69.3 | NA | NA | NA | NA |
| Padmavathy et al7, 2005 | 50 | Polyclonal anti-BCG | 0 | 68 | NA | NA | NA | NA |
| Purohit et al8, 2007 | 120 | Ani-MPT and anti-BCG | 50 | 80 | 13 | NA | 92 | 97 |
| Humphrey and Weiner21, 1987 | 59 | Polyclonal anti-BCG | NA | 77.7 | NA | NA | NA | NA |
| Oliveira et al22, 2004 | 3 | NA | NA | 100 | NA | NA | NA | NA |
| Goel and Budhwar14, 2007 | 36 | Monoclonal | NA | 100 | NA | NA | NA | NA |
| Present study (on BAL and BW) | 203 | Rabbit polyclonal antibody | 5.91 | 24.63 | 57 | 100 | 81 | 82 |
NA, not available; BAL, bronchoalveolar lavage; BW, bronchial washings; IHC, immunohistochemistry; ZN, Ziehl-Neelsen; Anti-BCG, anti-Bacillus Calmette-Guérin
The sensitivity and specificity of ZN stain was 57.1 and 100 per cent, respectively, whereas for IHC staining these were 81 and 81.9 per cent, respectively. The combined ZN stain and IHC results had a sensitivity of 69 per cent and specificity of 90.1 per cent. The likelihood ratio positive and likelihood ratio negative for IHC and ZN stains are included in Table I.
The sensitivity of IHC depends on various factors such as distribution of mycobacterial antigen within the granuloma, the clinical stage of disease, duration of anti-tubercular treatment received prior to sampling and specificity of the primary antibody18. The reason for 100 per cent positivity in few previous studies could be the use of monoclonal antibody.
Most of the studies published in literature have done studies on tissue sections, where PCR was used for tuberculosis as a standard for comparison, unlike the present study in which fluids were used as samples to compare ZN and IHC results with AFB culture, which is considered a gold standard in the diagnosis of tuberculosis. Culture with the new and robust BACTEC machine is not only considered reliable but also quick as compared to conventional culture methods. Studies comparing the utility of IHC and ZN stain in detecting mycobacteria in tissue sections are included in Table II.
According to literature, IHC staining gives superior results as compared to the ZN staining, with a positivity of 100 per cent using monoclonal antibody13. In our study also, the sensitivity of IHC (81%) was found to be better than ZN staining (57.10%). But as the morphology of mycobacteria on IHC stain is varied, distinguishing these from contaminants was a challenge and the main limitation of this study. The false-positive results were high, thus limiting the use of this method alone for the detection of mycobacteria in fluids.
Overall, based on the study findings, AFB culture as a gold standard, neither IHC nor ZN stain appears to be useful as independent methods for the detection of mycobacteria, but these can be used as adjuncts to each other along with other diagnostic tests. A combination of tests can give better and more accurate results for the detection of mycobacteria in bronchoscopic fluids and aid in timely institution of therapy. Further studies with a larger sample size are, however, needed to validate the findings of this study and also to validate the use of IHC as an independent diagnostic test in the diagnosis of tuberculosis.
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- New vision for Revised National Tuberculosis Control Programme (RNTCP): Universal access - Reaching the un-reached. Indian J Med Res. 2012;135:690-4.
- [Google Scholar]
- Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999;282:677-86.
- [Google Scholar]
- The biology of mycobacterium tuberculosis infection. Mediterr J Hematol Infect Dis. 2013;5:e2013070.
- [Google Scholar]
- Bancroft's theory and practice of histological techniques (8th ed). london: Churchill Livingstone Elsevier; 2013. p. :536.
- Immunohistochemical demonstration of mycobacterial antigens in intracranial tuberculoma. Indian J Exp Biol. 1991;29:641-4.
- [Google Scholar]
- Mycobacterial antigen in tissues in diagnosis of cutaneous tuberculosis. Indian J Tuberc. 2005;52:31-5.
- [Google Scholar]
- Immunohistochemical diagnosis of abdominal and lymph node tuberculosis by detecting Mycobacterium tuberculosis complex specific antigen MPT64. Diagn Pathol. 2007;2:36.
- [Google Scholar]
- BACTEC versus Loenstein-Jensen media for isolation of mycobacterium tuberculosis. Eur Res J. 2013;42:P4439.
- [Google Scholar]
- Comparison of recoveries of Mycobacterium tuberculosis using the automated BACTEC MGIT 960 system, the BACTEC 460 TB system, and Löwenstein-Jensen medium. J Clin Microbiol. 2000;38:2395-7.
- [Google Scholar]
- Internalization by HeLa cells of latex beads coated with mammalian cell entry (Mce) proteins encoded by the mc3 operon of Mycobacterium tuberculosis. In: J Med Microbiol. Vol 56. 2007. p. :1145-51.
- [Google Scholar]
- Immunohistochemistry using a Mycobacterium tuberculosis complex specific antibody for improved diagnosis of tuberculous lymphadenitis. Mod Pathol. 2006;19:1606-14.
- [Google Scholar]
- Immunohistologic analysis of mycobacterial antigens by monoclonal antibodies in tuberculosis and mycobacteriosis. Hum Pathol. 1989;20:1078-83.
- [Google Scholar]
- Immunohistochemical localization of mycobacterium tuberculosis complex antigen with antibody to 38 kDa antigen versus Ziehl Neelsen staining in tissue granulomas of extrapulmonary tuberculosis. Indian J Tuberc. 2007;54:24-9.
- [Google Scholar]
- Use of BACTEC MGIT 960 for recovery of mycobacteria from clinical specimens: multicenter study. J Clin Microbiol. 1999;37:3578-82.
- [Google Scholar]
- How liquid based microbiology can change the workflow in the microbiology laboratories. Adv Microbiol. 2013;3:504-10.
- [Google Scholar]
- Rapid and specific diagnosis of tuberculous pleuritis with immunohistochemistry by detecting mycobacterium tuberculosis complex specific antigen mpt64 in patients from a hiv endemic area. Appl Immunohistochem Mol Morphol. 2008;16:554-61.
- [Google Scholar]
- Relative value of immunohistochemistry in detection of mycobacterial antigen in suspected cases of tuberculosis in tissue sections. Indian J Pathol Microbiol. 2014;57:574.
- [Google Scholar]
- Immunohistochemical detection of mycobacterial antigen in tuberculous lymphadenitis. Indian J Tuberculosis. 2002;213:213-16.
- [Google Scholar]
- Immunohistochemical demonstration of mycobacterial antigen. Zhonghua Jie He He Hu Xi Za Zhi. 1990;13:360-2. 381-2
- [Google Scholar]
- Mycobacterial antigen detection by immunohistochemistry in pulmonary tuberculosis. Hum Pathol. 1987;18:701-8.
- [Google Scholar]
- Orbital tuberculosis diagnosed by immunohistochemistry: case reports. Rev Inst Med Trop Sao Paulo. 2004;46:291-4.
- [Google Scholar]






