Translate this page into:
The side effects of immune checkpoint inhibitor therapy on the endocrine system
For correspondence: Dr Paresh Dandona, Diabetes & Endocrinology Research Center of WNY, 1000 Youngs Road, Suite 105, Buffalo, NY 14221, USA e-mail: pdandona@kaleidahealth.org
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Immune checkpoint inhibitors (ICIs) are a relatively newer class of drugs approved for the treatment of malignancies such as melanoma, renal, bladder and lung cancer. Immune-related adverse events (IrAEs) involving the endocrine system are a common side effect of these drugs. The spectrum of endocrine adverse events varies by the drug class. Cytotoxic T-lymphocyte–associated antigen-4 inhibitors commonly cause hypophysitis/hypopituitarism, whereas the incidence of thyroid disease is higher with programmed cell death (PD)-1/ ligand (PD-L) protein 1 inhibitors. The focus of this review is to describe the individual endocrinopathies with their possible mechanisms, signs and symptoms, clinical assessment and disease management. Multiple mechanisms of IrAEs have been described in literature including type II/IV hypersensitivity reactions and development of autoantibodies. Patients with pre-existing autoimmune endocrine diseases can have disease exacerbation following ICI therapy rather than de novo IrAEs. Most of the endocrinopathies are relatively mild, and timely hormone replacement therapy allows continuation of ICIs. However, involvement of the pituitary–adrenal axis could be life-threatening if not recognized. Corticosteroids are helpful when the pituitary–adrenal axis is involved. In cases of severe endocrine toxicity (grade 3/4), ICIs should be temporarily discontinued and can be restarted after adequate hormonal therapy. Endocrinologists and general internists need to be vigilant and maintain a high degree of awareness for these adverse events.
Keywords
Adrenal insufficiency
autoimmune diabetes
autoimmune endocrinopathy
corticosteroids
immune checkpoint inhibitors
hormone replacement
hypophysitis
hypothyroidism
Introduction
Despite advances in chemotherapy and targeted molecular therapies, survival in some cancer patients remains poor. Immunotherapy has expanded the therapeutic options in our fight against cancer1. Cytotoxic T-lymphocyte–associated antigen-4 (CTLA-4) is present on T cells and competes with CD28 to bind with B7 protein on the antigen presenting cell, which in turn leads to the inhibition of the T cell2 (Fig. 1). Programmed cell death protein (PD)-1 is expressed on mature T and B cells, macrophages and thymocytes. It binds with its ligand PD ligand 1 (PD-L1), expressed extensively on tumour cells, suppressing T cell effector function3 (Fig. 2). T cells found in tumour microenvironment exhibit increased expression of PD-1. Thus, CTLA-4 and PD-1/PD-L1 pathways lead to exhausted T cells in a tumour and promote proliferation and survival of cancer cells. The blockade of these immune checkpoints by specific monoclonal antibodies (mAbs) leads to unrestrained T cell activation (Figs 1 and 2) - this interaction results in antitumour activity and improved survival in some tumours4.
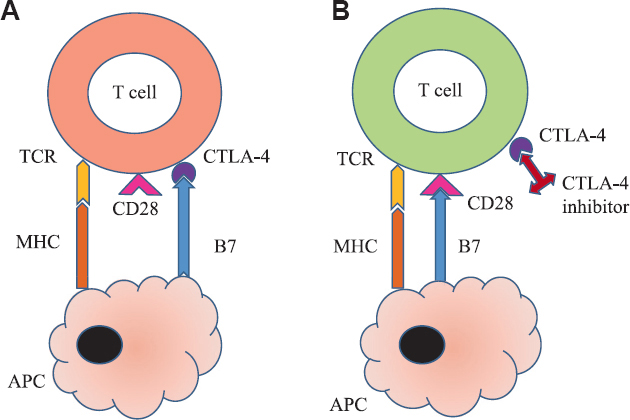
- CTLA-4 inhibitors mechanism of action. (A) Inactivated T cell: Binding of B7 with CTLA-4 instead of CD28 keeps T cell inactivated by blocking co-stimulation. (B) Activated T cell: CTLA-4 inhibitors like ipilimumab bind with CTLA-4 on T cells thereby releasing B7 to bind with CD28 for co-stimulating and activating T cells. TCR, T cell receptor; MHC, major histocompatibility complex; APC, antigen presenting cell; CTLA-4, cytotoxic T-lymphocyte–associated antigen-4. Source: Refs 2,3.
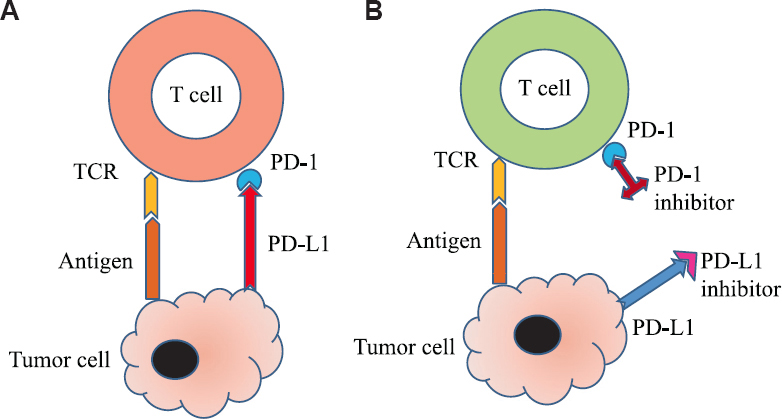
- PD-1/PDL-1 inhibitors mechanism of action. (A) Inactivated T cell: Binding of PD-1 on T-cell with PD-L1 on tumour cell keeps T cell inactivated. (B) Activated T cell: Anti PD-1 or PD-L1 antibodies prevent binding of PD-1 with PD-L1 to keep T cell activated. PD-1, programmed cell death protein-1; PD-L1, PD ligand-1. Source: Refs 3,4.
Immune checkpoint inhibitors (ICIs) are a relatively newer class of drugs that have been approved for the treatment of certain solid tumours such as melanoma, non-small cell lung carcinoma (NSCLC) and renal, ovarian and bladder cancer5. The three main categories of agents approved by the Food and Drug Administration (FDA) are CTLA-4 inhibitors such as ipilimumab (Ipi), PD-1 inhibitors such as nivolumab and pembrolizumab, dostarlimab and cemiplimab-rwlc and PD-L1 inhibitors such as atezolizumab, avelumab and durvalumab (Table I). CTLA-4 inhibitors act centrally at the lymph node level to increase T cell proliferation, whereas PD-1/PD-L1 inhibitors work more peripherally in the tumour microenvironment1. However, these drugs lead to the development of atypical toxicities, which are quite different from traditional anticancer therapies. Since the immune checkpoint molecules protect against autoimmunity and inflammation, inhibiting these checkpoints causes increased T cell activation. This leads to autoimmune manifestations against multiple organ systems [known as immune-related adverse events (IrAEs)], and particularly, concerning is the involvement of the endocrine system25.
| Drug class | Drug dose (FDA approved) | Endocrine dysfunction | Incidence of endocrine dysfunction (all grade) % | Incidence of endocrine dysfunction (Grade 3/4)5 |
|---|---|---|---|---|
| Anti-CTLA-4 | Ipilimumab: 1-10 mg/kg q3wks | Hypophysitis | 0-25567891011 | 0-4 per cent |
| Hypothyroidism | 0-15.256 | Rare | ||
| Hyperthyroidism | 1.0-2.356 | Rare | ||
| PAI and T1DM | Rare | NA | ||
| Anti-PD-1 (nivolumab, pembrolizumab) | Nivolumab: 3 mg/kg, 240 mg q2wks, 480 mg q4wks Pembrolizumab: 2 mg/kg, 200 mg q3wks and 400 mg q6wks | Hypophysitis | 0.5-1.156121314151617181920 | 0-3.0 per cent |
| Hypothyroidism | 0-405612 | 0-2.5 per cent | ||
| Hyperthyroidism | 0-7.75612 | Rare | ||
| PAI | 0-26 | NA | ||
| T1DM | 0-26 | NA | ||
| Ipilimumab + nivolumab | 1 mg/kg + 3 mg/kg | Hypophysitis | 3.8-11.75612212223 | 0-2.1 per cent |
| Hypothyroidism | 3.8-16.056 | Rare | ||
| Hyperthyroidism | 3.8-9.956 | Rare | ||
| PAI | 0-5624 | NA | ||
| T1DM | NA | NA | ||
| Anti-PD-L1 | Durvalumab: 10 mg/kg every 2 wks, 1500 mg fixed dose in some NSCL Avelumab: 800 mg q2wks Atezolizumab: 840 mg q2wks, 1200 mg q3wks and 1680 mg q4wks |
Hypophysitis | Rare | |
| Hypothyroidism | 0-5.656 | |||
| Hyperthyroidism | 0-2.356 | |||
| PAI | 0-1.16 | |||
| T1DM | 0-1.46 |
FDA, Food and Drug Administration; PAI, primary adrenal insufficiency; T1DM, type 1 diabetes mellitus; NA, not available; CTLA-4, cytotoxic T-lymphocyte associated antigen-4; PD-1, programmed cell death protein-1; PD-L1, programmed death ligand
In the endocrine system, cases of hypophysitis resulting in hypopituitarism, thyroid dysfunction (hypothyroidism, hyperthyroidism and thyroiditis), primary adrenal insufficiency (PAI) and type 1 diabetes mellitus (T1DM)/autoimmune diabetes have frequently been reported in various clinical trials and meta-analyses56122627 as well as in clinical practice with ICIs. Hypercalcaemia and hypoparathyroidism are less often reported. The involvement of the pituitary gland and thyroid gland is more commonly associated with ICI use than that of adrenals and pancreas. Individual ICIs are associated with different endocrinopathies: hypophysitis being frequent with Ipi, whereas thyroid dysfunction is more frequent with PD-1/PD-L1 inhibitors5626. The combination of these agents increases the risk of individual endocrine IrAEs compared to ICI monotherapy5626.
There is a lot of interest and excitement among the physicians and other healthcare providers, regarding the checkpoint inhibitor use and associated toxicities. In a recent review article, the authors have answered ten general questions that healthcare providers can come across while treating patients receiving checkpoint inhibitor therapy25. This review focuses on the toxicity of checkpoint inhibitors on the endocrine system and describes the individual endocrinopathies with their incidence (Table I), possible underlying mechanisms, signs and symptoms and proper clinical assessment and disease management (Table II). Multiple mechanisms underlying the development of endocrine adverse effects following ICI therapy have been described in the literature, such as type II/IV hypersensitivity reactions and/or development of autoantibodies, but exact pathogenesis of ICI drug toxicities has not been established32. The clinical manifestations of autoimmune endocrine complications associated with checkpoint inhibitor therapy may differ from those without immunotherapy. Patients with pre-existing autoimmune endocrine diseases such as autoimmune thyroid disease or diabetes mellitus (DM) can have an exacerbation of their disease after starting ICI therapy rather than de novo IrAEs. Management of most autoimmune endocrinopathies revolves around timely institution of hormone replacement therapy3032. In mild-to-moderate cases, ICIs are usually continued although, in severe cases, checkpoint inhibitor therapy should be temporarily discontinued and can be restarted after adequate hormonal therapy is in place. More research is required to identify the biological mechanisms of these toxicities and to recognize factors predicting endocrine toxicity.
| Endocrine gland | Disorders | Signs and symptoms | Labs/imaging | Treatment/follow up |
|---|---|---|---|---|
| Pituitary gland | Hypophysitis | Fatigue, nausea, dizziness, headache, loss of appetite, insomnia, labile mood, vision disturbances, nausea72829 | 8.00 AM ACTH, cortisol, TSH, T4, Testosterone, oestradiol, FSH, LH, prolactin30 MRI brain/pituitary to rule out brain metastasis729 |
Central adrenal insufficiency If associated with severe symptoms such as vision loss or severe headaches, use high-dose IV methylprednisolone 1-2 mg/kg/day for 3-4 days and then transition to physiological hydrocortisone about 20 mg PO in morning and 10 mg in late afternoon28303132 If less severe symptoms then start physiological dose of hydrocortisone303132 Central hypothyroidism Start levothyroxine 1.6 mcg/kg/day after ruling out adrenal insufficiency or 2-3 days after starting steroids (if there is adrenal insufficiency)303132 Central hypogonadism Best referred to endocrinologist as outpatient for management303132 |
| Thyroid gland | Thyroiditis/thyrotoxicosis | Tachycardia palpitation, hot intolerance, anxiety, tremors, hyperdefecation, increased perspiration3334 | TSH, T4, T3, Thyroid stimulating immunoglobulins or TBII, Radioiodine uptake scan (if needed)3536 | Thyroiditis/thyrotoxicosis Monitor thyroid function serially, Beta blocker for symptoms control303132 Steroids are not indicated37 |
| Hypothyroidism | Fatigue, bradycardia, cold intolerance, dry skin, constipation3338 | TSH, T4, Thyroid peroxidase and Thyroglobulin antibodies3536 | Hypothyroidism Usually a sequel of thyroiditis Treat with levothyroxine (if subclinical and no symptoms-observe)303132 |
|
| Adrenal gland | Primary adrenal insufficiency | Fatigue, nausea, vomiting, electrolyte changes, mental status changes394041 | Random cortisol levels, 8.00 AM cortisol and ACTH levels, cosyntropin stimulation test30 | If severe adrenal insufficiency, treat with high dose IV steroids for 2-3 days and then transition to more physiological PO steroid doses. If less severe, use physiological hydrocortisone or prednisone replacement to start with303132 Use fludrocortisone if electrolytes or blood pressure abnormalities persist. Use smallest dose to start with303132 |
| Pancreas | Diabetes mellitus, usually presenting such as type 1 diabetes mellitus | Elevated blood glucose, polyuria, polydipsia, dry mouth, dizziness, nausea, vomiting4243 | Basic metabolic panel, urinary and blood ketones, blood gas (if needed), urinalysis, hemoglobin A1c, c-peptide, | Hospitalization and IV fluids/insulin if DKA. If no DKA, start insulin therapy with basal–bolus regimen30314143 Endocrine follow up as outpatient |
| Rare DKA | Autoantibodies (GAD-65, Islet cell antibodies, insulin autoantibodies, ZNt8 antibodies)3034 |
TSH, thyroid stimulating hormone; TBII, TSH binding inhibitory immunoglobulin; ACTH, adrenocorticotropic hormone; FSH, follicle stimulating hormone; LH, luteinizing hormone; DKA, diabetic ketoacidosis; GAD, glutamic acid decarboxylase; ZNt8, zinc transporter 8; MRI, magnetic resonance imaging; T4, thyroxin; IV, intravenous; PO, per oral
Hypophysitis
There are substantial differences in the reported incidence of ICI-induced hypophysitis among various clinical trials and meta-analyses (Table I). The difference in the incidence rates is mainly attributable to the type of ICI used and the cancer type. Pituitary dysfunction is more commonly associated with anti-CTLA-4 mAbs than with other categories of ICI562628. In 2011, the US FDA licensed Ipi for the treatment of advanced melanoma45. The incidence of hypophysitis with hypopituitarism with Ipi has been reported to be 0-25 per cent among different trials and meta-analyses567891011. There is a lack of clarity on whether the incidence of hypophysitis from Ipi is dose related (3 vs. 10 mg/kg). One study showed that the cumulative Ipi dose or use of a higher dose (10 mg/kg) did not affect the rates of development of Ipi-induced hypophysitis7. However, a phase 3 trial comparing 10 mg/kg dose of Ipi to 3 mg/kg in patients with advanced melanoma showed higher rates of hypophysitis (6.6 vs. 3.3%) between the two doses11. The occurrence of Ipi-induced hypophysitis when used with other anticancer agents also remains ill defined. Hypophysitis was not diagnosed in most of the studies464748 using the combination of Ipi and chemotherapy, except one49. Similarly, it was not reported in a group of patients with advanced cutaneous melanoma pre-treated with radiotherapy for brain metastasis50. This was probably due to the depletion of immune cells by cytotoxic chemotherapy and radiotherapy.
The incidence of hypophysitis associated with nivolumab (a PD-1 inhibitor) is quite low and is reported to be about 0.5-0.9 per cent131415. Nivolumab has been evaluated in phase III trials and is approved for the treatment of advanced melanoma, NSCLC, head and neck squamous cell carcinoma and renal cell cancer4. The incidence of hypophysitis with pembrolizumab (another PD-1 inhibitor) as a single agent has also been reported to be <1 per cent in patients with melanoma and NSCLC in various studies1617181920. However, the incidence increases by combining ICIs with different mechanisms of action. In phase II/III trials, comparing Ipi and nivolumab combination therapy versus monotherapy, all grade hypophysitis occurred in 8-12 per cent in the combination arm, 4-7 per cent in Ipi arm and <1 per cent in nivolumab arm212223. Similarly, in one meta-analysis, the incidence of hypophysitis was found to be more in the combination arm; 3.8 per cent with Ipi, 1.1 per cent with PD-1 inhibitors but 8.0 per cent in the combination arm5. However, this study was restricted to include only subsets with melanoma.
Although the exact mechanism of underlying toxicity of these drugs has not been defined, autoimmunity is the most likely proposed mechanism. Iwama et al51 demonstrated the lymphocytic infiltration of the pituitary gland and also circulating pituitary antibodies by repeatedly injecting CTLA-4 blocking antibody into SJL/J or C57BL/6J mice. In the same study, they also found anti-pituitary autoantibodies in seven patients who developed hypophysitis after Ipi administration, although not in the other 13 patients without hypophysitis51. CTLA-4 expression was seen in the murine pituitary glands at both RNA and protein levels. When these mice were injected with CTLA-4 blocking antibody, complement deposition was seen in the pituitary gland, suggesting a type II hypersensitivity reaction51. In an autopsy series, high levels of CTLA-4 expression were seen on the pituitary cells of a patient who had clinical and pathologic evidence of hypophysitis after treatment with CTLA-4 blocking antibodies52. This study proposed type IV (T-lymphocyte dependent) and type II (immunoglobulin-mediated) immune mechanisms as a cause of hypophysitis after treatment with CTLA-4 inhibitors. The mechanism of hypophysitis with anti-PD-1/PD-L1 is unknown. Unlike CTLA-4, PD-1 is not expressed in the pituitary and anti-pituitary antibodies produced by anti-PD-1 are not known either.
Clinical presentation and management
Ipi-induced hypophysitis usually occurs around 8-16 wk (median time interval 11 wk) from the first treatment but can occur as early as four weeks or have delayed presentation as well285354. Some studies have shown increased male preponderance than females for ICI-induced hypophysitis7828, while other studies have shown similar occurrence between males and females. The male preponderance of ICI-induced hypophysitis needs to be further evaluated in long-term prospective studies. As in classic lymphocytic hypophysitis, patients with ICI-induced hypophysitis usually present with non-specific symptoms. Common presenting symptoms include fatigue, headache and less frequently nausea, vomiting, dizziness, vertigo, confusion, anorexia, insomnia, labile mood, temperature intolerance, loss of libido and erectile dysfunction. However, unlike classic lymphocytic hypophysitis, visual impairment such as diplopia is rare as optic structures are rarely involved28. Some patients can present with initial signs and symptoms of adrenal crisis as well, a life-threatening condition characterized by hypotension, shock, confusion and severe electrolyte abnormalities. Although pituitary enlargement on the magnetic resonance imaging (MRI) is variable and has been reported in 12-88 per cent of patients with Ipi-induced hypophysitis29, symptoms due to mass effect are rare. The pituitary enlargement may be mild to moderate (unlike classic lymphocytic hypophysitis), may be homogeneous or heterogeneous and associated with thickening of the stalk728.
Diagnosis is made when a patient presents with one or more of the above-mentioned symptoms with biochemical evidence of hypopituitarism and with/without pituitary enlargement on brain imaging. Other causes such as sepsis, brain metastasis and non-endocrine adverse effects due to ICIs should also be ruled out due to the overlapping symptoms in advanced cancer. Hypopituitarism secondary to ICI-induced hypophysitis can present with multiple anterior pituitary hormone deficiencies. Central hypothyroidism is the most common hormone deficiency followed by central adrenal insufficiency (AI) and hypogonadotropic hypogonadism92853. Unlike other forms of hypophysitis, ICI-induced hypophysitis does not seem to involve the posterior pituitary. Only a few cases of diabetes insipidus or syndrome of inappropriate antidiuretic hormone secretion have been described in the literature535556. When hypophysitis is suspected, it is necessary to measure pituitary as well as target tissue hormones for an appropriate and timely diagnosis. Laboratory evaluation with thyroid-stimulating hormone (TSH) and free T4 levels, early morning adrenocorticotropic hormone (ACTH), cortisol level and cosyntropin stimulation test (may be normal in early course), and gonadal hormones (testosterone in men, oestradiol in pre-menopausal women), follicle-stimulating hormone (FSH) and luteinizing hormone (LH) should be obtained to screen for hormonal deficiencies before starting the treatment30 (Table II). Prolactin and IGF-1 (insulin growth factor - 1) levels could be measured as well. MRI pituitary should be obtained, especially in those with headaches, visual changes, altered mental status or symptoms related to mass effect.
It is extremely crucial to start treatment of ICI-induced hypophysitis without any delay; as untreated, it can lead to an acute life-threatening adrenal crisis. Treatment of ICI-induced hypophysitis mainly entails early glucocorticoid treatment and hormone replacement (Table II). Glucocorticoids and thyroid hormone replacement should be started as soon as possible after diagnosis, while gonadal hormone replacement can be instituted later if hypogonadism persists303132. However, for patients presenting in adrenal crisis, therapy must be started immediately even before the availability of results of diagnostic tests. Consultation with an endocrinologist should be done in all cases of ICI-induced hypophysitis. With more clinical experience and recent studies, it has been proposed that physiologic (10-30 mg of hydrocortisone) or moderately supra-physiologic glucocorticoid doses can be used for the treatment of secondary AI from ICI-induced hypophysitis. Higher doses might be required in the presence of severe headache, significant hyponatraemia, life-threatening adrenal crisis or pituitary enlargement threatening the optic apparatus783132. Treatment with high doses of steroids did not seem to improve the likelihood of pituitary function recovery or affect overall survival8. If the patient has central hypothyroidism, levothyroxine replacement should also be started. However, if AI and hypothyroidism coexist, steroids should be started before initiating levothyroxine to prevent triggering an adrenal crisis303132.
Acute symptoms disappear within a few days of starting steroids and hormone replacement in most of the patients. Rapid pituitary shrinkage on the MRI corresponds to the efficacy of steroids, but pituitary enlargement decreases gradually over 4-12 wk, despite a more rapid reduction of the symptoms53. Recovery of pituitary function is variable, thyroid and gonadotropin axis recover more often than the adrenal axis. The thyrotropin function may recover in 37-50 per cent of patients, gonadotropic function in 57 per cent of men but recovery of the pituitary–adrenal axis is rare295758. Many patients remain on long-term glucocorticoid treatment because of persistent secondary AI. Patients treated with ICIs and those who develop hypophysitis need to be monitored for endocrine hormonal deficiencies while on and after stopping ICI therapy as delayed toxicities can occur. Routine monitoring with early morning ACTH and cortisol levels should be considered every month for six months, then every three months for six months and after that every six months for one year after treatment completion30.
Glucocorticoid administration used for the treatment of hypophysitis does not seem to impact survival in these patients negatively. In a study of 298 patients with metastatic melanoma treated with Ipi, 103 patients were treated with systemic steroids for IrAEs (endocrine and non-endocrine); treatment with steroids did not affect the overall survival in these patients59. In another study of Ipi for the treatment of melanoma, treatment with steroids did not appear to impair antitumour response60. Relationship of hypophysitis and cancer prognosis has been the topic of research, and improved overall survival with reduced mortality rates was recently shown in patients with hypophysitis due to ICIs61. This suggests its possible role as a marker for better efficacy of ICIs against malignancy. Development of hypophysitis does not imply discontinuation of ICI therapy; however, it should be temporarily discontinued for some cases of grade 3 toxicity and all cases of life-threatening grade 4 toxicity (Table III). Checkpoint inhibitor therapy can be restarted after adequate hormonal replacement therapy is in place.
| Adverse event | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|---|
| Hypophysitis | Asymptomatic or mild symptoms; clinical or diagnostic observations only; intervention not indicated | Moderate; minimal, local or non-invasive intervention indicated; limiting age-appropriate instrumental ADL | Severe or medically significant but not immediately life-threatening; hospitalization or prolongation of existing hospitalization indicated; disabling; limiting self-care ADL | Life-threatening consequences; urgent intervention indicated | Death |
| Thyroid Hypothyroidism Hyperthyroidism |
Asymptomatic; clinical or diagnostic observations only; intervention not indicated | Symptomatic; thyroid replacement/suppression indicated; limiting instrumental ADL | Severe symptoms; limiting self-care ADL; hospitalization indicated | Life-threatening consequences; urgent intervention indicated | Death |
| Adrenal insufficiency | Asymptomatic; clinical or diagnostic observations only; intervention not indicated | Moderate symptoms; medical intervention indicated | Severe symptoms; hospitalization indicated | Life-threatening consequences; urgent intervention indicated | Death |
Source: Table adapted with permission from U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute, Version 4.0 published on 28 May 2009. ADL, activities of daily living
Thyroid disorders
Thyroid disorders (hypo- and hyperthyroidism) are commonly reported with CTLA-4 inhibitors and PD-1/PD-L1 inhibitors (Table I). One meta-analysis found no difference in the incidence of hypothyroidism/hyperthyroidism between PD-1 inhibitors and CTLA-4 inhibitors27. However, another meta-analysis found statistically significant differences in the incidence of hypo- and hyperthyroidism between the two classes of ICIs5. With Ipi, the incidence of hypothyroidism (3.8%) was found to be more frequent than hyperthyroidism (1.7%). Similarly, with PD-1 inhibitors, the incidence of hypothyroidism (7.0%) is more common than hyperthyroidism (3.2%)5. Overall, thyroid dysfunction has been described to be more common in patients treated with PD-1/PD-L1 inhibitors than with CTLA-4 inhibitors56122626. Combination immunotherapy also increases the incidence of both hypothyroidism and hyperthyroidism compared to monotherapy521222326.
Patients with pre-existing autoimmune thyroid disease can have an exacerbation of their disease after starting ICI therapy rather than de novo IrAE. Typically, patients with pre-existing autoimmune disease have been excluded from the major clinical trials on ICIs. A systematic review was done by Abdel-Wahab et al62 on such reported cases of established diagnosis of autoimmune disease before receiving any dose of ICIs. As far as the endocrine system is concerned, 2/11 (17%) patients with pre-existing autoimmune thyroid disease were found to have worsening hypothyroidism after the start of therapy. These patients who had the exacerbation received either nivolumab or pembrolizumab and were managed by dose adjustment in hormonal therapy.
The mechanisms behind ICI-induced thyroid disorders remain unclear. Some studies suggest that genetic susceptibilities linked to HLA phenotypes or polymorphisms in CTLA-4/PD-1 genes with reduced function alter immune responses and, therefore, could be associated with the development of Graves’ disease and Hashimoto’s disease636465. The role of autoantibodies in the pathogenesis of ICI-induced thyroid dysfunctions remains questionable. One study showed the presence of antithyroid antibodies in the majority of patients who developed thyroid disease66, whereas another suggested an antibody-independent mechanism as anti-thyroid peroxidase (TPO) antibodies were not present in most of the patients with ICI-induced thyroid dysfunction67. However, there is increased inflammation within the thyroid gland due to destructive thyroiditis, likely autoimmune-mediated by cytotoxic T cells.
Clinical presentation and management
With Ipi, the time of onset of hypothyroidism varies from 2 to 4 infusions from the start of the therapy, but the timing of onset with PD-1/PD-L1 has not been well established. Thyroid disorders are more frequent among women as compared to men. ICI-induced thyroid dysfunction includes thyroiditis, hypothyroidism or hyperthyroidism3568. Many patients present as silent thyroiditis, transient thyroid dysfunction with no or minimal symptoms, which may, however, evolve into permanent hypothyroidism3369. Few cases of thyroid storm34 and severe myxoedema have also been published in the literature38. Graves’ ophthalmopathy has rarely been reported with normal thyroid function but elevated TSH-receptor antibodies6870.
Laboratory values consistent with elevated TSH and low level of free thyroxin (fT4) confirm overt hypothyroidism in the presence of classic clinical signs and symptoms36 (Table II). It is prudent to exclude secondary hypothyroidism from pituitary failure (due to hypophysitis), especially in the setting of low-to-normal TSH and low fT4. Thyrotoxicosis is diagnosed with laboratory values showing elevated serum levels of fT4, elevated total triiodothyronine (TT3) and low/non-existent levels of TSH along with classic signs and symptoms36 (Table II). Euthyroid sick syndrome, which is characterized by low TSH and low–normal fT4 and TT3, should also be ruled out in the setting of advanced malignancy. Hyperthyroidism from checkpoint inhibitor therapy could be secondary to two reasons; thyroiditis due to the destruction of the thyroid gland by autoantibodies which is generally temporary or due to the production of thyroid-stimulating antibodies causing Graves’ disease which is largely permanent35. A radioactive iodine uptake scan may be performed to distinguish between these two types of hyperthyroidism. However, it is to be noted that routine computed tomography scans performed in these patients with intravenous (IV) iodinated contrast might make this distinction challenging as iodine-based contrast can cause falsely low thyroid uptake. The presence of anti-thyroglobulin and anti-TPO antibodies may help in confirming the presence of autoimmune thyroiditis35. However, as mentioned above, the role of autoantibodies in ICI-induced thyroid dysfunction is not clear. Thyroid-stimulating immunoglobulin and/or TSH receptor antibody should be checked if the patient has signs and symptoms of Graves’ disease. Patients should be screened with a TSH and a free T4 before the start of immunotherapy and then every six weeks71.
Treatment depends on whether the patient develops hypo- or hyperthyroidism (Table II). For overt hypothyroidism, treatment with a usual weight-based dose of levothyroxine (0.8-1.6 μg/kg/day) is started, with a lower starting dose of 25-50 μg in the elderly and cardiac disease patients303132. ICIs are usually not discontinued as the symptoms are managed successfully with levothyroxine. After thyroid hormone replacement has been started, thyroid function tests should be repeated every 6-8 wk and then every 3-6 months once stable. The treatment of thyrotoxicosis depends on the underlying cause. In Graves’ disease, antithyroid drugs are used to block thyroid hormone synthesis. However, these have no role in the thyrotoxic phase of thyroiditis since it is caused due to the release of thyroid hormones from gland destruction and not increased thyroid hormone synthesis. Beta-blockers (propranolol or atenolol) can be used for symptomatic management of tachycardia, tremor and other symptoms of thyrotoxicosis303132. Thyroiditis can be transient or can evolve into permanent hypothyroidism; therefore, regular monitoring of thyroid function tests is needed, and if TSH is elevated, levothyroxine replacement should be initiated. The use of steroids is usually not recommended unless in rare cases of Graves’ ophthalmopathy that is unresponsive to the withdrawal of ICIs or in elderly patients with underlying cardiovascular disease who develop severe thyrotoxicosis3137. In a recent retrospective study of 186 patients with melanoma treated with checkpoint inhibitors, it was shown that thyroid IrAEs did not appear to be associated with change in survival nor did exposure to glucocorticoids72.
Primary adrenal insufficiency (PAI)
ICIs can also cause autoimmune adrenalitis leading to PAI and adrenal crisis. PAI has been reported, although rarely (0.8-2%) in clinical trials evaluating ICIs56. In a phase II trial of Ipi with/without nivolumab consisting of 142 advanced melanoma patients, PAI was observed in five per cent of patients receiving combination treatment and two per cent in the Ipi group624. However, no case of PAI was reported in a large phase III trial of advanced melanoma patients who received the same drugs as single agent or combination23. There are rare case reports describing PAI in patients on checkpoint inhibitor therapy3940. An autoimmune mechanism has been proposed to be the most likely pathogenesis of ICI-induced PAI. However, there are no studies that have evaluated the pathogenesis of ICI-induced adrenalitis. Adrenal gland destruction is likely driven by T cells. Gene polymorphisms in CTLA-4 have been shown to increase the genetic risk of autoimmune adrenalitis73.
Symptoms suggestive of PAI are nausea, weakness, anorexia and weight loss accompanied by hypotension, hyponatraemia, hyperkalaemia, fever and hypoglycaemia, resulting from lack of both glucocorticoids and mineralocorticoids41. When autoimmune adrenalitis is suspected, blood samples for serum cortisol, ACTH, aldosterone and renin must be obtained. A low early morning serum cortisol (<3 μg/dl) with high ACTH strongly suggests PAI. ACTH stimulation testing can be performed in indeterminate cases to confirm the diagnosis of PAI30. Serum electrolytes and glucose should also be checked. It is important to remember that PAI from autoimmune adrenalitis and central AI from hypophysitis can often coexist together. Treatment for PAI should include both glucocorticoid and mineralocorticoid replacement3132 (Table II). Oral hydrocortisone is most commonly used for glucocorticoid substitution aiming to mimic the physiological circadian rhythm. Patients should be counselled on sick day rules to increase glucocorticoid doses in acute stress situations. These patients should also wear medical bracelets for identification and should have emergency hydrocortisone injection kits.
Clinical symptoms of PAI can often be attributed to underlying malignancy or the treatment itself, often delaying the diagnosis and increasing the risk of an adrenal crisis. When it does happen, adrenal crisis is an emergency manifesting as hypotension, hypovolemic shock and electrolyte disturbances (hyponatraemia and hyperkalaemia) and other symptoms such as nausea, vomiting, confusion and coma41. When suspected, immediate hospitalization is necessary, requiring urgent aggressive IV fluids and IV glucocorticoids. It may be prudent to start the IV steroids immediately upon presentation even before obtaining laboratories for serum cortisol to reduce mortality and morbidity3132. In these situations, treatment with dexamethasone is preferred as it does not interfere with cortisol assays. Infection and sepsis should also be ruled out. Consultation with an endocrinologist is essential for acute management, differential diagnosis and long-term management of such patients after the resolution of acute crisis.
Diabetes mellitus (DM)
Autoimmune DM can occur with ICI therapy from autoimmune destruction of the pancreatic β cells, resulting in partial or absolute insulin deficiency. Similar to thyroid dysfunction, hyperglycaemia and development of DM are more common with PD-1/PD-L1 inhibitors with incidence ranging from 0 to 2 per cent56. A systematic review of published cases of autoimmune DM due to ICI therapy identified about 91 cases until 2018, with most patients treated with anti-PD-1 or anti-PD-L1 monotherapy42. Another meta-analysis of cases identified 71 cases from 2000 to 201874. The authors concluded that the majority of autoimmune diabetes develop within three months of starting checkpoint inhibitor therapy. Quite a few case reports of autoimmune DM and diabetic ketoacidosis (DKA) secondary to PD-1/PD-L1 inhibitor have also been described so far437576777879808182. HbA1c ranged from 6.8 to 9.8 per cent in these case reports. The pathogenesis behind the development of DM from ICI use is again considered to be autoimmune in nature. Pancreatic cells have been shown to express PD-L1, and the PD-1/PD-L1 interaction plays an essential role in protecting against autoimmunity83. Studies have shown that inhibiting the PD-1/PD-L1 interaction precipitates the onset of autoimmune diabetes in a non-obese diabetic mouse model848586. Therefore, PD-1/PD-L1 inhibitors by disinhibiting this pathway can cause expansion of the autoreactive T cells, leading to the destruction of pancreatic β cells and the development of T1DM.
The time of presentation of autoimmune diabetes from starting treatment has ranged from <1 month to as long as 12 months. DKA or severe abrupt hyperglycaemia (as high as >1000 mg/dl) is the most common initial presentation4243. Often patients present acutely with low or absent insulin and C-peptide levels, and because of the acuity of this disease, HbA1c levels might not be reflective of the blood glucose levels. ICI-induced diabetes mellitus is almost always permanent with insulin dependence persisting even after the acute hyperglycaemic phase. Many cases of DM due to checkpoint inhibitor therapy do not show the presence of diabetes-associated antibodies [anti-glutamic acid decarboxylase (GAD), anti-insulin, islet cell, or zinc transporter 8 antibodies]. An Australian case series identified 10 patients of checkpoint inhibitor-associated autoimmune DM; two had positive antibodies and 3/8 patients had high-risk HLA haplotypes87. A recent compilation of cases from various cohorts showed that islet autoantibodies were present variably (20-71%) in subjects who developed DM from ICI therapy44. Diabetes-associated autoantibodies and high-risk HLA (class II) typing need not be present for the diagnosis. However, some reported cases have shown the association of high-risk HLA genotypes for autoimmune diabetes after PD-1/PD-L1 inhibitor therapy757880. Although the data are not clear, it is suggestive that the onset of autoimmune diabetes mellitus with ICI therapy is more likely to happen in high-risk patients with certain HLA haplotypes. Predictive biomarkers have still not been identified, but HLA haplotypes could be one such biomarker to identify patients at risk for developing autoimmune diabetes from checkpoint therapy.
Autoimmune DM associated with checkpoint inhibitor therapy differs from the typical T1DM and latent autoimmune diabetes in adults (LADA). The age of onset for ICI-induced diabetes is later than T1DM and even LADA. It has an acute onset of hyperglycaemia with a rapid decline of C-peptide and sudden β cell failure after starting therapy, higher glycaemic variability and autoantibodies frequently testing negative4487. In patients with a diagnosis of LADA, which is diagnosed at a later age (after 40 yr of age), similar to ICI-induced DM, even though autoantibodies are almost always positive, the progression of β cell failure and insulin dependence is slow. This is in contrast to autoimmune DM from ICI use where acuity of onset is faster and β cell failure is rapid, making them insulin dependent4487.
Routine HbA1c and blood glucose should be tested in all patients before and during treatment with PD-1/PD-L1 inhibitors or when symptoms of diabetes develop30. As the use of ICIs continues to increase, it is necessary to diagnose and treat autoimmune diabetes promptly. It is easily treatable once identified but carries increased morbidity and mortality if not recognized and treated timely. Patients developing hyperglycaemia on immunotherapy should thus be referred to an endocrinologist for the timely institution of the treatment. Management of T1DM mainly includes insulin therapy in a basal–bolus regimen, education on insulin use, and recognition of hypo/hyperglycaemia303144 (Table II). Steroids are not indicated in this condition71. For patients presenting with DKA, appropriate management with insulin infusion, IV fluids and electrolyte monitoring should be undertaken.
In our clinical experience with about 40 patients who had checkpoint inhibitor toxicities, Ipi alone or in combination with nivolumab caused more hypophysitis than either nivolumab or pembrolizumab (unpublished data). Interestingly, hypophysitis was seen to be a late complication at 33-60 wk after starting pembrolizumab. More cases of thyroiditis were seen with pembrolizumab compared to other checkpoint inhibitors. Recently, we also observed ICI-induced rapid-onset diabetes with anti PD-1 and PDL-1 inhibitor therapy in our clinical practice with one geriatric patient developing diabetes after only one cycle of pembrolizumab (anti-PD-1) with high anti-GAD antibodies.
Conclusions
ICIs are relatively newer drugs for the treatment of certain solid tumours such as melanoma and NSCLC and will likely be used in the future for additional cancer types. IrAEs, as a result of autoimmunity, are common side effects and especially concerning is the involvement of the endocrine system. More common are the involvement of the pituitary and the thyroid gland than primary adrenal insufficiency or diabetes mellitus. Hypophysitis is more characteristic of CTLA-4 inhibitors, whereas thyroid disorders are more commonly seen with PD-1 inhibitors. Most of the time, these are relatively mild, and despite the irreversibility of most endocrinopathies, the timely institution of hormone replacement therapy allows the continuation of checkpoint inhibitor therapy. However, some of the conditions, particularly the involvement of the pituitary–adrenal axis, could be life-threatening if not recognized. Therefore, general internists, endocrinologists and oncologists need to be vigilant and maintain a high degree of awareness for the side effects of checkpoint therapy on the endocrine system.
Acknowledgment:
Authors acknowledge Mr Zahid Sayeed in helping with the manuscript submission process.
Financial support & sponsorship: None.
Conflicts of Interest: Dr Rajeev Sharma is one of the authors in the Society for Immunotherapy of Cancer consensus statement published in November 2017 (Ref. 30 in this article).
References
- Immune-related adverse events during anticancer immunotherapy:Pathogenesis and management. Oncol Lett. 2017;14:5671-80.
- [Google Scholar]
- CTLA-4 overexpression inhibits T cell responses through a CD28-B7-dependent mechanism. J Immunol. 2006;177:1052-61.
- [Google Scholar]
- Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion:Implications for tumor immunotherapy. Cancer Immunol Immunother. 2005;54:307-14.
- [Google Scholar]
- Current status and future directions of the immune checkpoint inhibitors ipilimumab, pembrolizumab, and nivolumab in oncology. Ann Pharmacother. 2015;49:907-37.
- [Google Scholar]
- Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens:A systematic review and meta-analysis. JAMA Oncol. 2018;4:173-82.
- [Google Scholar]
- A systematic review and meta-analysis of endocrine-related adverse events associated with immune checkpoint inhibitors. Horm Metab Res. 2019;51:145-56.
- [Google Scholar]
- Ipilimumab-induced hypophysitis:A detailed longitudinal analysis in a large cohort of patients with metastatic melanoma. J Clin Endocrinol Metab. 2014;99:4078-85.
- [Google Scholar]
- Systemic high-dose corticosteroid treatment does not improve the outcome of ipilimumab-related hypophysitis:A retrospective cohort study. Clin Cancer Res. 2015;21:749-55.
- [Google Scholar]
- Long-term follow-up of ipilimumab-induced hypophysitis, a common adverse event of the anti-CTLA-4 antibody in melanoma. Eur J Endocrinol. 2015;172:195-204.
- [Google Scholar]
- Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma:A multicenter single-arm phase II study. Ann Oncol. 2010;21:1712-7.
- [Google Scholar]
- Ipilimumab 10 mg/kg versus ipilimumab 3 mg/kg in patients with unresectable or metastatic melanoma:A randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2017;18:611-22.
- [Google Scholar]
- Endocrine toxicity in cancer patients treated with nivolumab or pembrolizumab:Results of a large multicentre study. J Endocrinol Invest. 2020;43:337-45.
- [Google Scholar]
- Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443-54.
- [Google Scholar]
- Nivolumab for metastatic renal cell carcinoma:Results of a randomized phase II trial. J Clin Oncol. 2015;33:1430-7.
- [Google Scholar]
- Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063):A phase 2, single-arm trial. Lancet Oncol. 2015;16:257-65.
- [Google Scholar]
- Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521-32.
- [Google Scholar]
- Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma:A randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109-17.
- [Google Scholar]
- Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002):A randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908-18.
- [Google Scholar]
- Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018-28.
- [Google Scholar]
- Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010):A randomised controlled trial. Lancet. 2016;387:1540-50.
- [Google Scholar]
- Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006-17.
- [Google Scholar]
- Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23-34.
- [Google Scholar]
- Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma:2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016;17:1558-68.
- [Google Scholar]
- Immune-Related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:158-68.
- [Google Scholar]
- Mapping endocrine toxicity spectrum of immune checkpoint inhibitors:A disproportionality analysis using the WHO adverse drug reaction database, VigiBase. Endocrine. 2020;69:670-81.
- [Google Scholar]
- Risk of endocrine complications in cancer patients treated with immune check point inhibitors:A meta-analysis. Future Oncol. 2016;12:413-25.
- [Google Scholar]
- Immunotherapy and hypophysitis:Clinical presentation, treatment, and biologic insights. Pituitary. 2016;19:82-92.
- [Google Scholar]
- Immune checkpoint inhibitor therapy associated hypophysitis. Clin Med Insights Endocrinol Diabetes. 2015;8:21-8.
- [Google Scholar]
- Managing toxicities associated with immune checkpoint inhibitors:Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) toxicity management working group. J Immunother Cancer. 2017;5:95.
- [Google Scholar]
- Endocrine-related adverse events related to immune checkpoint inhibitors:Proposed algorithms for management. Oncologist. 2020;25:290-300.
- [Google Scholar]
- Endocrine toxicities of immune checkpoint inhibitors. Nat Rev Endocrinol. 2021;17:389-99.
- [Google Scholar]
- Thyroid dysfunction as an unintended side effect of anticancer drugs. Thyroid. 2013;23:1345-66.
- [Google Scholar]
- Characterization of thyroid disorders in patients receiving immune checkpoint inhibition therapy. Cancer Immunol Res. 2017;5:1133-40.
- [Google Scholar]
- Ipilimumab-induced endocrinopathies:When to start corticosteroids (or not) Cancer Chemother Pharmacol. 2013;72:489-90.
- [Google Scholar]
- Immune checkpoint blockade anti-PD-L1 as a trigger for autoimmune polyendocrine syndrome. J Endocr Soc. 2019;3:496-503.
- [Google Scholar]
- Primary adrenal insufficiency from immune checkpoint inhibitors. AACE Clin Case Rep. 2018;4:e232-4.
- [Google Scholar]
- Diagnosis and treatment of primary adrenal insufficiency:An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016;101:364-89.
- [Google Scholar]
- Immune checkpoint inhibitors and type 1 diabetes mellitus:A case report and systematic review. Eur J Endocrinol. 2019;181:363-74.
- [Google Scholar]
- Immune checkpoint inhibitor-induced diabetic ketoacidosis:A report of four cases and literature review. Front Endocrinol (Lausanne). 2020;11:14.
- [Google Scholar]
- Immune checkpoint inhibitor diabetes mellitus:A novel form of autoimmune diabetes. Clin Exp Immunol. 2020;200:131-40.
- [Google Scholar]
- 2011. Available from:https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/125377s073lbl.pdf
- Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517-26.
- [Google Scholar]
- Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer:Results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol. 2013;24:75-83.
- [Google Scholar]
- Ipilimumab and fotemustine in patients with advanced melanoma (NIBIT-M1):An open-label, single-arm phase 2 trial. Lancet Oncol. 2012;13:879-86.
- [Google Scholar]
- Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer:Results from a randomized, double-blind, multicenter phase II study. J Clin Oncol. 2012;30:2046-54.
- [Google Scholar]
- Ipilimumab in patients with melanoma and brain metastases:An open-label, phase 2 trial. Lancet Oncol. 2012;13:459-65.
- [Google Scholar]
- Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci Transl Med. 2014;6:230ra45.
- [Google Scholar]
- Hypophysitis secondary to cytotoxic T-lymphocyte-associated protein 4 blockade:Insights into pathogenesis from an autopsy series. Am J Pathol. 2016;186:3225-35.
- [Google Scholar]
- Anti-CTLA-4 antibody therapy associated autoimmune hypophysitis:Serious immune related adverse events across a spectrum of cancer subtypes. Pituitary. 2010;13:29-38.
- [Google Scholar]
- Ipilimumab:A novel immunomodulating therapy causing autoimmune hypophysitis:A case report and review. Eur J Endocrinol. 2012;167:1-5.
- [Google Scholar]
- Hyponatremia associated with ipilimumab-induced hypophysitis. Med Oncol. 2012;29:374-7.
- [Google Scholar]
- Ipilimumab-induced hypophysitis in melanoma patients:An Australian case series. Intern Med J. 2015;45:1066-73.
- [Google Scholar]
- Pituitary dysfunction:A case series of immune checkpoint inhibitor-related hypophysitis in an emergency department. Ann Emerg Med. 2016;68:249-50.
- [Google Scholar]
- Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at memorial Sloan Kettering cancer center. J Clin Oncol. 2015;33:3193-8.
- [Google Scholar]
- Ipilimumab:Unleashing the power of the immune system through CTLA-4 blockade. Semin Oncol. 2010;37:440-9.
- [Google Scholar]
- OR32-02 immune checkpoint inhibitor-induced hypophysitis is associated with improved overall survival in cancer patients. J Endocr Soc. 2020;4(Suppl 1):OR32-02.
- [Google Scholar]
- Use of immune checkpoint inhibitors in the treatment of patients with cancer and preexisting autoimmune disease:A systematic review. Ann Intern Med. 2018;168:121-30.
- [Google Scholar]
- Pembrolizumab-induced thyroiditis:Comprehensive clinical review and insights into underlying involved mechanisms. J Clin Endocrinol Metab. 2017;102:2770-80.
- [Google Scholar]
- Association of cytotoxic T-lymphocyte-associated protein 4 (CTLA4) gene polymorphisms with autoimmune thyroid disease in children and adults:Case-control study. PLoS One. 2016;11:e0154394.
- [Google Scholar]
- Genetic susceptibility to autoimmune thyroid disease:Past, present, and future. Thyroid. 2010;20:715-25.
- [Google Scholar]
- Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol. 2017;28:583-9.
- [Google Scholar]
- Incidence of thyroid-related adverse events in melanoma patients treated with pembrolizumab. J Clin Endocrinol Metab. 2016;101:4431-9.
- [Google Scholar]
- Thyroid autoimmunity and ophthalmopathy related to melanoma biological therapy. Eur J Endocrinol. 2011;164:303-7.
- [Google Scholar]
- Expert opinion on thyroid complications in immunotherapy. Ann Endocrinol (Paris). 2018;79:555-61.
- [Google Scholar]
- Drug-induced Graves disease from CTLA-4 receptor suppression. Ophthalmic Plast Reconstr Surg. 2011;27:e87-8.
- [Google Scholar]
- Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy:American society of clinical oncology clinical practice guideline summary. J Oncol Pract. 2018;14:247-9.
- [Google Scholar]
- Thyroid dysfunction, recovery, and prognosis in melanoma patients treated with immune checkpoint inhibitors:A retrospective review. Endocr Pract. 2020;26:36-42.
- [Google Scholar]
- From genetic predisposition to molecular mechanisms of autoimmune primary adrenal insufficiency. Front Horm Res. 2016;46:115-32.
- [Google Scholar]
- Immune checkpoint inhibitor-induced type 1 diabetes:A systematic review and meta-analysis. Diabet Med. 2019;36:1075-81.
- [Google Scholar]
- Anti-programmed cell death-1 therapy and insulin-dependent diabetes:A case report. Cancer Immunol Immunother. 2015;64:765-7.
- [Google Scholar]
- Anti-PD-1 and anti-PDL-1 monoclonal antibodies causing type 1 diabetes. Diabetes Care. 2015;38:e137-8.
- [Google Scholar]
- Anti-PD1 pembrolizumab can induce exceptional fulminant type 1 diabetes. Diabetes Care. 2015;38:e182-3.
- [Google Scholar]
- Fulminant type 1 diabetes mellitus with anti-programmed cell death-1 therapy. J Diabetes Investig. 2016;7:915-8.
- [Google Scholar]
- Nivolumab, an anti-programmed cell death-1 antibody, induces fulminant type 1 diabetes. Tohoku J Exp Med. 2016;239:155-8.
- [Google Scholar]
- Association of serum anti-GAD antibody and HLA haplotypes with type 1 diabetes mellitus triggered by nivolumab in patients with non-small cell lung cancer. J Thorac Oncol. 2017;12:e41-3.
- [Google Scholar]
- Fulminant type I diabetes mellitus associated with nivolumab in a patient with relapsed classical hodgkin lymphoma. Int J Hematol. 2017;105:383-6.
- [Google Scholar]
- A case of pembrolizumab-induced type-1 diabetes mellitus and discussion of immune checkpoint inhibitor-induced type 1 diabetes. Cancer Immunol Immunother. 2017;66:25-32.
- [Google Scholar]
- Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203:883-95.
- [Google Scholar]
- The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med. 2003;198:63-9.
- [Google Scholar]
- Establishment of NOD-Pdcd1-/- mice as an efficient animal model of type I diabetes. Proc Natl Acad Sci USA. 2005;102:11823-8.
- [Google Scholar]
- Blockade of the programmed death-1 (PD1) pathway undermines potent genetic protection from type 1 diabetes. PLoS One. 2014;9:e89561.
- [Google Scholar]
- Checkpoint inhibitor-associated autoimmune diabetes is distinct from type 1 diabetes. J Clin Endocrinol Metab. 2019;104:5499-506.
- [Google Scholar]






