Translate this page into:
The impact of 30 ml/kg hydroxyethyl starch 130/0.4 vs hydroxyethyl starch 130/0.42 on coagulation in patients undergoing abdominal surgery
Reprint requests: Dr. C. Staikou, Department of Anesthesiology, Aretaieio Hospital, Medical School, University of Athens, 76 Vassilissis. Sophias Ave., 11528, Athens, Greece e-mail: c_staikou@yahoo.gr
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Hydroxyethyl starches (HES) 130/0.4 (Voluven®) and 130/0.42 (Venofundin®) impair coagulation less than older HES solutions with higher molecular weight and molar substitution. Thus, these may be used in high doses up to 50 ml/kg/day. The aim of this study was to investigate and compare the effects of HES 130/0.4 versus HES 130/0.42 on coagulation after the intraoperative infusion of 30 ml/kg in patients undergoing major abdominal surgery.
Methods:
Fifty two patients scheduled for elective major abdominal surgery were randomized to receive 30 ml/kg of HES 130/0.4 or HES 130/0.42 intraoperatively. Coagulation variables were assessed before and after infusion of the colloid solution using thrombelastography.
Results:
Data from 49 patients, 25 patients in the HES 130/0.4 and 24 in the HES 130/0.42 group, were analyzed. Measurements of reaction time, kinetic time, α-angle, maximum amplitude and coagulation index before and after colloid infusion did not differ between the groups. Within each group, after colloid infusion, reaction time did not change significantly, while α-angle, maximum amplitude and coagulation index values were significantly decreased (P<0.01, P<0.001 and P<0.001, respectively in HES 130/0.4 group and P<0.01, P<0.001 and P<0.01, respectively in HES 130/0.42 group). Kinetic time was significantly increased (P<0.001) in both the groups. In both groups, all thrombelastographic measurements after colloid infusion were found within normal limits.
Interpretation & conclusions:
HES 130/0.4 and HES 130/0.42 showed similar, not clinically significant effects on coagulation, as assessed by thrombelastography, when a dose of 30 ml/kg was administered in patients undergoing major abdominal surgery.
Keywords
Abdominal surgery
coagulation
colloids
hydroxyethyl starch
thombelastography
Colloids are effective plasma expanders, often used for intravascular volume replacement in patients undergoing major surgery. It is considered that the new hydroxyethyl starches (HES) have no adverse effects on renal function, reticular endothelial function or inflammatory response1, while these improve tissue oxygenation2.
HES preparations may differ in mean molecular weight (MW), degree of molar substitution (hydroxyethyl group number per mole glycose subunit), C2/C6 ratio (patern of hydroxyethylation at C2 and C6 carbon positions) or solvent (saline based or balanced)13. Molar substitution and C2/C6 ratio determine mainly the rate of HES degradation by serum α-amylase, while MW is considered less important14. HES preparations with MW more than 400 kDa, molar substitution more than 0.5 and C2/C6 ratio higher than 8 are degraded slowly and are associated with impaired coagulation15. Accumulation of the HES in plasma produces platelet dysfunction and decrease in factor VIII and von Willebrand factor (vWF)4. Other factors like calcium concentration in the solvent and HES derivation may also affect the coagulation profile of the colloid67. Corn derived HES impairs in vitro blood coagulation less than potato starch7.
It has been shown that new, rapidly degradable HES solutions with MW less than 200 kDa, molar substitution less than 0.5 and C2/C6 ratio lower than 8 do not affect significantly the coagulation mechanism18. Voluven® and Venofundin® are two new generation HES solutions with different origin and physico-chemical properties. Voluven® (Fresenius Kabi, Bad Homburg, Germany) is a 6 per cent HES solution based on waxy maize amylopectin, with MW 130 kDa, molar substitution 0.4 and C2/C6 ratio 9:1. Venofundin® (B.Braun, Melsungen AG, Germany) is a 6 per cent potato derived starch with MW 130 kDa, molar substitution 0.42 and C2/C6 ratio 6:1. Both are dissolved in saline solution (Na+ 154 mmol/l and Cl- 154 mmol/l) with a maximum daily dose of 50 ml/kg according to their manufacturers.
Our hypothesis was that the two colloid solutions, Voluven® or Venofundin®, have no clinical impact on coagulation of patients undergoing major abdominal surgery when administered intraoperatively in doses as high as 30 ml/kg. The coagulation profile of the two starches was compared using thrombelastography (TEG®).
Material & Methods
The study was approved by the Aretaieio Hospital Institutional Review Board and was conducted at Aretaieio Hospital, Department of Anesthesiology, Athens, Greece from April 2007 to October 2008. During this period, consecutive patients who were admitted to the surgery ward and underwent preanesthetic evaluation for elective major abdominal surgery were assessed for eligibility to be included in the study. Fifty two patients scheduled for gastrectomy, pancreatic surgery or colorectal surgery gave written informed consent to participate in this prospective randomized double blinded study. Exclusion criteria were physical status American Society of Anesthesiologists (ASA)>III, haemoglobin <10 g/dl, cardiac failure, myocardial infarction in the last 6 months, unstable angina, renal or hepatic dysfunction, coagulation disorders or oral anticoagulant medication, non-steroidal anti-inflammatory drugs (NSAIDs) within 24 h preoperatively, acetylsalicylic acid with in 5 days preoperatively, need for intraoperative administration of heparin, diabetes insipidus, chronic intake of corticosteroids, body weight more than 100 kg, pregnancy or lactation and allergy to starch. Low molecular weight heparin as standard prophylaxis for deep venous thrombosis was given the evening before the operation (12 or more hours preoperatively). Patients were randomly assigned to the Voluven® (n=26) or the Venofundin® (n=26) group by the use of sealed envelopes describing the group of assignment.
After positioning on the operating table, standard monitoring was applied to all patients (ECG, heart rate, pulse oximeter, blood pressure measurement (Datex-Ohmeda S/5™ Anaesthesia Monitor, Helsinki, Finland). The infusion of a Ringer's lactate (RL) solution was started at a rate 10 ml/kg/h, with further adjustments according to patient's needs. All patients received intravenously 10 mg of metoclopramide, 50 mg of ranitidine, 1-2 mg of midazolam and 50-100 μg of fentanyl. After establishing arterial blood pressure measurement via radial artery and central venous pressure (CVP) monitoring via the right internal jugular vein, the first blood sample was withdrawn and analyzed in the thombelastograph (TEG® 5000 Thrombelastograph® Hemostasis System, Haemoscope Corporation, Niles). An infusion of Voluven® or Venofundin® was then started at a basic rate of 500 ml/h, which was continuously adjusted so as the CVP to be maintained between 8 and 12 mmHg. The infusion of 30 ml/kg colloid solution was accomplished intraoperatively or shortly postoperatively.
Anaesthesia was induced with thiopental 4-5 mg/kg. Cis-atracurium 0.15 mg/kg was given to facilitate tracheal intubation. Sevoflurane 1-2 per cent end-tidal concentration in a nitrous oxide-oxygen mixture (FiO2: 0.4) along with incremental doses of cis-atracurium, fentanyl and morphine were administered to maintain anaesthesia. Mechanical ventilation was adjusted to maintain normocarbia (ETCO2 between 35-40 mm Hg). A urinary catheter and an oesophageal thermistor were inserted for urine output and core temperature monitoring, respectively. Air warming blankets and fluid warmers were used to prevent hypothermia. When the administration of 30 ml/kg HES was accomplished, a second blood sample was obtained and analyzed in the thrombelastograph. All blood samples were collected from the right internal jugular vein catheter after discarding the dead space.
Immediately after sampling, 1 ml of native whole blood was added to a kaolin vial (TEG® Hemostasis Analyzer Kaolin, Haemoscpe Corporation, Niles IL, USA) and mixed according to the manufacturer's guidelines. Subsequently, 360 μl of the blood/kaolin mixture were pipetted into disposable plastic cups (Disposable Cups and Pins, TEG® Hemostasis Analyzer) placed into the TEG® cupwells. All measurements were performed at 37°C by an investigator blinded to the colloid used. Reaction time (R, normal range 4-8 min), kinetic time (K, normal range 0-4 min ), α-angle (α, normal range 47-74°), maximum amplitude (MA, normal range 54-72 mm) and coagulation index (CI, normal range -3 to +3) were recorded.
Colloid and crystalloid infusion rates were adjusted in order to maintain normovolemia and haemodynamic stability. In case of reduction of urine output below 0.5 ml/kg/h or systolic blood pressure below 90 mmHg despite adequate intravascular volume replacement as guided by CVP, dopamine was added at 3-10 μg/kg/min. In case of severe haemodynamic instability phenylephrine was administered, dose titrated according to needs. The patients were transfused with red blood cells (RBC), if Hb <8 g/dl, with 1 unit of fresh frozen plasma (FFP) for 4 units of RBC or if diffuse oozing appeared in the surgical field, and with platelets (PLT) if their count was below 80000/μl. The duration of colloid infusion, the volume of RL administered, the volume of transfused blood products and any perioperative complications were recorded.
Statistical analysis: Sample size was determined based on power analysis of previously published studies about HES effects on TEG coagulation parameters910.
According to Kolmogorov-Smirnov test normality was maintained for age, duration of colloid infusion, volume of crystalloids, α, MA and CI values before the infusion, and also α and MA values after the infusion. Values for body weight, height, R and K before HES infusion and R, K and CI values when the predetermined HES volume was given did not follow normal distribution. For inter-group comparisons, t test was used to compare age, duration of colloid infusion, volume of crystalloids given, α, MA and CI values before and α and MA values after HES infusion. Mann-Whitney test was used for R and K values before and R, K and CI after HES infusion. Chi square was used to compare RBC and FPP transfused in each group.
Within the Voluven® group, comparisons between R, K, α, MA and CI values before and after the infusion were analyzed using the paired t test. In the Venofundin® group, Wilcoxon signed rank test was used for comparisons between R, K and CI values before and after infusion. The α and MA values before and after Venofundin® infusion followed normal distribution and were compared with the paired t test.
Results
Data obtained from 49 patients were analyzed. Complete data from three patients (one from Voluven® and two from Venofundin® group) were not obtained. Demographics, duration of colloid infusion, crystalloid volumes infused and units of RBC or FFP transfused did not differ between the two groups (Table I). No patient was transfused with platelets (PLTs). Patients remained haemodynamically stable during the protocol, so there was no need for use of inotropes or vasoactive drugs.
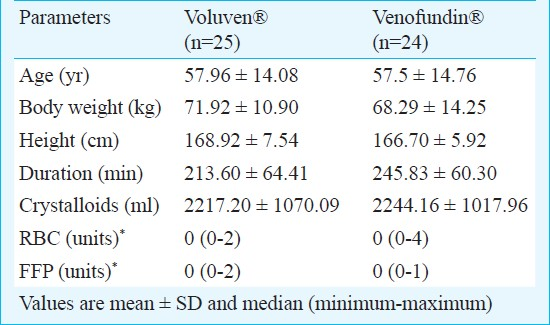
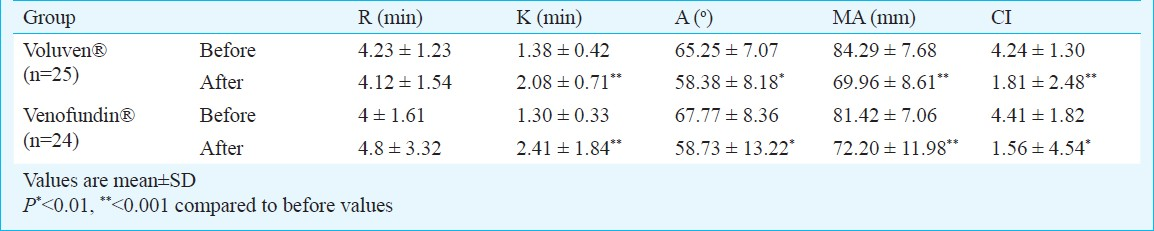
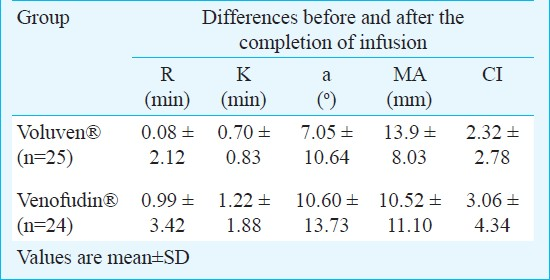
The R, K, α, MA and CI thrombelastographic values before colloid infusions did not differ between the two groups. The initial values of MA and CI were above the upper normal limit in both groups. In both groups colloid infusion was associated with a significant change in K, α, MA and CI values, while R was not significantly impaired (Table II). Despite the significant intra-group changes produced by the HES infusions, all the TEG® measurements obtained after the infusions were within normal range. Inter-group comparisons of the post-pre change of R, K, α, MA and CI values showed no significant differences between the two groups (Table III). None of the patients presented severe perioperative complications, anaphylactic reactions or adverse effects related to the colloids. Two patients in the Voluven® and one in the Venofundin® group had moderate to mild postoperative bleeding, which ceased spontaneously with no further surgical or conservative intervention.
Discussion
Our results demonstrated that intraoperative infusion of 30 ml/kg of Voluven® or Venofundin® in patients undergoing major abdominal procedures produced similar, clinically not significant changes in coagulation. Thrombelastography, a method that may be quite useful in the perioperative setting and advantageous over conventional coagulation laboratory tests was used11.
TEG® variables which represent a measure of clot formation kinetics were assessed. In fact, R indicates onset of coagulation, thus the time for initial fibrin formation, K represents the kinetics of clot development, α reflects the speed of clot strengthening, mostly affected by fibrinogen levels and less by platelet function and MA represents the maximum clot strength, mainly affected by platelet function/aggregation and to a lesser extent by fibrin. CI describes the patient's overall coagulation derived from the R, K and α. Addition of the reagent kaolin (hydrated aluminium silicate) to the in vitro sample was used to activate blood tracings and improve speed analysis.
The two colloids Voluven® and Venofundin® have similar MW, elimination half-lives and colloid osmotic effect12. The two colloids have different molar substitution, C2/C6 ratio, mean degree of branching (higher for Voluven®) and degree of esterification with phosphate groups (higher for Venofundin®)13. Compared to older HES solutions, Voluven® and Venofundin® are not characterized by a decreased volume expanding effect, since this is mainly affected by the number and not the size of molecules14. A significant characteristic is the rapid elimination as indicated by a less pronounced increase of α-amylase814. Rapid elimination accounts for the improved coagulation profile of these new starches8. Venofundin® is cleared from circulation even faster than Voluven®12.
The role of MW and molar substitution in the coagulation profile of HES solutions has attracted the interest of many investigators81516. Niemi et al15 found that medium-MW (200 kDa) HES with a high molar substitution (0.5) induces hypocoagulability when administered postoperatively in cardiac surgery patients, possibly by affecting coagulation factors rather than platelets. In this study, thrombelastographic parameters after HES infusion were outside normal range indicating a significantly slowed clot development15. Our results differ from these findings as we did not observe changes in the thrombelastographic parameters outside the normal range. Other studies also have shown that Voluven® has no significant effects on coagulation mechanism because it does not accumulate in plasma or tissues81718. Boldt et al16 found that Venofundin® even if combined with NaCl 0.9 per cent produced non significant prolongation of coagulation and clot formation time, while the maximum clot firmness was slightly decreased. It has also been found that the administration of Voluven® in doses 24-31 ml/kg does not affect partial thromboplastin time and reduces blood loss and homologous RBC transfusions compared to HES 200/0.5814. Kasper et al19 showed no increase in blood loss even with high doses (up to 50 ml/kg) of Voluven®. Voluven® has been shown to affect less than HES 200/0.5 the post-operative increase of factor VIII, which normally occurs as part of a post-operative physiological response814. In contrast to slowly degradable HES solutions, which even in doses below 25-50 ml/kg decrease factor VIII and vWF plasma levels by up to 80 per cent1, rapidly eliminated starches do not suppress the acute phase increases in factor VIII, vWF and ristocetin cofactor, even in doses as high as 70 ml/kg/day20.
In the present study large volumes of Voluven® or Venofundin® were infused in a relatively short time and it is possible that the tendency towards reduced coagulability found, may be in part attributed to haemodilution of clot proteins and platelets. In vitro studies have shown that infusion of large volumes of colloids results in dilutional coagulopathy with prolongation of coagulation and clot formation time21. HES physico-chemical characteristics and the electro-lyte composition of the solvent may also contribute to the coagulation profile of HES preparations616. The volume and type of crystalloid infused could also play a role in the intragroup coagulation changes seen. In our study the rate of colloid and crystalloid infusions was guided by the CVP and arterial pressure monitoring to avoid hypo-or hypervolemia and their possible impact on coagulation. Since RL has been shown to produce a mild hypercoagulability21, it might have attenuated the tendency towards hypocoagulability produced by the colloid infusions. Fries et al21 have demonstrated that the coagulation mechanism is significantly less affected when RL is infused along with colloids than with colloids alone.
It should be noted that while all measurements after colloid infusion were within normal range, the values of MA and CI before infusion were above the normal range, indicating a hypercoagulable state. However, the majority of the patients participated in the study suffered from malignant tumours and the coagulability of these patients as determined by thromboelastography is accelerated22.
One of the limitations of our study was the omission to measure International Normalized Ratio (INR), Activated Partial Thromboplastin Time (APTT) and factor levels along with TEG® measurements. Another point that should be noted is that the dose of colloids used (30 ml/kg) was not the maximum daily dose. The aim of the study was to investigate the coagulation effect of a large dose administered within a short time, thus in less than 4 h. Also, a direct comparison of the coagulation safety profile between these two HES preparations, with different origin and physicochemical properties, has not been reported in patients undergoing major abdominal surgery.
In conclusion, both HES 130/0.4 and HES 130/0.42 in the dose and for the duration of administration used in the present study were not associated with hypocoagulation or other side effects. Despite the differences regarding origin and physico-chemical properties, both produced similar effects on coagulation. Based on the thrombelastographic findings only, HES 130/0.4 and HES 130/0.42 were equally safe when administered in doses up to 30 ml/kg during major abdominal operations. More studies are required utilizing the entire range of tests on platelet functions and coagulation profiles to confirm our findings.
Conflict of interest : None.
References
- Colloids versus crystalloids and tissue oxygen tension in patients undergoing major abdominal surgery. Anesth Analg. 2001;93:405-9.
- [Google Scholar]
- Effects of hydroxyethyl starch solutions on hemostasis. Anesthesiology. 2005;103:654-60.
- [Google Scholar]
- Coagulation disorders caused by hydroxyethylstarch. Thromb Haemost. 1997;78:974-83.
- [Google Scholar]
- An international view of hydroxyethyl starches. Intensive Care Med. 1999;25:258-68.
- [Google Scholar]
- The effects of high molecular weight hydroxyethyl starch solutions on platelets. Anesth Analg. 2004;99:665-8.
- [Google Scholar]
- The effect of potato starch derived and corn starch derived hydroxyethyl starch on in vitro blood coagulation. Anaesthesia. 1998;53:638-44.
- [Google Scholar]
- Voluven, a lower substituted novel hydroxyethyl starch (HES 130/0.4), causes fewer effects on coagulation in major orthopedic surgery than HES 200/0.5. Anesth Analg. 2001;92:855-62.
- [Google Scholar]
- Molar substitution and C2/C6 ratio of hydroxyethyl starch: influence on blood coagulation. Br J Anaesth. 2006;96:455-63.
- [Google Scholar]
- Molecular weight does not determine the effect of hydroxyethyl starch on in vitro blood coagulation. Anesthesiology. 2004;101:A273.
- [Google Scholar]
- Coagulation monitoring: current techniques and clinical use of viscoelastic point-of-care coagulation devices. Anesth Analg. 2008;106:1366-75.
- [Google Scholar]
- Bioequivalence comparison between hydroxyethyl starch 130/0.42/6 : 1 and hydroxyethyl starch 130/0.4/9 : 1. Drugs R D. 2007;8:229-40.
- [Google Scholar]
- Differences in chemical structures between waxy maize- and potato starch-based hydroxyethyl starch volume therapeutics. Transfus Altern Transfus Med. 2007;l9:127-33.
- [Google Scholar]
- A novel hydroxyethyl starch (Voluven®) for effective perioperative plasma volume substitution in cardiac surgery. Can J Anesth. 2000;47:1207-15.
- [Google Scholar]
- Gelatin and hydroxyethyl starch but not albumin, impair hemostasis after cardiac surgery. Anesth Analg. 2006;102:998-1006.
- [Google Scholar]
- A total balanced volume replacement strategy using a new balanced hydroxyethyl starch preperation (6% HES 130/0.42) in patients undergoing major abdominal surgery. Eur J Anaesth. 2007;24:267-75.
- [Google Scholar]
- Hydroxyethyl starch (HES) [130/0.4], a new HES specification. Pharmacokinetics and safety after multiple infusions of 10% solution in healthy volunteers. Drugs R & D. 2003;4:149-57.
- [Google Scholar]
- Tissue storage of 14 C-labelled hydroxyethyl starch (HES) 130/0.4 and HES 200/0.5 after repeated intraveneous administration to rats. Drugs R D. 2003;4:337-8.
- [Google Scholar]
- Large-dose hydroxyethyl starch 130/0.4 does not increase blood loss and transfusion requirements in coronary artery bypass surgery compared with hydroxyethyl starch 200/0.5 at recommended doses. Anesthesiology. 2003;99:42-7.
- [Google Scholar]
- Repetitive large-dose infusion of the novel hydroxyethyl starch 130/0.4 in patients with severe head injury. Anesth Analg. 2003;96:1453-9.
- [Google Scholar]
- The effect of the combined administration of colloids and lactated Ringer's Solution on the coagulation system:an in vitro study using thrombelastograph coagulation analysis (ROTEG) Anesth Analg. 2002;94:1280-7.
- [Google Scholar]






