Translate this page into:
Synergism in dual functionality of cryptdin-2 in conjunction with antibiotics against Salmonella
Reprint requests: Dr Praveen Rishi, Department of Microbiology, Panjab University, Chandigarh 160 014, India e-mail: rishipraveen@yahoo.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
The emergence of multidrug-resistant Salmonella over the last two decades poses a major health risk. In this context, antimicrobial peptides have found a strategic place in the therapeutic armamentarium. Previously, we found that cryptdin-2 has the potential to augment the activity of conventional second- and third-generation anti-Salmonella antibiotics as evident by in vitro assays. In continuation to this, the present study was designed to evaluate the in vivo synergistic effects, if any, of cryptdin-2 in combination with ciprofloxacin and ceftriaxone against murine salmonellosis.
Methods:
Scanning electron microscopy (SEM) studies along with in vivo synergistic studies were performed using cryptdin- 2 and antibiotic combinations. In addition, peroxidative liver damage, levels of nitric oxide (NO) and antioxidant enzymes along with tumour necrosis factor-alpha (TNF-α) levels were also measured.
Results:
The SEM results revealed marked changes on the outer membrane of the bacterial cells treated with various combinations. Both the tested combinations demonstrated synergistic in vivo potency against S. Typhimurium as evident by reduction in the number of Salmonellae in the liver, spleen and intestine. Analysis of peroxidative liver damage, levels of NO and antioxidant enzymes along with TNF-α and nuclear factor-kappa B levels revealed that the tested combinations restored their levels to near normal. The most potent combination was found to be that of cryptdin-2 and ciprofloxacin in terms of direct killing and immunomodulatory potential.
Interpretation & conclusions:
These findings suggest that cryptdin-2 may act in conjunction with conventional antibiotics indicating the possibility of developing these combinations as additional therapeutic agents to combat Salmonella infections.
Keywords
Antimicrobial peptides
conventional antibiotics
cryptdin- 2
Salmonella
synergy
tumour necrosis factor-alpha
Salmonellae are Gram-negative bacterial pathogens capable of infecting a wide range of animals, which can result in manifestation of several diseases ranging from gastroenteritis to bacteraemia and enteric fever1. Antibiotics have been the mainstay of therapy to combat these infections. Multidrug-resistant (MDR) Salmonellae (both typhoidal and non-typhoidal) and spread of cephalosporin-resistant Salmonellae are a common health problem. Treatment failures due to acquisition of extended-spectrum β-lactamase or fluoroquinolone resistance genes in Salmonella isolates has prompted research on possible alternatives23. In this context, antimicrobial peptides (AMPs) have become the object of intense investigation to develop new therapeutic agents with specific activities such as antimicrobial activity and immunomodulatory functions. AMPs are found amongst all classes of life ranging from prokaryotes to humans4. One of the most well-characterized and highly expressed AMP families is that of the α-defensins (also named cryptdins in mice: crypt alpha-defensins). Cryptdins are characterized as broad-spectrum AMPs due to their ability to kill various bacteria, parasites as well as enveloped viruses56.
Synergistic effects of AMPs and conventional antibiotics have been reported to be beneficial in encountering difficult to treat infections7. Earlier, we had reported the synergism between cryptdin-2 and first-generation antibiotic (ampicillin) against Salmonella infection by direct killing (in vivo) and immunomodulatory potential (ex vivo studies)8. However, with nisin, less effect displayed by ampicillin was explained by the presence of ampicillin-inactivating enzyme such as β-lactamase, thereby making this combination ineffective. Interestingly, β-lactamases are not effective against higher generation cephalosporins with an oxyimino side chain such as cefotaxime, ceftazidime, ceftriaxone, or cefepime9. In our previous study, cryptdin-2 was found to have the potential to augment the activity of conventional, second and third generation anti-Salmonella antibiotics, which was evident by in vitro assays10. In continuation to this, here, we report in vivo synergism in dual functionality i.e., both in direct killing and immunomodulatory activity of cryptdin-2, a mouse Paneth cell alpha-defensin, in conjunction with second- and third-generation antibiotics i.e., ciprofloxacin and ceftriaxone, respectively, against Salmonella enterica serovar Typhimurium. The experiments were carried out with S. Typhimurium strain because in addition to its effects in human hosts, S. Typhimurium causes systemic disease in mice reminiscent of human typhoid or enteric fever caused by S. enterica serovar Typhi (S. Typhi). Because of this relationship, infection of mice with S. Typhimurium has been widely employed as a model of human infection with S. Typhi11.
Material & Methods
The experimental protocols were approved by the Institutional Animal Ethics Committee of Panjab University, Chandigarh, India and performed in accordance with the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India, on animal experimentation. Female BALB/c mice (18-22 g, 4-5 weeks old) were procured from Central Animal House, Panjab University, Chandigarh. The animals were housed under standard conditions with free access to food and water ad libitum.
Bacterial strain and growth medium: Standard strain of S. Typhimurium NCTC74, originally procured from Central Research Institute, Kasauli, India, and being maintained in our laboratory was used in the present study. Stock cultures were prepared and stored at -80°C in glycerol (20%). Purity of the strain was confirmed biochemically as well as serologically.
For preparation of bacterial cell suspension, bacterial cells grown overnight in nutrient broth (5.0 g/l peptone, 5.0 g/l NaCl, 1.5 g/l beef extract, 1.5 g/l yeast extract and pH 7.4 ± 0.2) were harvested by centrifugation (3783×g, 15 min), washed once with 10 mM sodium phosphate-buffered saline (PBS, pH 7.2) and resuspended in PBS to a final concentration of approximately 107 colony forming units (cfu)/ml.
Synthetic cryptdin-2 and antibiotics: Synthetic cryptdin-2 obtained from Genpro Biotech, New Delhi, India, was suspended in 0.01 per cent acetic acid, stored as a stock solution at 100 mg/l at -20°C and used within three weeks. Chloramphenicol (CHL), ciprofloxacin (CIP), ceftriaxone (CRO) and cefotaxime (CTX) powder were procured from Sigma-Aldrich (St. Louis, MO, USA). Chloramphenicol, ceftriaxone and cefotaxime were dissolved in distilled water, whereas ciprofloxacin was dissolved in 0.1 N HCl. Stock solutions of 1000 mg/l were prepared and used within one week.
Determination of minimum and fractional bactericidal concentrations (MBCs and FBCs): The MBCs and FBCs of all the test agents employed in the present study were evaluated as described earlier9. The MBC was defined as the concentration at which there was >99 per cent inhibition of growth. The FBCs were calculated after dividing the MBCs of the tested agents in combination by the MBCs of tested agents alone separately. The FBC index, obtained by adding both FBCs, was interpreted as indicating a synergistic effect when it was ≤0.5, an additive or indifferent effect when it was >0.5 and ≤2.0 and an antagonistic effect when it was >2.0.
Scanning electron microscopy (SEM): The ultrastructural changes, if any, induced by the combinations were studied by SEM as described earlier9. For the SEM study, washed and concentrated cells of S. Typhimurium from the log phase were incubated with various antimicrobial agents alone at their subinhibitory concentrations [at 0.25 times the MBC i.e., cryptdin-2 (5 μg/ml), CHL (32 μg/ml), CIP (1.5 μg/ml), CRO (4 μg/ml) and cefotaxime (3 μg/ml)] and in combinations at reduced concentrations [cryptdin-2 (5 μg/ml) + CHL (32 μg/ml), cryptdin-2 (2.5 μg/ml) + CIP (0.75 μg/ml), cryptdin-2 (5 μg/ml) + CRO (1 μg/ml) and cryptdin-2 (2.5 μg/ml) + cefotaxime (3 μg/ml)] for two hours at 37°C. Using agents alone at their MBCs, the damage observed was extensive. Therefore, the above mentioned concentrations were selected after standardization where the action of the agent(s) was best appreciated. Samples were processed and examined for the change in morphology by scanning electron microscope (Hitachi S-3400N model, Tokyo, Japan).
In vivo study using various combinations of cryptdin-2 and antibiotics against murine Salmonella infection: The magnitude of in vitro synergism observed between cryptdin-2-ciprofloxacin and cryptdin-2-ceftriaxone prompted us to further investigate the in vivo synergistic efficacy using a murine model. One hundred mice were infected with 0.25 ml of 107 cfu of S. Typhimurium orally. Seven days after the challenge, establishment of Salmonella infection was confirmed by bacterial translocation into the livers, spleens and intestines of the infected mice. At seven days post-infection, mice were divided into six groups and further into various subgroups each comprising at least six mice. A group of six mice was set aside which served as uninfected control.
The following treatment groups were made: (i) Control group: Mice in this group were injected with 0.1 ml sterile saline subcutaneously (s.c.) and served as the control (infected) group; (ii) Infected and ciprofloxacin treated: Mice in this group were divided into two subgroups. Group 2A mice were administered 20 mg/kg body weight ciprofloxacin s.c. and group 2B mice were administered 40 mg/kg body weight of ciprofloxacin s.c.; (iii) Infected and ceftriaxone treated: Mice were divided into two subgroups. Group 3A mice were administered 25 mg/kg body weight ceftriaxone s.c. and group 3B mice were administered 50 mg/kg body weight of ceftriaxone s.c.; (iv) Infected and cryptdin-2 treated: Mice in this group were administered cryptdin-2 s.c. at a single dose of 5 μg/mouse; (v) Infected and cryptdin-2+ciprofloxacin treated: Mice were divided into four subgroups. Group 5A mice were co-administered cryptdin-2 (5 μg/mouse, s.c.) and ciprofloxacin (20 mg/kg body weight, s.c.). Group 5B mice were co-administered cryptdin-2 (5 μg/mouse, s.c.) and ciprofloxacin (40 mg/kg body weight, s.c.). Group 5C mice were co-administered cryptdin-2 (2.5 μg/mouse, s.c.) and ciprofloxacin (10 mg/kg body weight, s.c.). Group 5D mice were co-administered cryptdin-2 (2.5 μg/mouse, s.c.) and ciprofloxacin (20 mg/kg body weight, s.c.); and (vi) Infected and cryptdin-2+ceftriaxone treated: Mice were divided into four subgroups. Group 6A mice were co-administered cryptdin-2 (5 μg/mouse, s.c.) and ceftriaxone (25 mg/kg body weight, s.c.), group 5B mice were co-administered cryptdin-2 (5 μg/mouse, s.c.) and ceftriaxone (50 mg/kg body weight, s.c.), group 5C mice were co-administered cryptdin-2 (2.5 μg/mouse, s.c.) and ceftriaxone (12.5 mg/kg body weight, s.c.), group 5D mice were co-administered cryptdin-2 (2.5 μg/mouse, s.c.) and ceftriaxone (25 mg/kg body weight, s.c.).
Cryptdin-2 was administered s.c. at a single dose, whereas CIP and CRO were injected in four doses s.c. after 12 h interval individually and in combination. The doses used were selected on the basis of pilot studies. At 48 h post-therapy, mice were sacrificed, and their livers, spleens and small intestines were removed aseptically, rinsed in isotonic saline solution and weighed. Ten per cent (w/v) of tissue homogenates were prepared in sterile PBS using a Potter-Elvehjem homogenizer. Serial 10-fold dilutions of each homogenate were plated on MacConkey agar medium and bismuth sulphite agar medium (HiMedia, Mumbai) for enumeration of cfu per organ after incubation at 37°C for 24 h.
Post-mitochondrial supernatant preparation: One subgroup from each treatment group was selected on the basis of reduction in bacterial loads in different target organs. Livers removed aseptically from all the selected groups were rinsed in 0.05M phosphate-buffered saline (pH 7.4; PBS). A 10% (w/v) tissue homogenate in each case was prepared in PBS. An aliquot of the liver homogenate was used for the estimation of lipid peroxidation. For the estimation of nitrite, superoxide dismutase (SOD) and catalase activities, post-mitochondrial preparation was made. For this, the remaining tissue homogenates were centrifuged at 8900×g for 20 min at 4°C. The supernatants thus obtained were called post-mitochondrial supernatants (PMSs). PMS was preferred to avoid any interference of reactive oxygen and reactive nitrogen species which are otherwise generated during the essential cellular metabolic processes at the mitochondrial site.
Extent of peroxidative liver damage: The quantitative measurement of lipid peroxidation in liver was performed according to the method of Wills12 as described earlier13. The results were expressed as nanomoles of malondialdehyde (MDA) per milligram of protein, using the molar extinction coefficient of chromophore (1.56 × 105/M/cm). The protein content of tissue homogenates was calculated as described previously13.
Estimation of enzymatic antioxidants activity: The SOD activity was assayed according to the method of Kono14 and was expressed as units of SOD per milligram of protein where one unit of activity was defined as the amount of SOD required to inhibit the rate of reduction of nitroblue tetrazolium by 50 per cent. The catalase activity was assayed by the method of Luck15 and was expressed as millimoles of H2O2 decomposed per minute per mg of protein using the molar extinction coefficient of the chromophore (0.0394/mM/cm).
Estimation of nitrite levels: The amount of nitric oxide (NO) was determined by Griess reaction, as described earlier13. Absorbance was measured at 546 nm. Nitrite levels in all the samples were quantified according to the standard graph of sodium nitrite.
Tumour necrosis factor-alpha (TNF-α) assay: Assay for TNF-α was performed by ELISA in the liver homogenates using commercially available Cytokine Assay Kit (RayBiotech, Norcross GA, USA) as described earlier13. Absorbance was taken at 450 nm. The results were expressed as pg/ml of the TNF-α released.
Assay for NF-κB p50 subunit: Expression of TNF-α is directly related with nuclear factor-kappa B (NF-κB) levels because upon activation, NF-κB results in the expression of inflammatory mediators including cytokines [particularly TNF-α and interleukin-6 (IL-6)], chemokines, lipid mediators, enzymes and adhesion molecules16. Likewise, blocking of NF-κB activation may further downregulate TNF-α as has been reported earlier13. Therefore, assay for NF-κB p50 subunit in the nuclear extracts was performed in cryptdin-antibiotics treated groups (alone and in combination) by commercially available Transcription Factor Assay Kit (Upstate Biotechnology, NY, USA) according to the manufacturer's instructions. Absorbance was read at 450 nm. Positive and negative controls were run simultaneously.
Statistical analysis: Statistical analysis was done by Student's unpaired t test and one-way analysis of variance, followed by pairwise comparison procedures (Tukey test), using Jandel SigmaStat statistical software version 2.0 (Systat Software Inc., USA).
Results
Scanning electron microscopy: In contrast to control samples, marked changes were evident on the outer membranes of S. Typhimurium treated with various combinations. However, cryptdin-2-CIP, cryptdin-2-CRO and cryptdin-CTX combinations seemed to extensively damage S. Typhimurium as evident by pronounced changes in their morphology. It is indicated that all these combinations could lead to wrinkling, surface roughing and disruption of bacterial membrane after two hours of treatment period (Fig. 1).
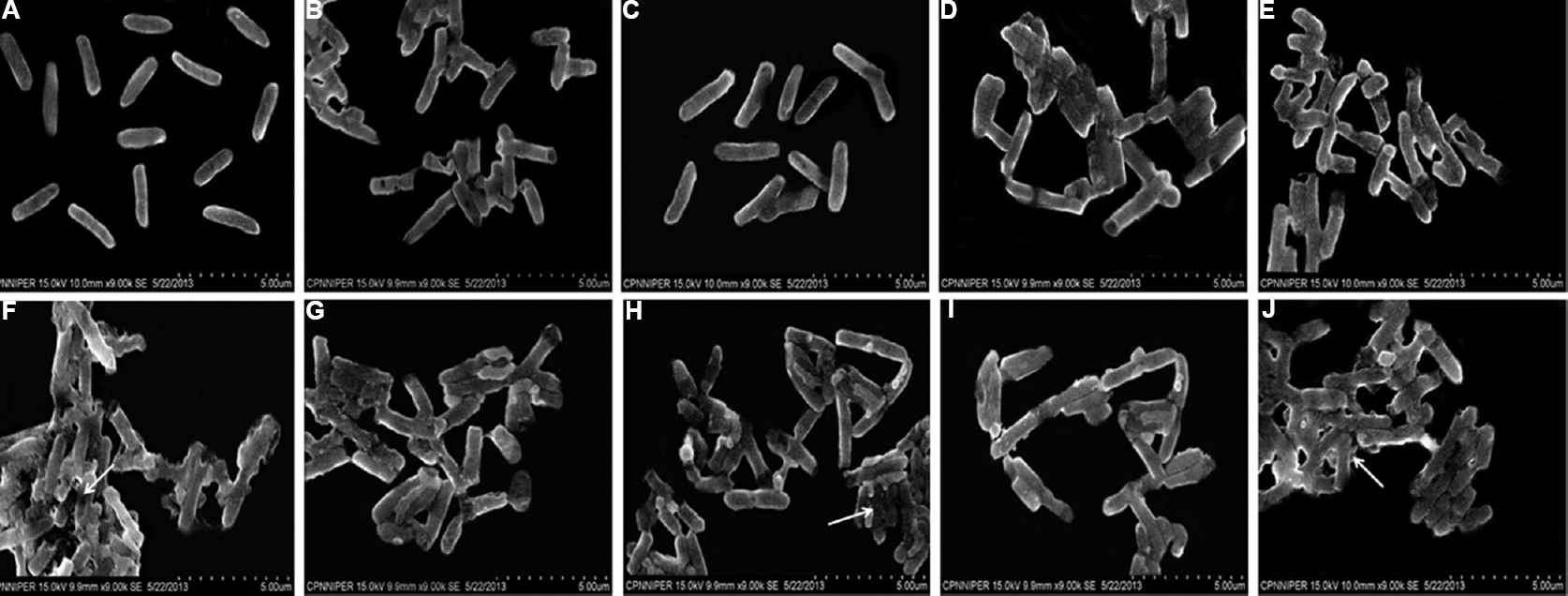
- Scanning electron micrographs of Salmonella Typhimurium in the presence of (A) normal saline, (B) cryptdin-2 (5 μg/ml), (C) chloramphenicol (32 μg/ml), (D) cryptdin-2 (5 μg/ml) + chloramphenicol (32 μg/ml), (E) ciprofloxacin (1.5 μg/ml), (F) cryptdin-2 (2.5μg/ml) + ciprofloxacin (0.75 μg/ml), (G) ceftriaxone (4 μg/ml), (H) cryptdin-2 (5 μg/ml) + ceftriaxone (1 μg/ml), (I) cefotaxime (3 μg/ml), (J) cryptdin-2 (2.5 μg/ml) + cefotaxime (3 μg/ml) (White arrows showing significant damage to the bacterial cells).
Therapeutic potential and synergistic efficacy of cryptdin-2 and antibiotics against experimental salmonellosis: Therapeutic efficacy of antimicrobial agents alone and in conjunction was investigated in terms of reduction in the number of Salmonellae in different target organs of mice infected with S. Typhimurium after 48 h of first dose of chemotherapy.
In the livers, separately CIP (20 mg/kg body weight and 40 mg/kg body weight), CRO (25 mg/kg body weight and 50 mg/kg bodyweight) at both the doses and cryptdin at 5 μg/mouse significantly decreased the bacterial load (P<0.05) compared to the infected group. However, co-administration of cryptdin with CIP and CRO at the same and even lower concentrations resulted in a much larger decrease in bacterial load (P<0.05 (Figs 2A and 3A). In spleens of mice, tested agents alone reduced the bacterial load significantly (P<0.05) compared to the infected group. Similarly, the adjunct therapy of cryptdin with CIP and CRO was found to be more effective (P<0.05), as higher log unit decreases in bacterial load were observed at all the tested concentrations (Figs 2B and 3B). A similar trend was observed in the bacterial load recovered from the small intestines also (Figs 2C and 3C).
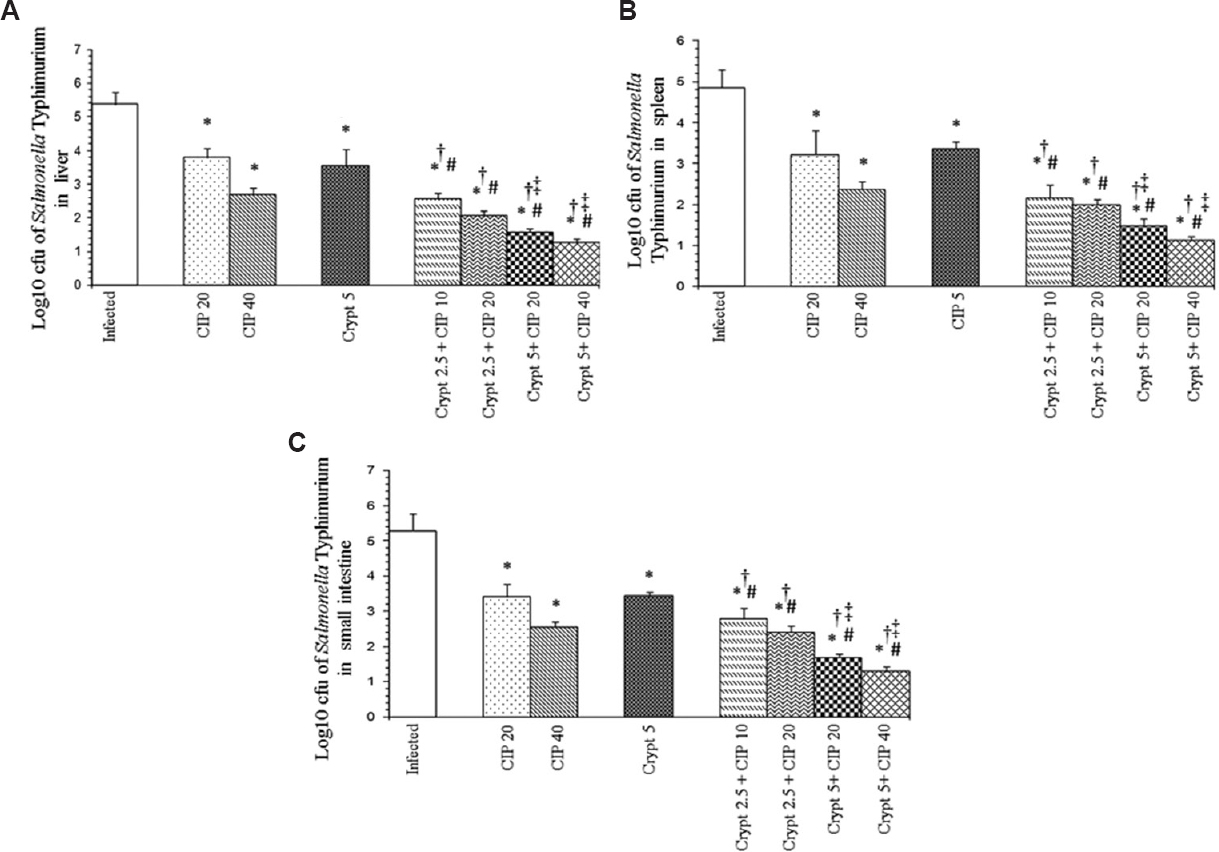
- Log10 cfu of Salmonella Typhimurium in (A) liver, (B) spleens and (C) small intestines of infected mice after 48 h of therapy with cryptdin-2 (cry) and ciprofloxacin (CIP) separately and in combination. Values are expressed as mean ± standard deviation of three independent experiments. *P<0.05 compared to untreated mice (infected-control); #P<0.05 compared to mice treated with cryptdin-2 (5 μg/mouse); †P<0.05 compared to mice treated with CIP (20 mg/kg body weight); ‡P<0.05 compared to mice treated with CIP (40 mg/kg body weight). Uninfected control group comprising six mice was also used in the study (not shown as no bacterial load was recovered from this group).
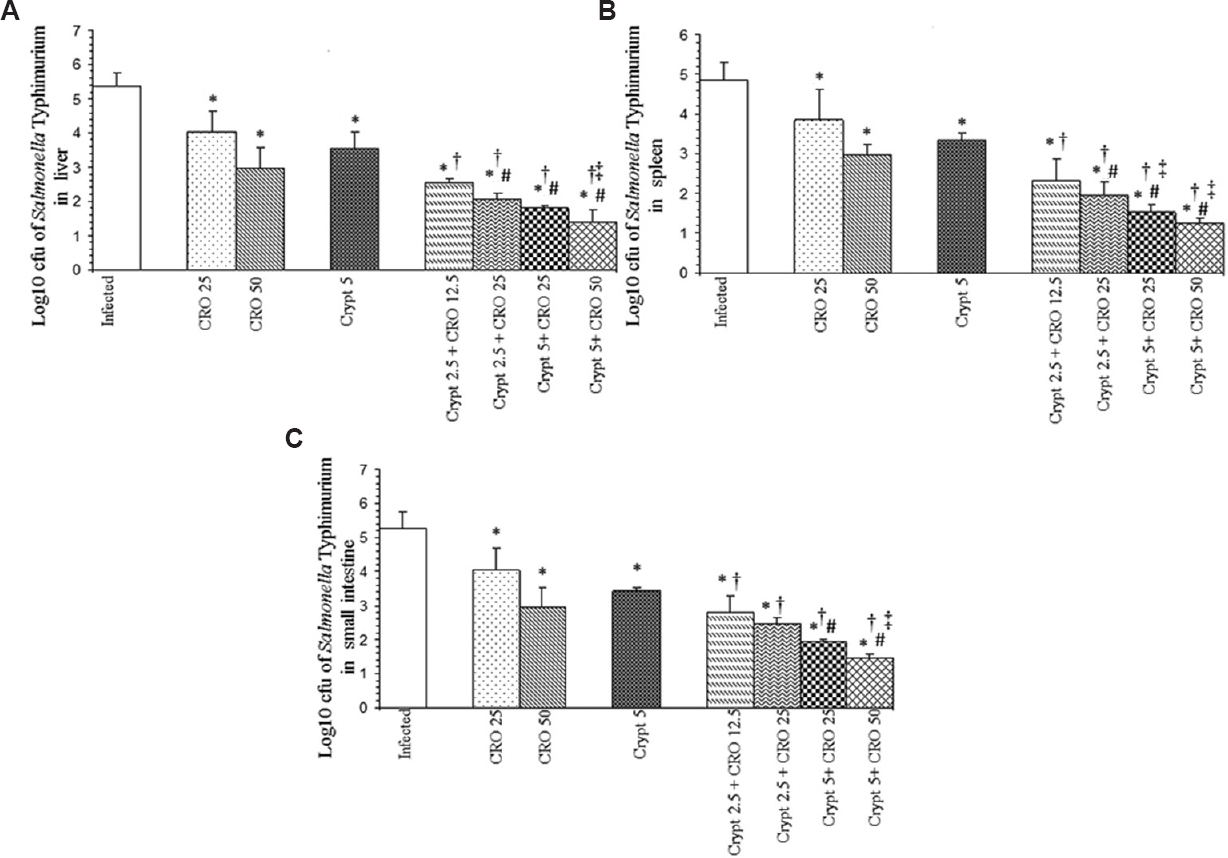
- Log10 cfu of Salmonella Typhimurium in (A) livers, (B) spleens and (C) small intestines of infected mice after 48 h of therapy with cryptdin-2 (cry) and ceftriaxone (CRO) separately and in combination. Values are expressed as mean ± standard deviation of three independent experiments. *P<0.05 compared to untreated mice (infected-control); #P<0.05 compard to mice treated with cryptdin-2 (5 μg/mouse); †P <0.05 compared to mice treated with CRO (25 mg/kg body weight); ‡P<0.05 compared to mice treated with CRO (50 mg/kg body weight). Uninfected control group comprising six mice was also used in the study (not shown as no bacterial load was recovered from this group).
Lipid peroxidation (malondialdehyde) levels: Infection with S. Typhimurium-induced significant (P<0.05) lipid peroxidation in the infected mice, as indicated by increased MDA levels compared to those of uninfected mice (202.73±27.02 nmoles/mg protein in infected controls vs. 95.04±10.47 nmoles/mg protein in uninfected controls). However, a significant decrease in the levels of MDA was observed when mice were treated separately with cryptdin, CIP and CRO compared to untreated infected group. Further, a much larger reduction in MDA levels was observed when mice were treated with the tested combinations of cryptdin-CIP (111.93±5.95 nmol/mg protein) and cryptdin-CRO (108.64±6.72 nmol/mg protein) (Table).
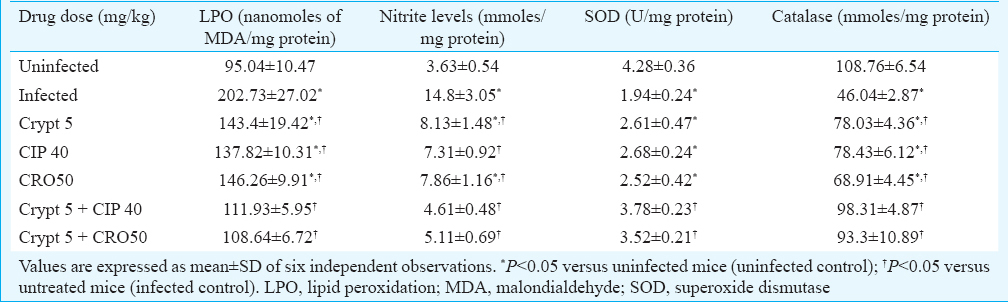
Estimation of enzymatic antioxidants activity: Infection with S. Typhimurium-induced a significant decrease (P<0.05) in the activity of enzymatic antioxidants (SOD and catalase) as compared to the uninfected control group. However, mice treated with the combinations showed a restoration of SOD and catalase activity, which attained near uninfected control values (Table).
Estimation of nitrite levels: There was a significant decrease (P<0.05) in the nitrite levels treated with cryptdin (8.13±1.48 μmol/mg protein) and antibiotics alone (7.31±0.92 μmol/mg protein in CIP-treated group and 7.86±1.16 μmol/mg protein in CRO-treated group) compared to the infected control group (14.8±3.05 μmol/mg protein). However, a more pronounced effect was observed in groups treated with combinations of cryptdin-CIP and cryptdin-CRO and levels were restored to near normal (Table).
TNF-α levels: Infection with S. Typhimurium-induced significant TNF-α generation (P<0.05) (277.51 ± 22.5 pg/ml) compared to the uninfected group (68.26 ± 8.25 pg/ml). Groups which were treated with tested antimicrobial agents alone showed a significant (P<0.05) decrease, but groups which were treated with tested combinations showed more pronounced effect (91.87±6.27 pg/ml in cryptdin-CIP treated group and 98.88±5.84 pg/ml in cryptdin-CRO treated group) (Fig. 4).
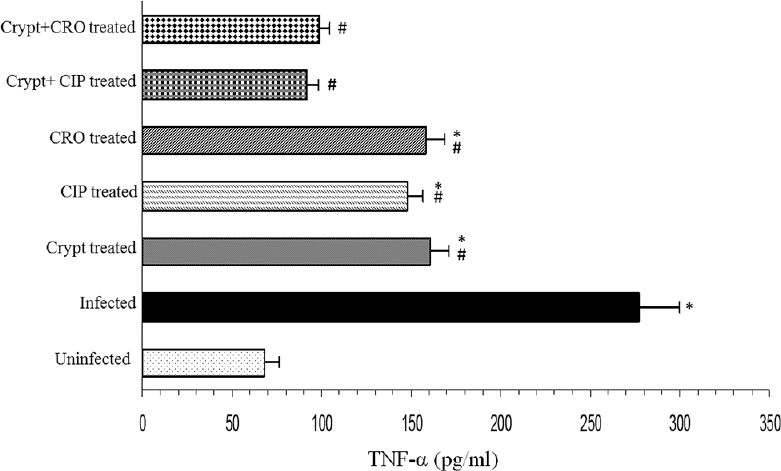
- Modulatory effects of cryptdin-2 (crypt), ciprofloxacin (CIP) and ceftriaxone (CRO) alone and in combination on hepatic tumour necrosis factor-alpha (TNF-α) levels of mice infected with Salmonella Typhimurium. Values are expressed as mean ± standard deviation of three independent observations. *P<0.05 versus uninfected mice (uninfected-control); #P<0.05 versus untreated mice (infected-control).
Assay for NF-κB p50 subunit: Levels of NF-kB p50 subunit were significantly (P<0.05) elevated in the group infected with S. Typhimurium as compared to the uninfected control group (Fig. 5). However, groups which were treated with tested antimicrobial agents alone (cryptdin-2, CIP and CRO alone) showed a significant attenuation in the activation of NF-κB. Interestingly, levels of NF-κB p50 subunit observed in cryptdin-CIP and cryptdin-CRO treated groups were comparable to the levels observed in alone treated groups (Fig. 5).
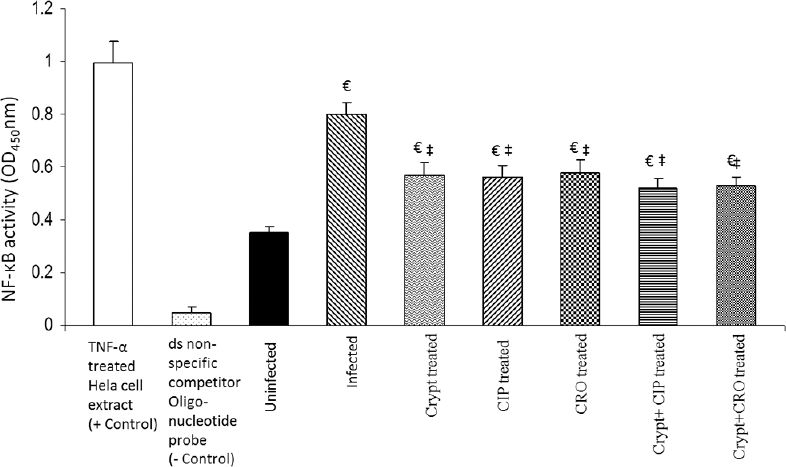
- Effect of cryptdin-2 (crypt), ciprofloxacin (CIP), ceftriaxone (CRO) alone and in conjunction on Salmonella-induced activation of nuclear factor-kappa B (NF- kB) in liver. Values are expressed as mean ± standard deviation of five different observations. €P<0.05 versus uninfected mice (uninfected control); ‡P<0.05 versus untreated mice (infected-control). Positive control (+control) refers to the TNF-α treated Hela whole cell extract; negative control (-control) refers to the biotinylated double stranded non-specific competitor oligonucleotide probe which does not contain the NFkB consensus sequence.
Discussion
Earlier, chloramphenicol, ampicillin and co-trimoxazole were used to treat Salmonella infections. Later on with the emergence of MDR Salmonella strains, ciprofloxacin became the drug of choice. However, reports of increasing MIC values suggested that ciprofloxacin therapy would not remain effective for much longer23. Therefore, synergism observed between cryptdin and antibiotics provided additional therapeutic choices by allowing the use of conventional antibiotics in conjunction with natural antibiotics against MDR Salmonella.
The most common explanation for synergism is permeabilization of the bacterial membrane, which would facilitate the penetration of the antibiotic into the cell1718. To substantiate the membrane-dependent action of the tested combinations, SEM studies were carried out. The findings revealed by SEM confirmed the synergy which was evident by more intense changes in cell morphology (clubbing and disruption of bacterial membrane) induced by the combinations as compared to each agent alone at subinhibitory concentrations. These observations were in concordance with our earlier checkerboard and time-kill assay findings suggesting faster disruption of the bacterial membrane910.
Further, the results of animalstudies indicated synergism between cryptdin-ciprofloxacin and cryptdin-ceftriaxone, as more clearance of Salmonellae from the target organs was observed using the combinations. These results were similar to an earlier report suggesting a synergistic effect of AMPs with β-lactam antibiotics and ciprofloxacin19. The interesting finding was that when cryptdin-2 was used in conjunction with ciprofloxacin and ceftriaxone, the log unit decreases in the number of Salmonellae in all target organs were observed to be comparable to the decreases observed when the agents (ciprofloxacin and ceftriaxone) were used alone at two-fold higher concentrations. Therefore, it reduced the therapeutic dosage of both agents to half (from 40 to 20 mg/kg of ciprofloxacin and from 50 to 25 mg/kg of ceftriaxone) while maintaining the increased therapeutic efficacy. This increased therapeutic efficacy might be attributed to multitargeted task approach of the combinations.
There is some evidence that during Salmonella infection, the antigenic stimulation activates the immune system of the host, thereby causing an excessive production of reactive oxygen species (ROS), leading to peroxidation of lipids and affects the antioxidant system and finally to tissue damage20. However, in the present study, a significant decrease in the extent of lipid peroxidation (in terms of MDA level) and restoration of the SOD level and catalase activity were also observed in the mice treated with the combinations of cryptdin-ciprofloxacin and cryptdin-ceftriaxone. These results indicated that the combinations might exhibit the synergistic effect by scavenging free radicals (efficient detoxification of H2O2) and by upregulating the antioxidant activity, thus counteracting the oxidative stress, thereby providing protection against oxidative damage.
NO is an important signalling molecule which is produced in large quantities during host defence and immunologic reactions. However, excessive amounts of NO are potentially toxic and have been implicated in numerous pathological situations and chronic inflammation21. The estimation of nitrite levels is an indirect measure of NO content. Elevated level of nitrite in Salmonella- infected mice group might be attributed to the fact that NO in conjunction with superoxide radical has been reported to form a potent oxidant peroxynitrite, which may stimulate the production of TNF-α and other pro-inflammatory cytokines leading to tissue injury22. However, a significant decrease was observed in groups treated with both the tested combinations in comparison to the groups treated with the tested agents alone. It might be possible that these agents neutralize the Salmonella-induced release of NO. In agreement with our findings, it has been reported that ciprofloxacin and ceftriaxone attenuate TNF-α levels2324, which might have decreased the NO levels following treatment in the present study. In addition, the lower levels of nitrite observed in groups treated with cryptdin-2 might be attributed to the anti-inflammatory activity of cryptdin, as has been reported for various other AMPs25. These two mechanisms might have been mutually responsible for the significant immunomodulatory effects obtained in the groups treated with combinations.
Further, to substantiate the role of TNF-α in altering the nitrite levels and antioxidant levels, TNF-α levels were estimated. The levels of TNF-α after Salmonella infection were related to the increased level of peroxidation and decreased activities of hepatic antioxidants. However, mice treated with the combinations managed to restore the normal levels, which were comparable to uninfected controls. These effects might be due to the immunomodulatory effects of the combinations in the treated mice.
Expression of TNF-α is directly related with NF-κB levels because upon activation, NF-κB results in the expression of inflammatory mediators including cytokines (particularly TNF-α and IL-6), chemokines, lipid mediators, enzymes and adhesion molecules26. Similarly, blocking of NF-κB activation may further downregulate TNF-α as has been reported earlier27. Therefore, effect of cryptdin-antibiotic combinations on NF-κB levels was also checked. Cryptdin-ciprofloxacin and cryptdin-ceftriaxone did not show any significant change in NF-κB levels compared to groups treated with single agent. On binding to its receptors, TNF-α regulates several intracellular signalling cascades i.e., NF-κB, c-Jun N-terminal kinases (JNK) and ROS pathways that influence cell survival and death. Although TNF-α may act as a potent activator of both pro-inflammatory and pro-apoptotic pathways, these signalling pathways interact in a complex network at several levels, and activation of one pathway often depends on the inactivation of another pathway, suggesting that cells are capable of directing the TNF-α-induced signal towards the appropriate response28. The ability of TNF-α to bind to two different receptors, which transmit distinct intracellular signals with different affinities, adds yet another level of control over TNF-α-induced cellular responses28. It might be possible that cryptdin-antibiotic combinations may have acted on p38 mitogen-activated protein kinases and JNK pathway along with ROS pathways instead of NF-κB pathway by regulating TNF-α levels. An earlier study with AMPs also indicated interrelatedness of JNK signalling with AMP gene expression29.
The most potent combination was found to be that of cryptdin and ciprofloxacin in terms of direct killing and immunomodulatory potential. The observed synergy between cryptdin and CIP might be attributed to the fact that cryptdin makes pores in the cell membranes, which allows increased amounts of ciprofloxacin to enter the cell and block DNA synthesis. Further, it could be attributed to the fact that besides acting directly on bacterial cell, quinolones have also been reported to sensitize Gram-negative bacteria to cationic AMPs by displacement of divalent cations from their LPS-binding sites thereby increasing the binding of AMPs to outer membrane30. These two mechanisms might have acted mutually making this combination more effective than the other.
In conclusion, strong synergism in terms of dual functionality (direct killing and immunomodulatory activity) was observed between the two tested combinations. These findings suggest that strategy of using cryptdin-2 in conjunction with conventional antibiotics having a direct effect on the pathogen might prove to be beneficial in designing alternative anti-infective therapeutic agents.
Acknowledgment
The authors acknowledge the Council of Scientific and Industrial Research, New Delhi, for providing financial assistance in the form of Senior Research Fellowship (CSIR Award No. 09/135 (0584)/ 2008-EMR-1) to the first author APS.
Conflicts of Interest: None.
References
- Molecular characterization of antimicrobial resistance in non-typhoidal Salmonellae associated with systemic manifestations from India. J Med Microbiol. 2010;59:1477-83.
- [Google Scholar]
- Antimicrobial resistance in typhoidal Salmonellae. Indian J Med Microbiol. 2011;29:223-9.
- [Google Scholar]
- Revisiting eukaryotic anti-infective biotherapeutics. Crit Rev Microbiol. 2014;40:281-92.
- [Google Scholar]
- Antimicrobial activity of Paneth-cell derived cryptdin-2 against selected pathogens. Am J Biomed Sci. 2010;2:13-22.
- [Google Scholar]
- Cryptdin-2: a novel therapeutic agent for experimental Salmonella typhimurium infection. J Antimicrob Chemother. 2010;65:991-4.
- [Google Scholar]
- Synergy among antibacterial peptides and between peptides and small-molecule antibiotics. Expert Rev Anti Infect Ther. 2010;8:703-16.
- [Google Scholar]
- In vitro and in vivo synergistic effects of cryptdin 2 and ampicillin against Salmonella. Antimicrob Agents Chemother. 2011;55:4176-82.
- [Google Scholar]
- Value addition in the efficacy of conventional antibiotics by Nisin against Salmonella. PLoS One. 2013;8:e76844.
- [Google Scholar]
- Efficacy of cryptdin-2 as an adjunct to antibiotics from various generations against Salmonella. Indian J Microbiol. 2014;54:323-8.
- [Google Scholar]
- Animal models of Salmonella infections: enteritis versus typhoid fever. Microbes Infect. 2001;3:1335-44.
- [Google Scholar]
- Mechanisms of lipid peroxide formation in animal tissues. Biochem J. 1966;99:667-76.
- [Google Scholar]
- Nisin/ß-lactam adjunct therapy against Salmonella enterica serovar typhimurium: a mechanistic approach. J Antimicrob Chemother. 2014;69:1877-87.
- [Google Scholar]
- Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys. 1978;186:189-95.
- [Google Scholar]
- Catalase. In: Bergmeyer HU, ed. Methods of enzymatic analysis (2nd ed). New York: New York Academic Press; 1971. p. :885-94.
- [Google Scholar]
- Overexpression of mitochondrial manganese superoxide dismutase promotes the survival of tumor cells exposed to interleukin-1, tumor necrosis factor, selected anticancer drugs, and ionizing radiation. FASEB J. 1993;7:361-8.
- [Google Scholar]
- Synergistic interaction between silver nanoparticles and membrane-permeabilizing antimicrobial peptides. Antimicrob Agents Chemother. 2009;53:3538-40.
- [Google Scholar]
- Antimicrobial action of the cyclic peptide bactenecin on Burkholderia pseudomallei correlates with efficient membrane permeabilization. PLoS Negl Trop Dis. 2013;7:e2267.
- [Google Scholar]
- Role of human neutrophil peptide-1 as a possible adjunct to antituberculosis chemotherapy. J Infect Dis. 2004;190:1476-80.
- [Google Scholar]
- Are the increases in local tumour necrosis factor and lipid peroxidation observed in pre-starved mice infected with Salmonella typhimurium markers of increased liver damage? Microbes Infect. 2006;8:1695-701.
- [Google Scholar]
- Peroxynitrite is an essential component of cytokines production mechanism in human monocytes through modulation of nuclear factor-kappa B DNA binding activity. J Biol Chem. 2002;277:2330-5.
- [Google Scholar]
- Effect of ciprofloxacin on lethal and sublethal challenge with endotoxin and on early cytokine responses in a murine in vivo model. J Antimicrob Chemother. 2002;50:51-8.
- [Google Scholar]
- Attenuation of cerebrospinal fluid inflammation by the nonbacteriolytic antibiotic daptomycin versus that by ceftriaxone in experimental pneumococcal meningitis. Antimicrob Agents Chemother. 2010;54:1323-6.
- [Google Scholar]
- Immune cells: free radicals and antioxidants in sepsis. Int Immunopharmacol. 2004;4:327-47.
- [Google Scholar]
- Catechin suppresses an array of signalling molecules and modulates alcohol-induced endotoxin mediated liver injury in a rat model. PLoS One. 2011;6:e20635.
- [Google Scholar]
- Mechanisms of liver injury. I. TNF-alpha-induced liver injury: role of IKK, JNK, and ROS pathways. Am J Physiol Gastrointest Liver Physiol. 2006;290:G583-9.
- [Google Scholar]
- Functional analysis of immune response genes in Drosophila identifies JNK pathway as a regulator of antimicrobial peptide gene expression in S2 cells. Microbes Infect. 2005;7:811-9.
- [Google Scholar]
- Quinolones sensitize Gram-negative bacteria to antimicrobial peptides. Antimicrob Agents Chemother. 2006;50:2361-7.
- [Google Scholar]






