Translate this page into:
Surveillance for enterotoxigenic & enteropathogenic Escherichia coli isolates from animal source foods in Northwest Iran
For correspondence: Dr Mohammad Ahangarzadeh Rezaee, Infectious & Tropical Diseases Research Center, Tabriz University of Medical Sciences, Tabriz, Tehran, Iran e-mail: rezaee@tbzmed.ac.ir
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Diarrhoeagenic Escherichia coli strains are common agents of diarrhoea particularly in developing countries. Food products of animal origin are considered as common carriers of E. coli. This study was undertaken to identify enterotoxigenic Escherichia coli (ETEC) and enteropathogenic E. coli (EPEC) pathotypes in animal-source foods (ASF).
Methods:
A total of 222 ASF samples were investigated. Based on the culture and biochemical tests, 109 E. coli isolates were identified. Duplex-polymerase chain reaction assay was used to detect ETEC and EPEC. The target genes selected for each category were the lt and st for the ETEC, and eae and bfp for the EPEC isolates.
Results:
The occurrence of E. coli in dairy and meat products was 45 and 52.5 per cent, respectively. Among the E. coli isolates, two ETEC, one typical EPEC and three atypical EPEC were detected in meat samples, whereas only one typical EPEC and one atypical EPEC were detected in dairy samples.
Interpretation & conclusions:
Our results showed presence of ETEC and EPEC strains in ASFs. The milk without pasteurization and traditional dairy products produced in unhygienic conditions are most likely the main sources of E. coli pathotypes and other zoonotic pathogens and thus can be considered a potential hazard to the health of the community.
Keywords
Animal source foods
enteropathogenic Escherichia coli
enterotoxigenic Escherichia coli
food borne diseases
food hygiene
Diarrhoeal disease is a global problem, particularly in developing countries. Various infectious agents cause diarrhoea, such as rotaviruses, coronaviruses, Campylobacter spp., Clostridium perfringens, Escherichia coli and Salmonella species12. Among the bacterial pathogens, diarrhoeagenic E. coli (DEC) strains are the most common agents of diarrhoea, especially in developing countries3. Based on the virulence factors, DEC is classified into six groups: enterotoxigenic E. coli (ETEC), enteropathogenic E. coli (EPEC), enterohaemorrhagic E. coli (EHEC), enteroinvasive E. coli (EIEC), enteroaggregative E. coli (EAEC) and diffusely adherent E. coli (DAEC)14. ETEC is a major cause of childhood diarrhoea in developing countries and in people who travel from industrialized countries to native regions567. The virulence factors of these bacteria are heat-stable and heat-labile enterotoxins that are encoded by the st and lt genes6. Another important pathotype of the species is EPEC, which is the primary cause of diarrhoea in infants, especially in developing countries. EPEC strains exist in two forms: typical EPEC or tEPEC (having eae and bfp genes) and atypical EPEC or aEPEC (lacking bfp gene)58.
Because the isolation of E. coli was easier than that of other enteric pathogens, it could be used as an indicator of faecal contamination and other faecal-origin microorganisms in food materials9. Ruminants, especially calves, are identified as a major source of pathogenic E. coli10. Food products of animal origin, such as fresh meat and raw milk, are considered as common carriers of E. coli. Meat and meat products may be contaminated in several ways, such as through direct contact with faeces or hides during slaughter11. Moreover, raw milk and milk products could be rich nutritious medium for many microorganisms. Poor sanitation during collection or storage can also cause contamination. Pathogenic E. coli has been identified as a major cause of foodborne diseases, because animal source foods (ASFs) are constituents of human diet12. Hence, it becomes necessary to determine the occurrence of E. coli in ASFs through a reliable and quick test. The aim of this study was to identify ETEC and EPEC as two common pathotypes of E. coli in raw meat and dairy products through duplex-polymerase chain reaction (PCR).
Material & Methods
This study was conducted from May to September 2016 in the laboratory of Microbiology, Food and Drug Research Center, Tabriz University of Medical Sciences, Tabriz, Iran. A total of 120 samples of meat products (fresh beef, ground beef and hamburger) and 102 dairy products (raw milk, traditional cheese, Doogh and yoghurt) were collected from markets in different localities of Northwest Iran. All specimens were immediately transferred to the laboratory under cold chain and sterile conditions.
Bacterial isolation: For the enrichment of the E. coli strains, all specimens were cultured in lauryl sulphate broth (Merck, Germany) overnight at 37°C and subsequently were streaked onto MacConkey agar (Merck, Germany) and incubated at 37°C for 24 h. The lactose-fermenting colonies were identified as E. coli using Gram staining and conventional biochemical tests as described previously13. Finally, E. coli isolates were stored at −80°C in trypticase soy broth supplemented with 20 per cent glycerol for further procedures.
DNA extraction: DNA extraction from overnight cultures was done using the Promega DNA extraction kit (A11125, USA), following the instructions given by the manufacturer.
Polymerase chain reaction assays: The DNA templates were examined by two separate duplex PCRs with specific primers (Table I). Duplex-PCR 1 and 2 were for the identification of lt and st genes (for detection ETEC) and eae and bfp genes (for detection EPEC), respectively141516. Both duplex-PCR assays were accomplished in a 25 μl reaction mixture, consisting of 2× PCR master mix [2× concentrated solutions of Taq DNA polymerase, reaction buffer, MgCl2 and dNTPs (CinnaGen Inc., Iran)] with a BioRad T100™ thermal cycler (Bio-Rad Laboratories, Inc., USA). The PCR Master Mix (CinnaGen Inc., Iran) contains all components for PCR, except DNA template and primers. Primers were provided by GeNetBio Inc., Korea. The first duplex-PCR conditions included an initial denaturation at 95°C for five minutes for one cycle followed by 35 cycles of 95°C for 45 sec, 49°C for 45 sec, 72°C for 45 sec and final extension at 72°C for seven minutes. The second duplex-PCR conditions comprised: An initial denaturation at 95°C for three minutes for one cycle followed by 38 cycles of 95°C for one minute, 53°C for one minute, 72°C for one minute and final extension at 72°C for 10 minutes. Amplified PCR products were observed after electrophoresis on one per cent agarose and staining with Safe Dye (CinnaGen Inc., Iran). The PCR products were visualized under ultraviolet UV transilluminator and photographed.
| Target organism | Target genes | Gene location | Primer sequences (5’→3’) | Product size (bp) | Reference |
|---|---|---|---|---|---|
| ETEC | st | Plasmid | F: ATTTTTMTTTCTGTATTRTCTT | 190 | 15 |
| R: CACCCGGTACARGCAGGATT | |||||
| lt | Plasmid | F: GGCGACAGATTATACCGTGC | 450 | 15 | |
| R: CGGTCTCTATATTCCCTGTT | |||||
| EPEC | eae | Chromosome | F: AGGCTTCGTCACAGTTG | 570 | 14 |
| R: CCATCGTCACCAGAGGA | |||||
| bfp | Chromosome | F: AATGGTGCTTGCGCTTGCTGC | 326 | 16 | |
| R: GCCGCTTTATCCAACCTGGTA |
Reference strains: The following standard strains were employed as positive controls: ETEC (H10407) and EPEC (2348/69). These strains were provided by R. Vidal, Microbiology and Mycology Program, Institute of Biomedical Sciences, Faculty of Medicine, University of Chile, Santiago, Chile.
Results
Among the 222 samples (120 meat samples, 102 diary samples) examined in this study, 109 (49%) E. coli isolates were obtained [63 (57.7%) from meat products and 46 (42.2%) from dairy products]. The ground beef and hamburger had the highest occurrence of contamination, followed by fresh beef. About dairy products, all of the E. coli isolates belonged to raw milk and traditional cheese samples. No E. coli isolate was identified in fermented dairy products i.e., Doogh, yoghurt.
Occurrence of ETEC and EPEC strains in meat and dairy products: Among E. coli isolates, two ETEC and four EPEC (1 typical EPEC and 3 atypical EPEC) were detected in meat samples while no cases of ETEC and only two EPEC (1 typical EPEC and 1 atypical EPEC) were identified in dairy samples. The distribution of virulence target genes is shown in Table II. Fifty per cent (4 of 8 isolates) of the pathogenic E. coli isolates harboured both eae and bfp genes and were considered as tETEC. Furthermore, no st and lt genes were identified in dairy products (Figs 1 & 2).
| Virulence gene(s) | E. coli strains with indicated virulence genes | ||
|---|---|---|---|
| Raw milk products | Raw meat products | Total | |
| st | 0 | 1 | 1 |
| lt | 0 | 0 | 0 |
| st+lt | 0 | 1 | 1 |
| eae | 1 | 1 | 2 |
| eae+bfp | 1 | 3 | 4 |
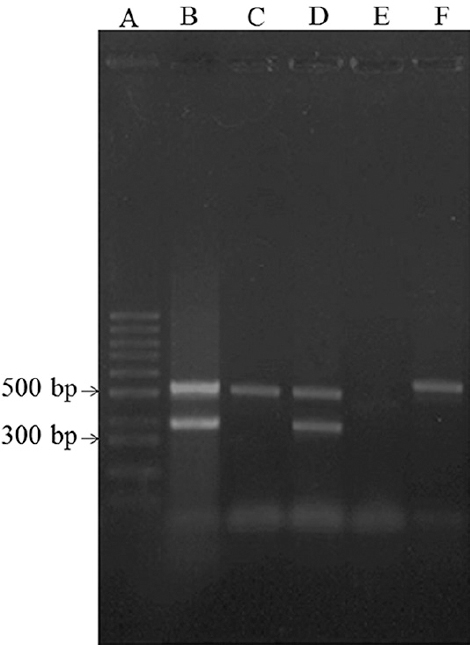
- (A) Ladder 1000 bp, (B) positive control for eae and bfp genes, (C) positive isolate for eae gene, (D) positive isolate for eae and bfp genes, (E) negative control, (F) positive isolate for eae gene.
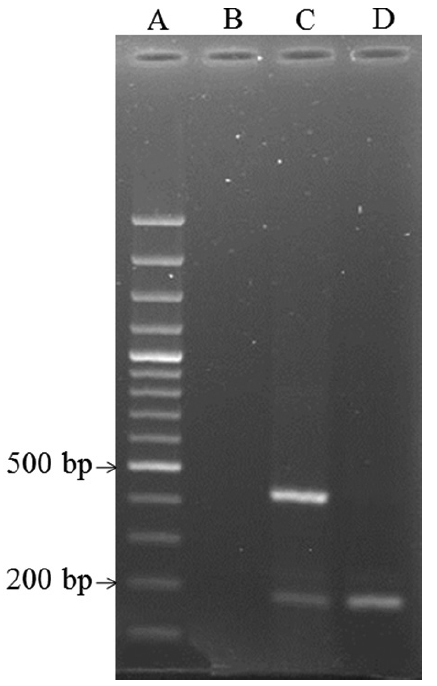
- (A) Ladder 1000 bp, (B) negative control, (C) positive isolate for lt and st genes, (D) positive isolate for st gene.
Discussion
Diarrhoea caused by different intestinal pathogens is one of the major concerns of public health. An important bacterial agent of diarrhoea is E. coli, which causes death among under five children and the elderly17. Animal food products, especially raw meat and dairy products that are rich in nutritional value are likely to get contaminated with spoilage and pathogenic bacteria, resulting in the transmission of infections to humans12. Therefore, use of rapid diagnostic techniques is important in reducing disease development rate and decreasing the financial burden of the disease on the community health2.
In the present study, the occurrence of E. coli was 42.2 and 57.7 per cent in raw milk/raw milk products and raw meat/raw meat products, respectively. In a similar study carried out by Rúgeles et al16, E. coli was isolated from 58 per cent of the meat samples. Badri et al18 reported the prevalence of E. coli in meat samples as 48 per cent. Several other studies have reported the presence of E. coli in raw milk and meat products12192021. It should be noted that in the studied dairy samples, no E. coli isolate was identified in Doogh and yoghurt samples. It could be due to acidic pH and high temperatures created during their fermentation process22. Moreover, majority of E. coli identified in meat products were isolated from ground meat and hamburger. It is probable, these products are more contaminated due to poor sanitation of grinder and high manipulation in their production process23. According to the results of the duplex-PCR of isolated E. coli, only two EPEC were found in raw milk and raw milk products, whereas two ETEC and four EPEC were identified in raw meat products. In similar studies518, no case of ETEC was found in raw meat products, while EPEC (mostly atypical) was the most isolated pathotype, thereby confirming the results of the present study. Mohammed11 showed that 15.63 per cent E. coli isolated from meat products were ETEC, which was higher than that of several previous studies. Holko et al24, reported 2.1 per cent ETEC and 3.09 per cent EPEC in traditional cheese samples made from sheep milk. A study conducted on raw milk materials, found one ETEC and 13 EPEC5. In another study on 206 samples of raw milk, 17 EPEC (8.25%) were identified.
In conclusion, our results showed that the use of unpasteurized milk in traditional dairy products and inadequate sanitation during the preparation, transport and/or storage of ASFs and personal hygiene could be a potential factor in the spread of E. coli pathotypes and other zoonotic pathogens in the community should be considered and as a threat to public health.
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- Prevalence of diarrhoeagenic Escherichia coli in children from León, Nicaragua. J Med Microbiol. 2009;58:630-7.
- [Google Scholar]
- Detection of diarrhoeagenic Escherichia coli from children with and without diarrhea in Salvador, Bahia, Brazil. Mem Inst Oswaldo Cruz. 2007;102:839-44.
- [Google Scholar]
- Detection and characterization of diarrhoeagenic Escherichia coli from young children in Hanoi, Vietnam. J Clin Microbiol. 2005;43:755-60.
- [Google Scholar]
- Novel multiplex PCR for detection of diarrhoeagenic Escherichia coli strains isolated from stool and water samples. Genet Mol Res. 2017;16 gmr16039760
- [Google Scholar]
- Prevalence and antibiotic resistance profiles of diarrhoeagenic Escherichia coli strains isolated from food items in Northwestern Mexico. Int J Food Microbiol. 2013;164:36-45.
- [Google Scholar]
- Enterotoxigenic Escherichia coli in developing countries: Epidemiology, microbiology, clinical features, treatment, and prevention. Clin Microbiol Rev. 2005;18:465-83.
- [Google Scholar]
- Expression of Escherichia coli heat-labile enterotoxin B subunit (LTB) in Saccharomyces cerevisiae. J Microbiol. 2005;43:354-60.
- [Google Scholar]
- Epidemiology of Escherichia coli O157:H7 outbreaks, United States, 1982-2002. Emerg Infect Dis. 2005;11:603-9.
- [Google Scholar]
- The distribution of Escherichia coli serovars, virulence genes, gene association and combinations and virulence genes encoding serotypes in pathogenic E. coli recovered from diarrhoeic calves, sheep and goat. Transbound Emerg Dis. 2013;60:69-78.
- [Google Scholar]
- Molecular characterization of diarrhoeagenic Escherichia coli isolated from meat products sold at Mansoura city, Egypt. Food Control. 2012;25:159-64.
- [Google Scholar]
- Bacterial quality of raw milk investigated by Escherichia coli and isolates analysis for specific virulence-gene markers. Food Control. 2009;20:913-7.
- [Google Scholar]
- Bailey & Scott's diagnostic microbiology (13th ed). St. Louis: American Society for Clinical Pathology; 2015.
- Phylogenetic of Shiga toxin-producing Escherichia coli and a typical enteropathogenic Escherichia coli strains isolated from human and cattle in Kerman, Iran. Int J Enteric Pathog. 2014;2:1-5.
- [Google Scholar]
- Evaluation of multiplex PCRs for diagnosis of infection with diarrheagenic Escherichia coli and Shigella spp. J Clin Microbiol. 2004;42:5849-53.
- [Google Scholar]
- Molecular characterization of diarrhoeagenic Escherichia coli strains from stools samples and food products in Colombia. Int J Food Microbiol. 2010;138:282-6.
- [Google Scholar]
- Pathotypes of diarrhoeagenic Escherichia coli in children attending a tertiary care hospital in South India. Diagn Microbiol Infect Dis. 2010;68:117-22.
- [Google Scholar]
- Prevalence of virulence genes in Escherichia coli isolated from food in Casablanca (Morocco) Food Control. 2009;20:560-4.
- [Google Scholar]
- Bacteriological quality of raw cow milk in Shahrekord, Iran. Vet World. 2014;7:240-3.
- [Google Scholar]
- Molecular characterization and antibiotic resistance of enterotoxigenic and entero-aggregative Escherichia coli isolated from raw milk and unpasteurized cheeses. Vet Res Forum. 2014;5:29-34.
- [Google Scholar]
- Occurrence of toxigenic Escherichia coli in raw milk cheese in Brazil. Arquivo Bras Med Vet Zootec. 2007;59:508-12.
- [Google Scholar]
- Virulence factors, serogroups and antimicrobial resistance properties of Escherichia coli strains in fermented dairy products. BMC Res Notes. 2014;7:217.
- [Google Scholar]
- Distribution patterns of Escherichia coli O157:H7 in ground beef produced by a laboratory-scale grinder. J Food Prot. 2002;65:1894-902.
- [Google Scholar]
- Virulence markers of Escherichia coli strains isolated from traditional cheeses made from unpasteurised sheep milk in Slovakia. Food Control. 2006;17:393-6.
- [Google Scholar]






