Translate this page into:
Stem cell therapy approaches for non-malignant diseases & non-haematological diseases in India: A systematic review
For correspondence: Dr Suman Ray, Department of Inclusive Health, CSIR-National Institute of Science Communication and Policy Research, Delhi 110 012, India e-mail: sumanitrc@gmail.com
-
Received: ,
Accepted: ,
Abstract
Background & objectives
Our study aims to provide the diversity of stem cell use for non-malignant, non-haematological diseases in India through the lens of clinical trials.
Methods
A PRISMA approach was used to evaluate the safety and efficacy of stem cell use for the period 2001-2021 in India. The outcomes were measured using each disease category, types of stem cells, the origin of stem cells, safety, and efficacy.
Results
Of the 9206 studies screened, 61 studies that were relevant to stem cell use for non-malignant diseases were included for analysis. Autologous stem cells (75%) were used predominantly compared to allogenic stem cells (18.33%), followed by mixed type (6.67%). Use of bone marrow-derived stem cells (51%) was dominant, followed by melanocytes (19%), adipose (7%), haematopoietic (12%), and (11%) other types of stem cells. The study revealed 37 randomized clinical trial studies conducted in the government research hospital compared to the non-government.
Interpretation & conclusions
Maintaining the gold standard for stem cell therapy requires randomized clinical trials with large sample sizes, control groups, failures, adverse effects, etc. It is important to have a monitoring and regulation system in stem cell clinical research activities with enough preclinical data and repeated exchanges between the bench and the bedside.
Keywords
Allogeneic stem cells
autologous stem cells
clinical outcomes
efficacy
non-malignant disease
safety
stem cell therapy
The World Health Organization (WHO) has expressed serious concerns regarding the increasing incidence of non-communicable diseases (NCDs) worldwide. WHO coordinates with each country to prevent and control NCDs through their leadership. However, WHO set the 2030 Agenda for Sustainable Development Goals (SDG target 3.4) to prevent and control NCDs1 An estimated 41 million people die due to non-communicable diseases each year1. Cardiovascular diseases, cancers, respiratory diseases, and diabetes; these four groups are majorly responsible for premature death1. India is now a major hub for NCDs2. To reduce the burden of NCDs, the Indian government has implemented the National Programme for Prevention and Control of Cancer, Diabetes, Cardiovascular Diseases and Stroke (NPCDCS)3. However, these efforts are limited to prevention and control, not the cure.
Stem cell therapy is an important branch of multidisciplinary regenerative medicine that primarily focuses on repairing, regenerating, or rejuvenating the body function4,5. Stem cell therapy is a new hope for individuals suffering from NCDs. This includes malignant and non-malignant diseases, and the treatment plan aims for cure. Because stem cells are defined by their uniqueness of self-renewal and differentiation, their therapeutic potential has been proven in basic research, and their utility in clinical settings is being explored. For example, the treatments of spinal cord injury, heart failure, retinal and macular degeneration, and type 1 diabetes have shown promising results as injecting stem cells at the target may help in reverting to normal functioning4-6, but large clinical trials on these are still lacking so far. However, the emergence of ‘unproven stem cell therapy’ through unauthorized clinics that claim the importance of stem cell therapy as ‘magic cells or snake oil’ has raised concern regarding the safety and efficacy of stem cell therapy7,8. Moreover, various adverse effects of stem cell injection have been noted historically9. For example, during the treatment of macular degeneration, patients lose their vision10. Thus, we need more studies on the mechanisms of action, toxicological studies, and standardization and characterization of transplanted cells11,12.
For the promotion and regulation of stem cell therapy, the Indian Council for Medical Research (ICMR) and the Department of Biotechnology (DBT) initially released the National Guidelines for Stem Cell Research and Therapy in 200713. It was subsequently modified in 2013 and 2017. The guidelines were renamed as National Guidelines for Stem Cell Research (NGSCR) in 2013 by removing the word therapy and was retained as is in 2017. The NGSCR 2017 is comprehensive and continued to emphasize on consideration of stem cell-based therapy as a drug indicated in NGSCR 201314,15. Therefore, needs to go through rigorous clinical trial procedures. This guideline also provides a list of approved indications where there is no perceived need for clinical trials, and it mainly includes nearly all haematological diseases, whether malignant or not. Additionally, more comprehensive guidelines for haematological diseases are mentioned in the National Guidelines for Hematopoietic Cell Transplantation (NGHCT) 2021, released by ICMR16. Considering stem cells as a drug in NGSCR 2017 was not effective, hence the New Drugs and Clinical Trials Rules (NDCTR), 2019 implemented. Since then legal provisions have been made available for stem cells as a drug. It is hence now necessary to check the status of stem cell therapy based on the outcome of clinical trials17.
The treatment of haematological diseases using allogeneic or autologous bone marrow/blood stem cell transplantation is already established as part of medical treatment through historical development18-21. However, stem cell treatments for non-malignant and non-haematological diseases have not yet been established, and current progress is unknown in the Indian context. Hence, the authors in this study have done a comprehensive systematic analysis to study the outcomes of the clinical trials using stem cell therapy for non-malignant diseases and non-haematological diseases in India.
Materials & Methods
A systematic review was undertaken as per the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) checklist22. This review focused on previously published studies from India. Figure 1 describes the PRISMA diagram for search strategy and study selection.
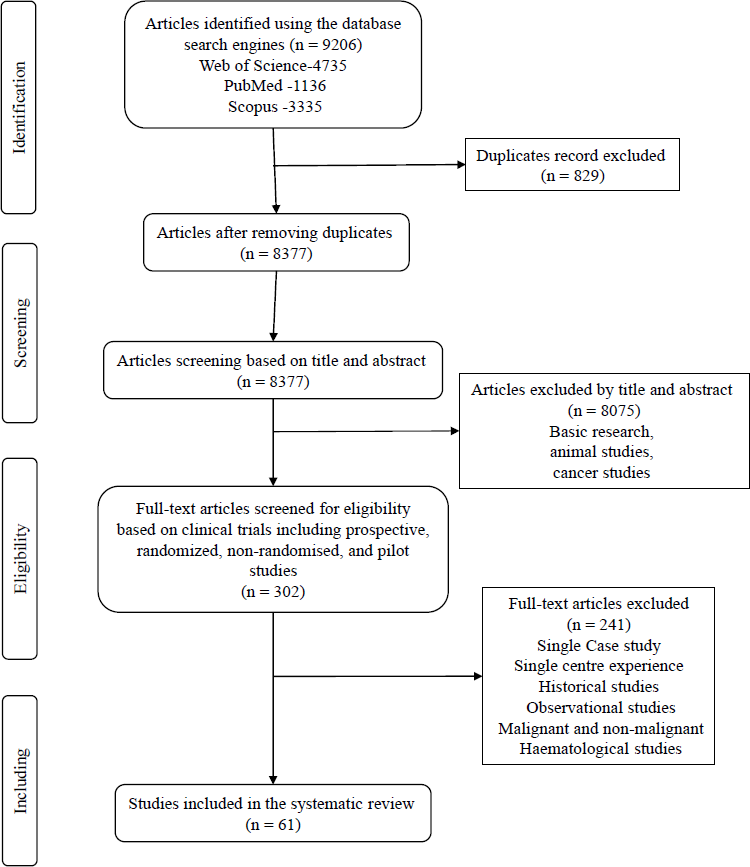
- PRISMA diagram for search strategy and study selection.
Search strategy
We performed systematic searches with language restriction (only English) using PubMed, Web of Science, and Scopus databases. The period chosen was between 2001 and 2021, and the search was performed on January 31, 2022. The main keywords were ‘stem cell therapy’, ‘clinical trials’ and ‘India’. These keywords and their allied keywords were used for data extraction from the above databases. For example, on the Web of Science, we used search strings as (Stem OR cell OR cells) AND (Therapy OR treatment OR cure OR intervention OR therapeutic) AND (Clinical) (Topic) and INDIA (Countries/Regions) and Article (Document Types) and English (Languages). This search string was modified for Scopus and PubMed databases.
Eligibility criteria
Research articles included were related to prospective, randomized, non-randomized controlled trials and other uncontrolled clinical trials, including single-arm trials that examined the safety and efficacy of stem cell therapy in Indian adults or mixed adult and paediatric participants. Full-text articles that were available in the chosen databases were included.
Exclusion criteria
We excluded all animal studies in the first step, and malignant diseases were excluded in the next step. Non-malignant haematological diseases were also excluded because NGSCR 2017 exempted these. Single case reports, observational studies, and single-centre experience studies were excluded.
Screening and article selection
The first and second authors independently screened the selected studies and extracted the data using a standardized form. Doubts and discrepancies were fixed by discussing with the third author.
Search results
With screening and applying exclusion criteria, 8075 articles were excluded, and the remaining 302 articles were considered for full-text selection. Of the total (n=9206) articles screened, 61 articles were included for further analysis (Fig. 1).
Articles particularly related to clinical trials, including prospective, randomized, non-randomized, and pilot studies were included. Of these, single case studies, single centre experiences, historical studies, observational studies, cancer or malignancies, and haematological diseases were all excluded. Among the 61 studies, finally five were included for further analysis recorded before, and 56 were recorded after the release of the National Guidelines for Stem Cell Research and Therapy 2007.
For the purpose of discussion, the studies were sorted on the basis of organ-specificity and disease characteristics (Table)23-83. This included: dental (1 article having 15 participants), diabetes (7 articles having 153), eye (4 articles having 24 participants), heart (9 articles having 484 participants), kidney (4 articles having 539 participants), liver (2 articles 80 participants), neurological (15 articles having 794 participants), musculoskeletal (4 articles having 156 participants) and skin (15 articles 482 participants). Table I describes the main characteristics and outcomes of stem cell therapy (SCT) use for non-malignant non-haematological diseases in India based on this systematic review analysis.
| Author name | Disease | Category | Total sample | Age (yr) | Follow up (months) | Purpose of study | Cell origin | Types of cells | Outcome | Side effects |
|---|---|---|---|---|---|---|---|---|---|---|
| U et al23, 2019 | Cystic maxillofacial bony defects | Dental | 15 | . | 6 | Evaluate the role of BMA in regenerating new bone | Autologous | Bone marrow-derived Stem Cells | 1) Bone defect volume reduction was statistically significant; 2) No tooth mobility; 3) Faster wound healing | . |
| Bhansali et al24, 2009 | T2DM | Diabetes | 10 | . | 6 | Efficacy of Autologous Bone Marrow–Derived Stem Cell | Autologous | Bone Marrow-derived Stem Cells | 1) Insulin requirements reduced; 2) c-peptide stimulated | 1) Self-limiting nausea; 2) Vomiting; 3) Hematoma |
| Vanikar et al25, 2010 | T1DM | Diabetes | 11 | 13 to 43 | 12 | Efficacy and safety of combined | Mixed | Adipose tissue-derived insulin-secreting mesenchymal stem cells (IS-AD-MSC) and cultured bone marrow (CBM) | 1) c-peptide assay- increased gradually; 2) Insulin requirement decreased; 3) HBA2c level - decreased; 4) GAD antibodies - decrease in some and others not | . |
| Dave et al26, 2015 | T1DM | Diabetes | 10 | 8 to 45 | 31.71 | Safety and efficacy | Mixed | Adipose tissue-derived MSC-differentiated insulin-secreting cells (ISC) with hematopoietic stem cells (HSC). | 1) c-peptide level increased; 2) HbA1c improved; 3) Insulin requirement reduced; 4) GAD ab - positive | No untoward effect |
| Thakkar et al27, 2015 | T1DM | Diabetes | 20 | 8 to 45 | 12 | Compare & assess - safety & efficacy | Mixed | Adipose-derived MSC and Bone marrow-derived HSC | 1) Insulin requirement reduced; 2) HBA1c reduced; 3) GAD antibody decreased; 4) Autologous SCT improved better than allogenic SCT for C-peptide | No untoward effect, morbidity (pulmonary embolism, sepsis) or mortality |
| Thakkar et al28, 2016 | T1DM | Diabetes | 20 | 8 to 45 | 27+ | Efficacy and safety of coinfusion | Mixed | Adipose tissue-derived insulin-secreting mesenchymal stem cells and bone marrow-derived hematopoietic stem cells | 1) Mean GAD antibody - decreased 2) Mean insulin requirement decreased 3) absence of DKA episodes in all 4) c-peptide level - increased | . |
| Sood et al29, 2017 | T2DM | Diabetes | 42 | 30 to 70 | 6 | To find out optimal routes for deliveryof stem cells | Autologous | Bone Marrow-derived Mononuclear Cells | 1) C-peptide assay - difference remained statistically non-significant across all groups; 2) Insulin sensitivity indices of HOMA IR and HOMA B did not show any significant differences; 3) Decrease in Insulin dosages except for peripheral intravenous route; 4) HbA1c - non-significant change | . |
| Bhansali et al30, 2017 | T2DM | Diabetes | 40 | 30 to 60 | 12 | Efficacy and safety of ABM-MSCs and ABM-MNCs transplantation | Autologous | Bone marrow-derived mesenchymal stem cells and mononuclear cells | 1) Insulin requirement reduction; 2) HbA1c reduction; 3) Improvement in c-peptide response; 4) Insulin sensitivity also improved | . |
| Sangwan et al31, 2012 | Unilateral limbal stem cell deficiency | Eye | . | . | . | Novel simplified technique of limbal transplantation | Autologous | Limbal epithelial cells | Epithelialised, avascular and stable corneal surface | . |
| Sharma et al32, 2013 | Total limbal stem cell deficiency | Eye | 4 | 8 - 12 | 26 | Clinical outcome with the phenotype of rejuvenated corneal epithelium | Autologous | Limbal epithelial cell (cultured) | 1) Epithelial transparency increased; 2) Reduction or absence of corneal vascularization and conjunctivalization; 3) No sign of signs of recurrent LSCD; 4) ocular the surface remained stable and visual acuity improved | . |
| Titiyal et al33, 2015 | Ocular burns | Eye | 20 | . | 6 | Outcomes of live-related limbal allograft (Lr-CLAL) versus cadaveric keratolimbal allograft (KLAL) in limbal stem cell deficiency (LSCD) | Allogenic | Limbal stem cell (live and cadaveric) | Lr-CLAL shows better results than KLAL regarding vision gain and ocular surface restoration. | . |
| Kaliki et al34, 2017 | Ocular surface squamous neoplasia | eye | 8 | . | 12 | Compare the surgical outcomes with and without p-SLET | Autologous | Limbal epithelial cells | None of them developed LSCD or tumor recurrence | . |
| Kaparthi et al35, 2008 | Dilated cardiomyopathy | Heart | 5 | 20-65 | . | Safety and efficacy | Autologous | Bone marrow-derived mononuclear cells | 1) Procedure was safe; 2) improved myocardial contractility and LV function | . |
| Guhathakurta et al36, 2009 | Cellular Cardiomyoplasty | Heart | 40 | . | 6 | Safety of protocol | Mixed | Bone marrow mononuclear cells and peripheral blood-derived endothelial precursor cells | Marginal improvement in myocardial function | . |
| Prasad et al37, 2012 | Stroke | Heart | 11 | 30 to 70 | 12 | Feasibility, safety and clinical outcome | Autologous | Bone marrow-derived mononuclear cells | 1) Study was feasible; 2) Safe - no evidence of tumour formation; 3) All scores - statistically significant | . |
| Bhasin et al38, 2013 | Stroke | Heart | 40 | 18-65 | 6 | Safety, feasibility and efficacy | Autologous | Bone marrow-derived mesenchymal stem cells and mononuclear cells | Only the modified Barthel Index was statistically significant | . |
| Prasad et al39, 2014 | Ischemic Stroke | Heart | 58 | 18 to 75 | 6 | Efficacy and safety of autologous BMSCs | Autologous | Bone marrow-derived mononuclear cells | No beneficial effect of treatment on stroke outcome | . |
| Chullikana et al40, 2015 | Acute myocardial infarction | Heart | 20 | . | 24 | Safety and efficacy of intravenous administration | Allogenic | Bone marrow-derived mesenchymal stem cell | Not significant outcomes compared with placebo | 1) 39 treatment-emergent adverse events; 2) SASEs - ventricular tachycardia, pericardial effusion and AMI |
| Nair et al41, 2015 | Acute myocardial infarction | Heart | 250 | 20-65 | 6 | Efficacy of stem cells in the improvement of left ventricular function | Autologous | Bone marrow-derived mononuclear cells | 1) Improvement was not significant; 2) Cell dose more than 5x10^8 shows positive impact | 1) Chest pain, dyspnoea and other symptoms; 2) One died due to acute stent thrombosis with acute LV failure |
| Patel et al42, 2015 | Ischemic heart failure or non-ischemic heart failure | Heart | 60 | . | 12 | Safety and feasibility | Autologous | Bone marrow-derived mononuclear cells | Not powered to demonstrate statistical significance | 1) Elevated troponin levels; 2) Catheterization site hematomas; 3) Bleeding at the marrow aspiration site; 4) Pain at the aspiration site; 5) Congestive heart failure exacerbation requiring hospital admission; 6) Ventricular arrhythmia; 7) Hematomas at the catheterization site and elevatedserum creatinine |
| Bhatia et al43, 2018 | Subacute Ischemic Stroke | Heart | . | . | 6 | Evaluate the Safety and the efficacy of intra-arterial infusion | Autologous | Bone marrow-derived mononuclear cells | 1) Good clinical outcomes; 2) modified Rankin Scale score also improved | . |
| Trivedi et al44, 2002 | Pediatric renal transplant | Kidney | 44 | . | 18 | To achieve zero-rejection status in pediatric renal allograft recipients, | Allogenic | Peripheral blood stem cell | 1) 100% graft survival with sustained low serum creatinine value; 2) Absence of graft vs. host disease | 1) Appearance of CMV |
| Trivedi et al45, 2003 | Renal Allograft | Kidney | 43 | . | 12 | Tolerance in Living Related Renal Allografts | Allogenic | Bone Marrow-derived Stem Cells | 2) Better graft function but not statistically significant | 1) Single acute rejection; 2) Appearance of CMV disease; 3) Serum creatine not significant level; 4) No GVHD5) rise of donor-specifi cytotoxic allo-antibodies |
| Trivedi et al46, 2007 | Chronic kidney disease | Kidney | 357 | . | 36 | Induce tolerance against MHC barriers | Allogenic | Bone marrow (BM)-derived and peripheral blood stem cell (PBSC) | 1) Significantly better allograft function with low serum creatinine value | 1) Acute rejection episode; 2) Acute vascular plus tubulointerstitial rejection; 3) Systemic infections; 4) Patients died |
| Vanikar et al47, 2014 | end-stage renal disease | Kidney | 95 | . | 7 | Safety, efficacy and benefits | Mixed | Adipose-derived mesenchymal stem cells (AD-MSC) + hematopoietic stem cells (HSC) | 1) No side effects; 2) Survival rate is high; 3) safe and effectivestrategy for minimization of immunosuppression | . |
| Khan et al48, 2010 | Cirrhosis | Liver | 25 | . | . | Safety and efficacy of human fetal liver-derived stem cell | Allogenic | Human fetal liver-derived stem cell | 1) Decrease MELD score; 2) Improve clinical and biochemical parameters; 3) No episodes related to hepatic encephalopathy recurred | No other clinical complications were observed after follow up |
| Sharma et al49, 2015 | Liver cirrhosis | Liver | 55 | 18-70 | 3 | Effect of peripheral CD+ cells | Autologous | Peripheral blood CD34+ cell | 1) Procedure was safe; 2) Statistically significant - improve live function; 3) helps to delay liver transplantation | . |
| Kumar et al50, 2009 | Spinal cord injury | Neuro | 297 | . | . | Safety and primary efficacy | Autologous | Bone marrow derived mononuclear cell | 1) One-third patients show perceptible improvements; 2) No correlation between level of injury and improvements; 3) Number of CD34+ cells injected has direct correlation to outcomes | In some - fever, Headache, Tingling sensation, Neuropathic sensory symptoms |
| Pal et al51, 2009 | Spinal cord injury | Neuro | 30 | . | 3 | Growth kinetics of BM MSC, safety and functional improvement | Autologous | Bone marrow-derived mesenchymal stem cell | 1) Protocol is safe; 2) uncontrolled nature of the trial does notpermit demonstration of the effectiveness | . |
| Venkataramana et al52, 2010 | Parkinson’s Disease | Neuro | 7 | . | 36 | Safety and feasibility of BM-MSCs | Autologous | Bone marrow-derived mesenchymal stem cell | 1) Improvements in the UPDRS scale; 2) H&Y and S&E score also improved; 3) PD medication reduced | . |
| Srivastava et al53, 2011 | Cerebral palsy | Neuro | 30 | 5-25 | 12 | To evaluate the feasibility, safety, therapeutic potential | Autologous | Bone marrow-derived mononuclear cells | 1) Twopatient - fever; 2) Protocol - Safe; 3) mBI score - significantly improved; 4) MRC, Ashworth scale - significantly improved | . |
| Bhanot et al54, 2011 | Spinal cord injury | Neuro | 13 | 18-51 | 12 | Safety and efficacy of | Autologous | Bone marrow-derived mesenchymal stem cells | Only few patients shows improvement | 1) 50% patients reported - a transient increase in spasticity; 2) In some - Fever, vomiting, general body ache, tingling/burning girdle sensation |
| Sharma et al55, 2012 | Muscular dystrophy, spinal cord injury, cerebral palsy, and miscellaneous | Neuro | 71 | . | 15 | Outcomes of autologous stem cell therapy | Autologous | Bone marrow derived mononuclear cell | Shows improvements and also improves quality of life | No adverse event |
| Venkataramana et al56, 2012 | Parkinson’s Disease | Neuro | 12 | 37–69 | 12 | Safety, feasibility, and efficacy of allogenic | Allogenic | Bone marrow-derived mesenchymal stem cells | Subjective improvement observedreported clarity in speech, reduction in tremors, rigidity, and freezing attacks | . |
| Aggarwal et al5,7, 2012 | Posttraumatic Facial Nerve Paralysis | Neuro | 8 | 18-60 | 6 | Safety profile and role | Autologous | Bone marrow-derived mononuclear cells | 1) Significant improvement in ENoG amplitude; 2) statistically significant both for eyeclosure and for deviation of angle of mouth | . |
| Sharma et al58, 2013 | Autism | Neuro | 32 | 3-33 | 26 | Safety, efficacy, and clinical effects | Autologous | Bone marrow-derived mononuclear cells | 1) Statistically significant in CGI-I score and total ISAA score; 2) Not significant in FIM score and Wee-FIM scores; 3) CGI-II scale - global improvement | 1) Seizures after therapy controlled using antiepileptic drugs; 2) In some - headache, nausea, vomiting backache, pain at the site of injection, aspiration; 3) Increase in hyperactivity at minimal and persistent level but not interfere with the global clinical improvement |
| Sharma et al59, 2013 | Muscular dystrophy | Neuro | 150 | 2.11-8 | Mean 12 | Safety and Efficacy | Autologous | Bone marrow-derived mononuclear cells | Neurological improvements in trunk muscle strength, limb strength | No adverse events |
| Sharma et al60, 2015 | Cerebral palsy | Neuro | 40 | 17 months-22 yr | 6 | to evaluate the efficacy | Autologous | Bone marrow-derived mononuclear cells | 95% of patients showed improvements | 1) The beneficial effect of MNC (stem cell instillation) onhip survival. Spinal headache, nausea, vomiting, pain at the site of injection, suffered diarrhoea |
| Rajput et al61, 2015 | Muscular Dystrophy, Duchenne | Neuro | 11 | . | 36 | Role in the cellular therapy | Allogenic | Human umbilical cord mesenchymal stem cells | 1) Provide muscle stability; 2) Provide muscle strength in the distal and proximal lower limb; 3) Stability in muscle function of other body parts | . |
| Sharma et al,62, 2015 | Traumatic Brain Injury | Neuro | 14 | 12-65 | 6 | To promote angiogenesis, axonal remodelling, neurogenesis and synaptogenesis | Autologous | Bone marrow-derived mononuclear cells | 1) Improvements - speech, trunk, upper limb activity, muscle tone, voluntary control, ambulation, gait pattern, posture, balance, psychological status, cognition, memory, Adls; 2) improved functional outcome and enhanced quality of life | Side effect noted - seizure |
| Chhabra et al63, 2016 | Spinal cord injury, acute | Neuro | 21 | . | 12 | The safety and feasibility | Autologous | Bone marrow-derived stem cells | 1) No significant adverse effects; 2) No significant improvements; 3) procedure is safe and feasible; 4) No efficacy demonstrated | . |
| Sharma et al64, 2018 | Intellectual disability | Neuro | 58 | 4-45 | . | Safety, efficacy and clinical effects of autologous bone marrow mononuclear cell | Autologous | Bone marrow-derived mononuclear cells | 1) Symptomatic improvements in the intervention the group showed after transplantation compared with rehabilitation | 1) No adverse events were recorded; 2) In some - Fever, headache, vomiting |
| Sen et al65, 2012 | Femoral Head Osteonecrosis | Skeletomuscular | 40 | . | . | Evaluates the early results of BMNC instillation into the femur head | Autologous | Bone marrow-derived mononuclear cells | 1) Statistically, significant differences in HHS and its domains (pain, function, deformity, and motion); 2) the beneficial effect of MNC on hip survival. | . |
| Gupta et al66,2013 | critical limb ischemia | Skeletomuscular | 20 | . | 24 | Safety and efficacy | Allogenic | Bone marrow derived mesenchymal stem cell | 1) Improvements - rest pain scores in both the arms | SAE - death but not related to stem cells |
| Gupta et al67, 2016 | osteoarthritis | Skeletomuscular | 60 | . | 12 | Safety and efficacy | Allogenic | Bone marrow mesenchymal stromal cells | 1) Trend towards improvement in subjective parameters; 2) Not statistically significant with placebo | Knee pain and swelling |
| Gupta et al68, 2017 | Critical limb ischemia (CLI) due to Buerger’s disease | Skeletomuscular | 36 | 38-42 | 24 | Efficacy and safety of i.m. injection of allogenic BMMSC | Allogenic | Bone marrow-derived mesenchymal stem cells | Benefit in both the primary endpoints (rest pain relief and ulcer healing) and most secondary endpoints (improvement in total walking distance, ankle brachial pressure index, and quality of life). | 1) Two deaths were reported; 2) administered allogeneic cells did not adversely alter the immunological and lymphocytic profile |
| Mulekar69, 2003 | Vitiligo | Skin | 122 | 12-70 | 12 | To evaluate the usefulness of epidermal cell transplantation | Autologous | Melanocyte-keratinocyte | 1) Excellent repigmentation; 2) Recurrence also observed | . |
| Tegta et al70, 2006 | Vitiligo | Skin | 20 | . | 3 | Efficacy of Autologous melanocyte | Autologous | Melanocyte | 210-250 cells/mm2 required for satisfactory repigmentation | . |
| Dash et al71, 2009 | Nonhealing Ulcers | Skin | 24 | . | 3 | Assess the efficacy and feasibility | Autologous | Bone marrow-derived mesenchymal stem cells and mononuclear cells | Significant improvement in pain-free walking distance and reduction in ulcer size | . |
| Mohanty et al72, 2011 | Vitiligo | Skin | 14 | . | . | To evaluate the efficacy of a novel surgical method | Autologous | Melanocyte | Greatly achieved repigmentation | . |
| Sahni et al73, 2011 | Vitiligo | Skin | 25 | . | . | Compare results of autologous melanocyte trnasplanation with saline and serum | Autologous | Melanocyte | 1) Own serum shows better results than saline; 2) Statistically significant DLQI score | 1) Mild adverse events; 2) Halo phenomenon and infection at site of injection;3) Hyperpigmentation; 4) scarring at the donor site |
| Budania et al74, 2012 | Vitiligo | Skin | 41 | . | 4 | Comparison of techniques | Autologous | Melanocyte | 1) Excellent re-pigmentation observed; 2) NCES better than SBEG | . |
| Singh et al75, 2013 | Vitiligo | Skin | 30 | . | 4 | Compare NCES and NCORSHFS | Autologous | Melanocyte | 1) Excellent repigmentation; 2) reduction in DLQI score; 3) Both Safe and effective; 4) NCES is superior to NCORSHFS | None any adverse events reported |
| Vinay et al76, 2015 | Vitiligo | Skin | 30 | . | 6 | Clinical characteristics and treatment variables | Autologous | Melanocytes and hair follicle stem cells | 1) Achieving optimum repigmentation; 2) a strong correlation between repigmentation at 24 week and number of melanocytes and HFSC transplanted; 3) absence of dermal inflammation | . |
| Donaparthi & Chopra,77, 2016 | Vitiligo | Skin | 11 | . | 6 | Comparative efficacy | Autologous | Melanocyte epidermal | 1) >90% repigmentation; 2) safe and effective method; 3) Smaller patches repigmented better than larger ones | . |
| Kumar et al78, 2018 | Vitiligo | Skin | 25 | . | 6 | Clinical Efficacy, Viability and cell compositions of suspension | Autologous | Melanocyte-keratinocyte | 1) More than 50% repigmentation; 2) More than 80% cell viability | Recipient site infection |
| Thakur et al79, 2018 | Vitiligo | Skin | 40 | . | 6 | Efficacy of transplantation of NCES and NDCS vs NCES | Autologous | Melanocyte-keratinocyte | 1) Combination of NCES and NDCS resulted in excellent response than NCES alone | 1) Mild hyperpigmentation or hypopigmentation; 2) Post surgery perilesional halo developed |
| Sahoo et al80, 2019 | localized facial volume loss | Skin | 10 | 15-24 | 6 | Safety and efficacy | Autologous | Dermal mesenchymal stem cells | 1) Improvement in dermal atrophy and lipoatrophy | 1) Erythema, edema and mild to moderate pain at the site of injection |
| Gupta et al81, 2019 | Vitiligo | Skin | 32 | 13-31 | 6 | Compare the two techniques | Autologous | Melanocyte-keratinocyte | 1) 49% repigmentation achieved; 2) No statistically significant between two techniques | Reported 1) Hyperpigmentation; 2) Scarring; 3) achromatic fissures; 4) reactivation of disease |
| Mrigpuri et al82, 2019 | Vitiligo | Skin | 30 | . | 4 | Efficacy of NCES | Autologous | Melanocyte-keratinocyte | 1) Good repigmentation | . |
| Gunaabalaji et al83, 2020 | Vitiligo | Skin | 20 | . | . | Comparison of efficacy | Autologous | Melanocyte hair follicle cell suspension and noncultured epidermal cell and | 1) ECS was better than HFCS in repigmentation of leukotrichia and vitiligo, although the difference was not statistically significant | . |
Outcome measure
The safety of the studies was measured based on the treatment protocol reported for stem cell therapy mentioned in these selected studies. This included obtaining stem cells from donors or patients, purification of stem cells (cultured or non-cultured), and injecting to the patients at the targeted site. The efficacy measured in the form of the outcome of the studies included clinical, biochemical, and behavioural parameters or the overall outcome of the study. Adverse events were recorded as: (i) no adverse events, (ii) mild or treatable adverse events, and (iii) serious adverse events, including death or malignancy.
Results
Overall, autologous stem cells (75%) were used dominantly for stem cell therapy as compared with allogenic stem cells (18.33%) followed by mixed type (6.67%). The bone marrow-derived stem cells (51%) were used prominently, followed by melanocytes (19%), adipose (7%), haematopoietic (12%), limbal (6%), dermal (2%), fetal liver (2%) and umbilical cord (1%) derived stem cells (Fig. 2).
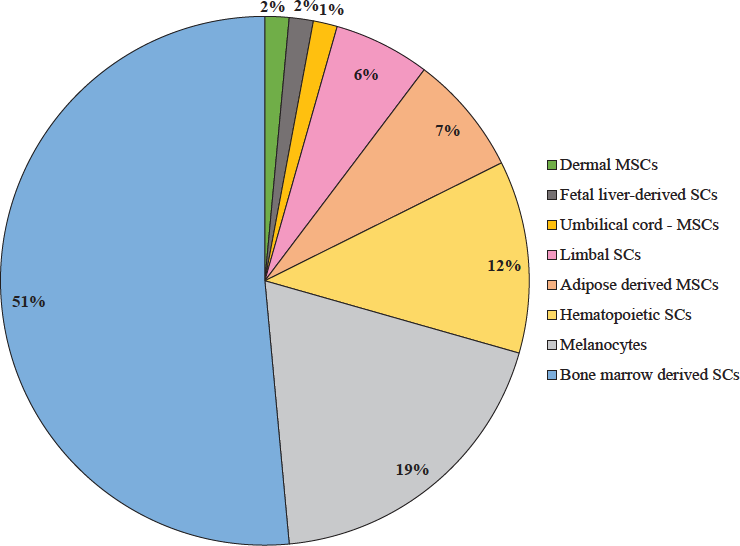
- Types of stem cells. MSCs, mesenchymal stem cells; SCs, stem cells.
Vitiligo (22%) emerged as the disease with a predominant use of stem cell therapeutics, followed by type 1 diabetes mellitus (8%), stroke (8%), spinal cord injury (8%), cerebral palsy (5%), muscular dystrophy (5%), type 2 diabetes mellitus (3%), limbal stem cell deficiency (3%), acute myocardial infarction (3%), cellular cardiomyoplasty (3%), kidney disease (3%), renal allograft (3%), cirrhosis (3%), Parkinson’s disease (3%), critical limb ischemia (3%), cystic maxillofacial bony defects (2%), autism (2%), ocular burns (2%), intellectual disability (2%), traumatic brain injury (2%), posttraumatic facial nerve paralysis (2%), femoral head osteonecrosis (2%), osteoarthritis (2%), facial volume loss (2%) and non-healing ulcers (2%). Figure 3 describes the use of stem cells for each disease category.
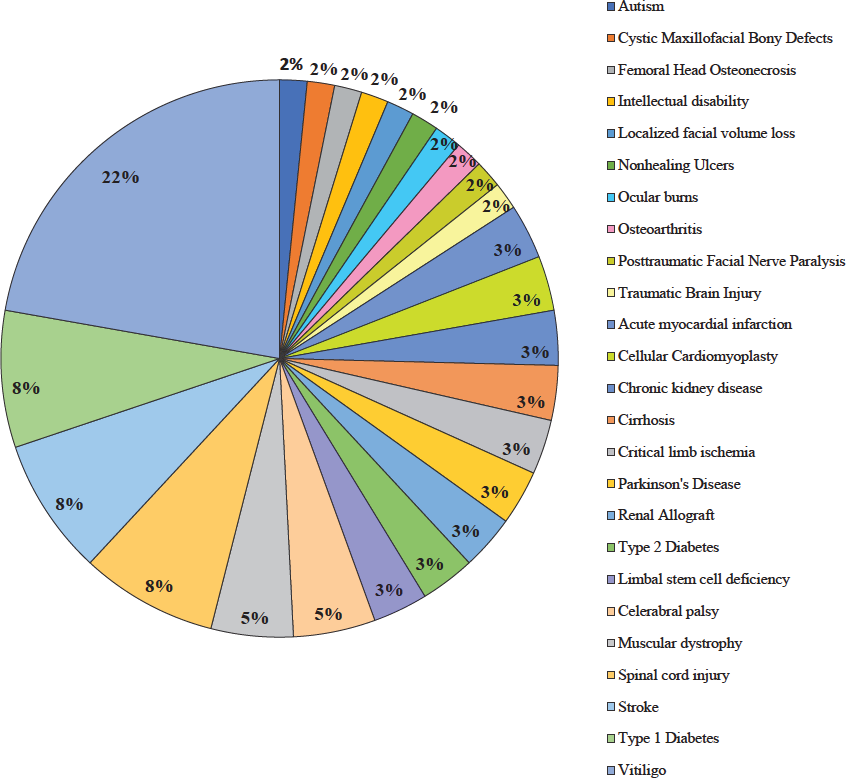
- Types of diseases treated using stem cells during clinical trials.
Lack of enough randomized clinical trials
There were lack of randomized clinical trials in the selected studies. Many studies were conducted without randomisation, had low sample sizes, and control groups were mostly absent (Table)23-83.
Status of selected clinical studies
Out of 61 selected studies, 37 studies reported clinical trials that were conducted in government research hospitals, and 24 clinical trials were conducted in non-government ones, based on the authors and their affiliated institutes mentioned in the research papers. However, only 16 studies mentioned the clinical trial registration number from the Clinical Trials Registry of India (CTRI number) or from clinicaltrials.gov. (National Clinical Trial (NCT) number). Of 61 selected studies, 56 mentioned approval or clearance from the Institutional Review Board, the Institutional Ethics Committee, or the Institutional Ethics Committee for Stem Cell Research (ICSCR).
Uses of stem cells for non-malignant diseases in terms of safety and efficacy
Stem cell therapy can be safe as a treatment protocol mentioned in the studies selected for this systematic review. The treatment protocol includes stem cell extraction from the patient or donor to reinjection of the stem cell aspirate at the specific site in the patient. During this procedure, selected studies did not mention any life-threatening adverse effects of this treatment protocol. However, few exceptions, such as infection or pain at the injection site or aspiration site, were reported32,43,49,79. The efficacy level of stem cell therapy varied according to disease type.
Skin diseases
Stem cell therapy for skin diseases like vitiligo, non-healing ulcers, and localized facial volume loss, etc., showed good safety and efficacy69-75. Though improvement in dermal atrophy and lipoatrophy was observed for localised facial volume loss, adverse effects like erythema, oedema, and pain at the injection site were also observed81. Non-healing ulcers treated using stem cells reported significant pain-free walking and reduced ulcer size71. In vitiligo, repigmentation was good, and percentages of repigmentation depended on the applied technique and cell concentration. Mild adverse events were also noted in a few studies, such as hyperpigmentation, halo phenomenon, infection at the site of injection, scarring at the donor site, achromatic fissures, and reactivation of vitiligo73,80-82.
Diabetes
Among diabetic trials, type 1 as well as type 2 diabetes mellitus, the use of stem cells showed increased c-peptide level, reduced HbA1c (haemoglobin A1c or glycated haemoglobin) level, and reduced insulin requirement, but GAD (glutamic acid decarboxylase) antibodies showed mixed results24-30. Bone marrow-derived stem cell transplantation type 2 diabetes mellitus patients did not show major complications. However, minor complications like nausea, vomiting, and hematoma were reported24. The type 1 diabetes showed significant outcomes in terms of reduction in insulin dose and HbA1c levels, and increased c-peptide levels24-27.
Dental diseases
In the dental field, the solitary trial outcome showed that the cystic maxillofacial defects were treated using autologous bone marrow-derived stem cells. After six months of followup, bone defect volume was reduced, tooth mobility was not observed, and faster wound healing was achieved23.
Eye diseases
Studies on eye diseases showed that limbal stem cell deficiency could be treated with limbal stem cells, but after a followup period, it showed increased epithelialized, avascular, stable corneal surface and visual acuity31-34. Live limbal stem cells showed better results in allogeneic transplantation than cadaveric limbal stem cells33.
Cardiovascular diseases
Stem cell therapy was used for stroke, myocardial infarction, cardiomyoplasty, cardiomyopathy, and heart failure35-43. For stroke, stem cell treatment showed improvement based on the Rankin scale and Barthel Index, which was statistically significant38. No significant outcomes were compared with the placebo; procedure-level safety was found, but serious adverse events were also noted37. The adverse events included ventricular tachycardia, pericardial effusion, chest pain dyspnoea, thrombosis, haematoma at the catheterization site, pain at the aspiration site, ventricular arrhythmia, and elevated serum creatinine level39-41.
Kidney-related diseases
Stem cell therapy has been tried for kidney-related diseases too in India, and it includes chronic kidney disease renal transplants, and end-stage renal disease44-47. This therapy helped renal transplantation to minimise the chances of graft rejection through allogenic stem cell therapy, and it was also used for minimizing immunosuppression47. The side effects include acute rejection, CMV disease appearance, and donor-specific cytotoxic alloantibodies45.
Liver disorders
The use of stem cell therapy for the treatment of liver cirrhosis has also been reported. Reports suggest its beneficial effect in improving liver function, improved clinical and biochemical parameters, and provided support to delay liver transplantation48,49.
Neurological diseases
Stem cell therapy has been used for neurological diseases such as facial nerve paralysis, spinal cord injury, autism, cerebral palsy, traumatic brain injury, intellectual disability, Parkinson’s disease, muscular dystrophy, and the outcomes showed mixed results50-64.
For Autism, stem cell therapy showed a significant difference between CGI (clinical global impression) and ISAA (Indian Scale for Assessment of Autism) scores, but not for FIM (Functional Independence Measure) and Wee-FIM scores58. Significant improvement in the mBI (modified Barthel Index) score, MRC (Muscle Power Scale), and Ashworth scale was found for cerebral palsy49,53. Symptomatic improvement was found for intellectual disability64. Neurological improvements in limb strength and stability in muscle function of the body parts were shown in muscular dystrophy after stem cell treatment55,58,61.
The use of stem cells for Parkinson’s disease showed improvement in the UPDRS (Unified Parkinson’s Disease Rating Scale) scale, H&Y (Hoehn and Yahr), and S&E (Schwab and England) scores that helped in the reduction its medication, tremors, rigidity, and freezing attacks, improved clarity in speech and subjective improvement52,56. In spinal cord injury, stem cell therapy protocol was safe, but at the efficacy level, only a few patients showed improvement; there was no correlation between injury and improvements50,51,54,63. In traumatic brain injury, stem cell therapy helped in the improvement of speech, trunk, upper limb activity, muscle tone, voluntary control, posture balance, and psychological status62. No serious adverse were noted for neurological disorders, but seizures, headache, fever, nausea, vomiting, backache, pain at the site of injection, diarrhoea, spasticity, and tingling sensation were reported as minor adverse events50,54,58,60,64.
Musculoskeletal disorders
In critical limb ischemia, stem cell therapy helped relieve pain and heal ulcers65-68. Serious adverse events were reported, resulting in two deaths, during the use of allogeneic stem cell therapy68. SCT also shows statistically significant results in HHS (Harris Hip Scale/Score) in hip survival, and subjective improvements were observed in osteonecrosis65.
Discussion
The findings of this study indicated that India is taking great interest in the benefits of melanocytes (extracted from hair follicle cell suspension) for treating vitiligo. Injection of hair follicle cell suspension containing melanocytes and keratinocytes was found to be useful in managing vitiligo76,77,79. Although there was a small population size in some studies, there was a trend towards an improvement in symptoms and disease outcomes in individuals who had received BM-MSC compared with controls56,57,66. Two deaths were also reported as serious adverse events when using allogeneic stem cell therapy68. On the other hand, statistically significant results were also shown that helped in hip survival, with subjective improvements in osteonecrosis65.
The findings from this systematic review address important gaps in stem cell therapeutics for non-malignant diseases in India. Study indicates that stem cell therapy could be safe for treating non-malignant, non-haematological diseases, but the smaller number of participants in these clinical trials is a cause of concern. This study highlights important factors that are expected to shape the future of stem cell research and therapy in India. It may include standardization, regulations, basic research and clinical trials support, trained human resources, and infrastructure84. Maintaining the gold standard for stem cell therapy requires randomized clinical trials with a large sample size to study success, failures, adverse effects, etc8,85,86.
Future trials would need to incorporate more robust outcome measures that are patient-centered, and RCTs should be done instead of cohort studies and clinical trials with a small number35,57,59,62. Studies on assessing potential barriers and enablers to both patient participation and physician involvement in early-phase clinical trials are limited. This is an important knowledge gap that needs to be addressed for safety outcomes in stem cell therapy and research. This study also revealed that government hospitals published more studies than non-government hospitals.
India is aware of the potential of stem cell science, and the key question is, to what extent is India sensitive to the emerging challenges or barriers to stem cell therapeutic commercialization, its clinical implications, and its position in the global scenario? The other question arises: What should be done about desperate patients paying out of pocket for unproven treatments? The factors that affect such clinical practice and research in the public arena need to be identified. There is a need to synthesize more knowledge in stem cell research and therapeutics.
The study limitations include inability to use meta-analysis because of the qualitative outcome of all studies, the smaller number of clinical trials, the low sample size in some disease categories, the significant diversity of diseases, or study heterogeneity.
Overall, we need more clinical studies and the stakeholders’ perspectives on stem cell therapy to shift from experimental interventions into routine clinical practice. Despite the potential of stem cell and regenerative medicine research for safety and efficacious outcomes, there is potential for stem cell treatment in non-haematological diseases. However, well-controlled, randomized, large-scale trials are required to establish safety and efficacy. Clinical trials need to be reviewed by IC-SCR, and prospective interventional trials need to be registered with CTRI. Our findings are a call to action to stakeholders (clinicians, industries, policymakers, researchers, etc.) to identify approaches for stem cell therapy that are best suited for treating non-malignant diseases and non-haematological and accordingly can plan to invest resources for further research and development for a particular disease.
Acknowledgments
The authors acknowledge the Director, CSIR-NIScPR, New Delhi, India, for providing the support and guidance needed to complete the manuscript.
Financial support & sponsorship
None.
Conflicts of Interest
None.
Use of Artificial Intelligence (AI)-Assisted Technology for manuscript preparation
The authors confirm that there was no use of AI-assisted technology for assisting in the writing of the manuscript and no images were manipulated using AI.
References
- Non-Communicable Diseases. Available from: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases, accessed on October 19, 2023.
- India’s escalating burden of non-communicable diseases. Lancet Glob Health. 2018;6:e1262-63.
- [CrossRef] [PubMed] [Google Scholar]
- National programme for prevention and control of NCDs. Available from: https://ncd.nhp.gov.in/ncdlandingassets/aboutus.html, accessed on December 20, 2023.
- Concise review: Human embryonic stem cells-what have we done? What are we doing? Where are we going? Stem Cells. 2017;35:17-25.
- [CrossRef] [PubMed] [Google Scholar]
- Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: First clinical case report. Eur Heart J. 2015;36:2011-7.
- [CrossRef] [PubMed] [Google Scholar]
- Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: Follow-up of two open-label phase 1/2 studies. Lancet. 2015;385:509-16.
- [CrossRef] [PubMed] [Google Scholar]
- Stem cell-based therapy for human diseases. Signal Transduct Target Ther. 2022;7:272.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The large grey area between ‘bona fide’ and ‘rogue’ stem cell interventions – ethical acceptability and the need to include local variability. Technol Forecast Soc Change. 2016;109:76-86.
- [Google Scholar]
- Unproven regenerative medical products have led to infections, disabilities, and deaths. Available from: https://www.pewtrusts.org/en/research-and-analysis/issue-briefs/2021/06/harms-linked-to-unapproved-stem-cell-interventions-highlight-need-for-greater-fda-enforcement, accessed on December 19, 2023.
- Vision loss after intravitreal injection of autologous “stem cells” for AMD. N Engl J Med. 2017;376:1047-53.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Translational prospects and challenges in human induced pluripotent stem cell research in drug discovery. Cells. 2016;5:46.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Human stem cells for cardiac disease modeling and preclinical and clinical applications-are we on the road to success? Cells. 2023;12:1727.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Guidelines for stem cell research and therapy 2007. Available from: https://main.icmr.nic.in/sites/default/files/guidelines/stem_cell_guidelines_2007_0.pdf, accessed on October 19, 2023.
- National guidelines for stem cell research 2013. Available from: https://main.icmr.nic.in/sites/default/files/guidelines/NGSCR%202013_0.pdf, accessed on October 19, 2023.
- National guidelines for stem cell research 2017. Available from: https://dbtindia.gov.in/sites/default/files/National_Guidelines_StemCellResearch-2017.pdf, accessed on October 19, 2023.
- National guidelines for hematopoietic cell transplantation 2021. Available from: https://main.icmr.nic.in/sites/default/files/upload_documents/Nat_Guide_HCT.pdf, accessed on October 19, 2023.
- Current scenario of clinical trials on stem cells as a drug in India: A clinical trials registry of India database analysis. Perspec Clin Res 2022
- [CrossRef] [Google Scholar]
- A history of haemopoietic cell transplantation. Br J Haematol. 1999;105:330-9.
- [CrossRef] [PubMed] [Google Scholar]
- History of hematopoietic cell transplantation: challenges and progress. Haematologica. 2020;105:2716-29.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Blood and Bone Marrow Transplantation in India: Past, Present, and Future. Indian J Med Paediatr Oncol. 2020;41:308.
- [CrossRef] [Google Scholar]
- History of bone marrow transplantation. In: Chandy M, Radhakrishnan VS, Sukumaran RK, eds. Contemporary Bone Marrow Transplantation. Cham: Springer International Publishing; 2021. p. :3-26.
- [Google Scholar]
- Preferred reporting items for systematic review and meta-analysis protocols (prisma-P) 2015: Elaboration and explanation. BMJ. 2015;349:g7647.
- [CrossRef] [Google Scholar]
- Bone marrow aspirate in cystic maxillofacial bony defects. J Craniofac Surg. 2019;30:e247-e251.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of autologous bone marrow-derived stem cell transplantation in patients with type 2 diabetes mellitus. Stem Cells Dev. 2009;18:1407-16.
- [CrossRef] [PubMed] [Google Scholar]
- Cotransplantation of adipose tissue-derived insulin-secreting mesenchymal stem cells and hematopoietic stem cells: A novel therapy for insulin-dependent diabetes mellitus. Stem Cells Int. 2010;2010:582382.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Novel therapy for insulin-dependent diabetes mellitus: Infusion of in vitro-generated insulin-secreting cells. Clin Exp Med. 2015;15:41-5.
- [CrossRef] [PubMed] [Google Scholar]
- Insulin-secreting adipose-derived mesenchymal stromal cells with bone marrow-derived hematopoietic stem cells from autologous and allogenic sources for type 1 diabetes mellitus. Cytotherapy. 2015;17:940-7.
- [CrossRef] [PubMed] [Google Scholar]
- Co-infusion of insulin-secreting adipose tissue-derived mesenchymal stem cells and hematopoietic stem cells: Novel approach to management of type 1 diabetes mellitus. Int J Diabetes Dev Ctries. 2016;36:426-32.
- [CrossRef] [Google Scholar]
- Autologous bone marrow derived stem cell therapy in patients with type 2 diabetes mellitus - defining adequate administration methods. World J Diabetes. 2017;8:381-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Efficacy of autologous bone marrow-derived mesenchymal stem cell and mononuclear cell transplantation in type 2 diabetes mellitus: A randomized, placebo-controlled comparative study. Stem Cells Dev. 2017;26:471-81.
- [CrossRef] [PubMed] [Google Scholar]
- Simple limbal epithelial transplantation (SLET): A novel surgical technique for the treatment of unilateral limbal stem cell deficiency. Br J Ophthalmol. 2012;96:931-4.
- [CrossRef] [PubMed] [Google Scholar]
- Phenotypic evaluation of severely damaged ocular surface after reconstruction by cultured limbal epithelial cell transplantation. Ophthalmic Res. 2013;50:59-64.
- [CrossRef] [Google Scholar]
- Live related versus cadaveric limbal allograft in limbal stem cell deficiency. Ocul Immunol Inflamm. 2015;23:232-9.
- [CrossRef] [PubMed] [Google Scholar]
- Concomitant simple limbal epithelial transplantation after surgical excision of ocular surface squamous neoplasia. American J Ophthal. 2017;174:68-75.
- [CrossRef] [Google Scholar]
- Autologous bone marrow mononuclear cell delivery to dilated cardiomyopathy patients: A clinical trial. Afr J Biotechnol. 2008;7:207-10.
- [Google Scholar]
- Stem cell experiments and initial clinical trial of cellular cardiomyoplasty. Asian Cardiovasc Thorac Ann. 2009;17:581-6.
- [CrossRef] [PubMed] [Google Scholar]
- Autologous intravenous bone marrow mononuclear cell therapy for patients with subacute ischaemic stroke: A pilot study. Indian J Med Res. 2012;136:221-8.
- [PubMed] [PubMed Central] [Google Scholar]
- Stem cell therapy: A clinical trial of stroke. Clin Neurol Neurosurg. 2013;115:1003-8.
- [CrossRef] [PubMed] [Google Scholar]
- Intravenous autologous bone marrow mononuclear stem cell therapy for ischemic stroke: A multicentric, randomized trial. Stroke. 2014;45:3618-24.
- [CrossRef] [PubMed] [Google Scholar]
- Randomized, double-blind, phase I/II study of intravenous allogeneic mesenchymal stromal cells in acute myocardial infarction. Cytotherapy. 2015;17:250-61.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of stem cell in improvement of left ventricular function in acute myocardial infarction--MI3 Trial. Indian J Med Res. 2015;142:165-74.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- REVIVE trial: Retrograde delivery of autologous bone marrow in patients with heart failure. Stem Cells Translational Medicine. 2015;4:1021-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Randomized assessment of the safety and efficacy of intra-arterial infusion of autologous stem cells in subacute ischemic stroke. Am J Neuroradiol. 2018;39:899-904.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- High-dose peripheral blood stem cell infusion: A strategy to induce donor-specific hyporesponsiveness to allografts in pediatric renal transplant recipients. Pediatr Transplant. 2002;6:63-8.
- [CrossRef] [PubMed] [Google Scholar]
- Mega dose unfractionated donor bone marrow-derived cell infusion in thymus and periphery-an integrated clinical approach for tolerance in living related renal allografts. Transplant Proc. 2003;35:203-6.
- [CrossRef] [PubMed] [Google Scholar]
- In pursuit of the ultimate: The initial Ahmedabad journey toward transplantation tolerance. Transplant Proc. 2007;39:653-7.
- [CrossRef] [PubMed] [Google Scholar]
- Co-infusion of donor adipose tissue-derived mesenchymal and hematopoietic stem cells helps safe minimization of immunosuppression in renal transplantation - single center experience. Ren Fail. 2014;36:1376-84.
- [CrossRef] [PubMed] [Google Scholar]
- Human fetal liver-derived stem cell transplantation as supportive modality in the management of end-stage decompensated liver cirrhosis. Cell Transplant. 2010;19:409-18.
- [CrossRef] [PubMed] [Google Scholar]
- Autologous mobilized peripheral blood CD34(+) cell infusion in non-viral decompensated liver cirrhosis. World J Gastroenterol. 2015;21:7264-71.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Autologous bone marrow derived mononuclear cell therapy for spinal cord injury: A phase I/II clinical safety and primary efficacy data. Exp Clin Transplant. 2009;7:241-8.
- [PubMed] [Google Scholar]
- Ex vivo-expanded autologous bone marrow-derived mesenchymal stromal cells in human spinal cord injury/paraplegia: A pilot clinical study. Cytotherapy. 2009;11:897-911.
- [CrossRef] [PubMed] [Google Scholar]
- Open-labeled study of unilateral autologous bone-marrow-derived mesenchymal stem cell transplantation in Parkinson’s disease. Transl Res. 2010;155:62-70.
- [CrossRef] [PubMed] [Google Scholar]
- Restorative therapy using autologous bone marrow derived mononuclear cells infusion intra-arterially in patients with cerebral palsy: An open label feasibility study. Neurol ASIA. 2011;16:231-9.
- [Google Scholar]
- Autologous mesenchymal stem cells in chronic spinal cord injury. Br J Neurosurg. 2011;25:516-22.
- [CrossRef] [PubMed] [Google Scholar]
- Administration of autologous bone marrow-derived mononuclear cells in children with incurable neurological disorders and injury is safe and improves their quality of life. Cell Transplant. 2012;21:S79-90.
- [CrossRef] [PubMed] [Google Scholar]
- Bilateral transplantation of allogenic adult human bone marrow-derived mesenchymal stem cells into the subventricular zone of Parkinson’s disease: A pilot clinical study. Stem Cells Int 2012:931902.
- [Google Scholar]
- Safety profile of bone marrow mononuclear stem cells in the rehabilitation of patients with posttraumatic facial nerve paralysis-a novel modality (phase one trial) J Neurol Surg B Skull Base. 2012;73:245-52.
- [CrossRef] [PubMed] [Google Scholar]
- Autologous bone marrow mononuclear cell therapy for autism: An open label proof of concept study. Stem Cells Int. 2013;2013:623875.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A clinical study shows safety and efficacy of autologous bone marrow mononuclear cell therapy to improve quality of life in muscular dystrophy patients. Cell Transplant. 2013;22:S127-38.
- [CrossRef] [PubMed] [Google Scholar]
- A clinical study of autologous bone marrow mononuclear cells for cerebral palsy patients: A new frontier. Stem Cells Int. 2015;2015:905874.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Human umbilical cord mesenchymal stem cells in the treatment of duchenne muscular dystrophy: Safety and feasibility study in India. J Stem Cells. 2015;10:141-56.
- [PubMed] [Google Scholar]
- Cell therapy attempted as a novel approach for chronic traumatic brain injury - a pilot study. Springerplus. 2015;4:26.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Autologous bone marrow cell transplantation in acute spinal cord injury-an Indian pilot study. Spinal Cord. 2016;54:57-64.
- [CrossRef] [PubMed] [Google Scholar]
- An open-label proof-of-concept study of intrathecal autologous bone marrow mononuclear cell transplantation in intellectual disability. Stem Cell Res Ther. 2018;9:19.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Early results of core decompression and autologous bone marrow mononuclear cells instillation in femoral head osteonecrosis: A randomized control study. J Arthroplasty. 2012;27:679-86.
- [CrossRef] [PubMed] [Google Scholar]
- A double blind randomized placebo controlled phase I/II study assessing the safety and efficacy of allogeneic bone marrow derived mesenchymal stem cell in critical limb ischemia. J Transl Med. 2013;11:143.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Efficacy and safety of adult human bone marrow-derived, cultured, pooled, allogeneic mesenchymal stromal cells (Stempeucel®): Preclinical and clinical trial in osteoarthritis of the knee joint. Arthritis Res Ther. 2016;18:301.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Administration of adult human bone marrow-derived, cultured, pooled, allogeneic mesenchymal stromal cells in critical limb ischemia due to buerger’s disease: Phase II study report suggests clinical efficacy. Stem Cells Transl Med. 2017;6:689-99.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Melanocyte-keratinocyte cell transplantation for stable vitiligo. Int J Dermatol. 2003;42:132-6.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of autologous transplantation of noncultured epidermal suspension in two different dilutions in the treatment of vitiligo. Int J Dermatol England. 2006;45:106-10.
- [CrossRef] [Google Scholar]
- Targeting nonhealing ulcers of lower extremity in human through autologous bone marrow-derived mesenchymal stem cells. Rejuvenation Res. 2009;12:359-66.
- [CrossRef] [PubMed] [Google Scholar]
- Noncultured extracted hair follicle outer root sheath cell suspension for transplantation in vitiligo. Br J Dermatol. 2011;164:1241-6.
- [CrossRef] [PubMed] [Google Scholar]
- Autologous noncultured melanocyte transplantation for stable vitiligo: Can suspending autologous melanocytes in the patients’ own serum improve repigmentation and patient satisfaction? Dermatol Surg. 2011;37:176-82.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison between autologous noncultured epidermal cell suspension and suction blister epidermal grafting in stable vitiligo: a randomized study. Br J Dermatol England. 2012;167:1295-301.
- [CrossRef] [Google Scholar]
- Comparison between autologous noncultured extracted hair follicle outer root sheath cell suspension and autologous noncultured epidermal cell suspension in the treatment of stable vitiligo: a randomized study. Br J Dermatol England. 2013;169:287-93.
- [CrossRef] [Google Scholar]
- Clinical and treatment characteristics determining therapeutic outcome in patients undergoing autologous non-cultured outer root sheath hair follicle cell suspension for treatment of stable vitiligo. J Eur Acad Dermatol & Venereol. 2015;29:31-7.
- [PubMed] [Google Scholar]
- Comparative study of efficacy of epidermal melanocyte transfer versus hair follicular melanocyte transfer in stable vitiligo. Indian J Dermatol. 2016;61:640-4.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Study of efficacy and safety of noncultured, extracted follicular outer root sheath cell suspension transplantation in the management of stable vitiligo. Int J Dermatol. 2018;57:245-9.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of transplantation of combination of noncultured dermal and epidermal cell suspension vs epidermal cell suspension alone in vitiligo: A randomized clinical trial. JAMA Dermatol. 2019;155:204-10.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Safety and efficacy of autologous noncultured dermal cell suspension transplantation in the treatment of localized facial volume loss: A pilot study. Indian J Dermatol Venereol Leprol. 2019;85:44-50.
- [CrossRef] [PubMed] [Google Scholar]
- Autologous noncultured melanocyte-keratinocyte transplantation in stable vitiligo: A randomized comparative study of recipient site preparation by two techniques. Indian J Dermatol Venereol Leprol. 2019;85:32-8.
- [CrossRef] [PubMed] [Google Scholar]
- Four compartment method as an efficacious and simplified technique for autologous non-cultured epidermal cell suspension preparation in vitiligo surgery: a randomized, active-controlled study. J Eur Acad Dermatol Venereol England. 2019;33:185-90.
- [Google Scholar]
- Comparison of efficacy of noncultured hair follicle cell suspension and noncultured epidermal cell suspension in repigmentation of leukotrichia and skin patch in vitiligo: a randomized trial. Int J Dermatol England. 2020;59:1393-400.
- [CrossRef] [Google Scholar]
- Are there specific translational challenges in regenerative medicine? Lessons from other fields. Regen Med. 2015;10:885-95.
- [CrossRef] [PubMed] [Google Scholar]
- Alter-standardizing clinical trials: The gold standard in the crossfire. Science as Culture. 2019;28:125-48.
- [CrossRef] [Google Scholar]
- Experimental heterogeneity and standardisation: Stem cell products and the clinical trial process. BioSocieties. 2011;6:401-19.
- [CrossRef] [Google Scholar]






