Translate this page into:
SoSoSo or its active ingredient chrysophanol regulates production of inflammatory cytokines & adipokine in both macrophages & adipocytes
Reprint requests: Dr Hyun-Ja Jeong, Prof., Biochip Research Center, Hoseo University, 165 Sechul-ri, Baebang-myun, Asan, Chungnam, Republic of Korea e-mail: hjjeong@hoseo.edu Dr Hyung-Min Kim, Prof., Department of Pharmacology, College of Oriental Medicine, Kyung Hee University, 1 Hoegi-dong, Dongdaemun-gu, Seoul, Republic of Korea e-mail: hmkim@khu.ac.kr
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Obesity is now considered as a major risk factor for the development of fatty liver diseases, cardiovascular diseases, and atherosclerosis. SoSoSo is a newly developed dietary supplement made of seven medicinal herbs. This study was aimed at examining the anti-obesity effect of SoSoSo or its active ingredient chrysophanol on the production of inflammatory cytokines and adipokine in macrophyage cell line RAW264 and 3T3-L1 adipocytes.
Methods:
No release was measured as a form of nitrite by Griess method. The production of inflammatory cytokines and adipokine were measured with the ELISA method. The m-RNA expression of each cytokine and adipokine were measured using RT-PCR. The nuclear proteins for NF-κB were analyzed with western blotting.
Results:
SoSoSo or chrysophanol significantly inhibited the nitric oxide production in lipopolysaccharide-stimulated RAW264 cells as well as in RAW264 cells-conditioned medium (CM)-treated 3T3-L1 cells. The production of interleukin (IL)-6 and tumour necrosis factor (TNF)-α were inhibited by SoSoSo or chrysophanol. In addition, SoSoSo or chrysophanol inhibited the activation of nuclear factor-κB in RAW264 cells. SoSoSo or chrysophanol inhibited the productions of IL-6, TNF-α, and monocyte chemoattractant protein-1 as well as the reduction of adiponectin production in CM-treated 3T3-L1 cells.
Interpretation & conclusions:
These results suggest a potential of SoSoSo or chrysophanol as a source of anti-inflammatory agent for obesity. Further in vivo studies would be required to confirm these findings.
Keywords
Adiponectin
chrysophanol
interleukin-6
monocyte chemoattractant protein-1
SoSoSo
tumour necrosis factor-α
Obesity in its various manifestations is now considered as a very serious health problem, affecting a significant proportion of people of all ages. Several studies have indicated that obesity is associated with a low-grade proinflammatory state, characterized by macrophage infiltration of muscle and adipose tissue, adipocyte dysfunction, and abnormal production of proinflammatory mediators1–3. Infiltrated macrophages are crucial contributors to inflammation stimulated through toll-like receptors (TLRs) and produce various inflammatory proteins including nitric oxide (NO) and tumour necrosis factor (TNF)-α. The nuclear factor (NF)-κB pathway plays an important role in TLR mediated inflammation4. Nuclear factor (NF)-κB is a key transcription factor required for the expression of many inflammatory genes including iNOS, TNF-α, and IL-65. NF-κB normally resides in the cytoplasm, where it is retained by association with IκB protein (α, β and γ), an endogenous inhibitor. However, once activated, it translocates to the nucleus and activates genes6.
NO plays a significant role in the pathogenesis of inflammation7. Plasma levels of NO metabolites correlate with body mass index and body fat mass8. TNF-α is known to disturb insulin signaling. Mice lacking TNF-α or TNF-α receptors are reported to be resistant to the development of obesity induced insulin resistance9. Interleukin (IL)-6 another cytokine similar to TNF-α, is overexpressed in the adipose tissue in obesity10.
The adipocyte is the primary site of energy storage and accumulates triacylglycerol during nutritional excess. Adipocytes also synthesize and secrete biologically active molecules called adipokines. The altered production of proinflammatory molecules by adipose tissue has been implicated in the metabolic complications of obesity11. Compared with the adipose tissue of lean individuals, the adipose tissue of obese individuals expresses increased proinflammatory proteins such as TNF-α, IL-6, and monocyte chemotactic protein-1 (MCP-1) as well as reduced adiponectin level12. The MCP-1 is increased by obesity13, and circulating levels of adiponectin a protein secreted from adipocytes are decreased in obesity14. Adiponectin itself may be anti-atherosclerotic factor, as it acts as an endogenous anti-thrombotic factor and inhibits macrophage activation and foam cell accumulation. Mice lacking adiponectin have reduced insulin sensitivity15.
We developed a new dietary supplement (SoSoSo) from seven medicinal herbs (Cassiae semen, Theae folium, Eucommiae cortex, Pine needle, garlic, Crataegi Fructus, and Artemisiae capillaris herba) and evaluated for anti-obesity effect. Cassiae semen was the main medicinal herb of SoSoSo. Chrysophanol is an active ingredient of Cassiae semen16, main component of SoSoSo. Chrysophanol also inhibited the production of TNF-α and IL-6 in mouse macrophages17, thus, we investigated the effect of SoSoSo or its active ingredient chrysophanol on the inflammatory reaction in macrophage cell line RAW264 cells and 3T3-L1 adipocytes.
Material & Methods
The study was carried out in Department of Pharmacology, Kyung Hee University, Seoul, Republic of Korea.
Reagents: Enzyme-linked immunosorbent assay (ELISA) capture and detection antibodies as well as recombinants (standard) were purchased from R&D Systems (Minneapolis, Minnesota, USA). Dulbecco's Modified Eagle's Medium (DMEM), lipopolysaccharide (LPS), 3-isobutyl-1-methylxanthine (IBMX), insulin, chrysophanol, N-(1-naphtyl)-ethylenediamine dihydrochloride, dexamethasone acetate, and sodium nitrite were purchased from Sigma (St. Louis, MO, USA). Western blot antibodies were obtained from Santa Cruz Biotechnology, Inc., USA. Foetal bovine serum (FBS) and other tissue culture reagents were purchased from Gibco BRL (Grand Island, NY, USA).
Preparation of SoSoSo and chrysophanol: SoSoSo (voucher No 201032) which is a mixture of seven medicinal herbs was obtained from Noa oriental pharmacy (Seoul, Republic of Korea). (constituents shown in Table). Extract of SoSoSo was prepared by decocting the dried medicinal herbs with boiling distilled water. The extraction decocted for approximately 3 h, filtered, lyophilized, and kept a 4°C. The yield of SoSoSo was about 5 per cent (w/w). Chrysophanol was prepared by dissolving with dimethyl sulphfoxide. Dilutions were made in phosphate buffered saline (PBS) pH and filtered through 0.22 μm syringe filter.

Cell culture and treatment: 3T3-L1 fibroblasts were grown in DMEM plus 10 per cent calf serum and plated for final differentiation in DMEM plus 10 per cent FBS. Two days after reaching confluence, the medium was changed to the differentiation medium containing IBMX (0.5 mM), dexamethasone (0.25 μM), and insulin (10 μg/ml). After 2 days, the cell culture medium was changed to DMEM containing 10 μg/ml insulin and 10 per cent FBS. The medium was replaced again with fresh DMEM containing 10 per cent FBS after 2 days. As shown in Fig. 1, adipocytes (3T3-L1 cells) (ATCC, USA) were used 6 - 8 days after the initiation of differentiation. On the other hand, RAW264 cells (KCLB, Republic of Korea) were maintained in DMEM containing 10 per cent FBS. The cells were treated with SoSoSo or chrysophanol under serum-free conditions, and then stimulated with 100 ng/ml of LPS for 24 h. Twenty four hours after LPS stimulation, RAW264 cells-conditioned medium (CM) was collected by centrifugation (1500 × g for 5 min at 4°C) and filtered using 0.22 μm syringe filter. Collected CM was used for NO, IL-6, and TNF-α assay, whereas harvested RAW264 cells were used for NF-κB assay. On the other hand, differentiated 3T3-L1 cells were incubated with 1.5 ml of each medium (CM, CM LPS, CM LPS S and CM LPS C) in 6-well plates for 24 h. Twenty four hours after incubation, collected supernatants were used for NO, IL-6, TNF-α, and MCP-1 assay, whereas harvested 3T3-L1 cells were used for mRNA expression assay.
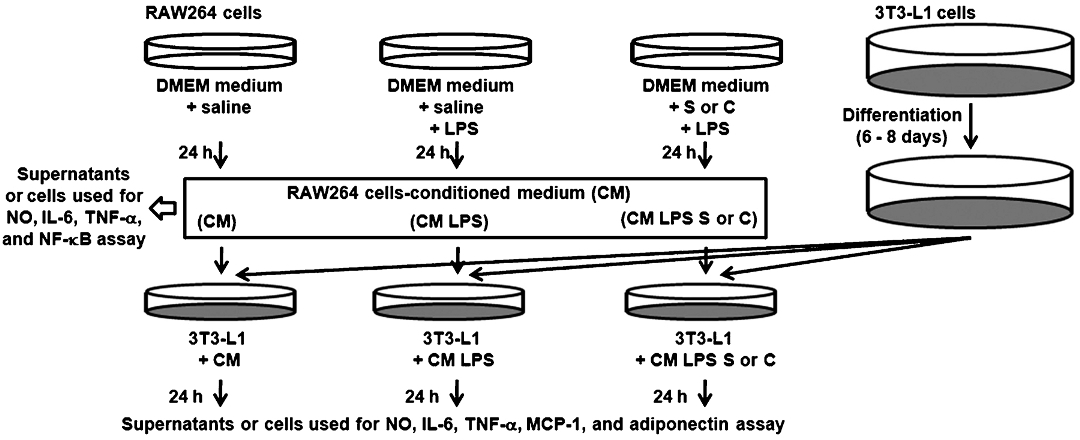
- Experimental protocol. CM, unstimulated RAW264 cells-conditioned medium; CM LPS, LPS-stimulated RAW264 cells-conditioned medium; CM LPS S, SoSoSo-pretreated and LPS-stimulated RAW264 cells-conditioned medium; CM LPS C, chrysophanol-pretreated and LPS-stimulated RAW264 cells-conditioned medium.
MTT assay: RAW264 cells were seeded in 24-well plates and exposed to various concentrations with SoSoSo or chrysophanol for 24 h. The cell survival fraction was determined with the MTT assay9. In brief, after incubation with SoSoSo or chrysophanol, MTT solution (5 mg/ml in PBS) was added (50 μl/well). Plates were further incubated for 4 h at 37°C, and the formazan crystals formed were centrifuged and the pellets dissolved by the addition of dimethyl sulphoxide. Absorption was measured by spectrometer at 540 nm.
Measurement of nitrite concentration: NO synthesis in cell cultures was measured by a microplate assay method, as previously described18. To measure nitrite, 100 μl aliquots were removed from CM and incubated with an equal volume of Griess reagent [1% sulphanilamide, 0.1% N-(1-naphtyl)-ethylenediamine dihydrochloride, 2.5% H3PO4] at room temperature for 10 min. The absorbance at 540 nm was determined by an automatic microplate reader (Molecular Devices Corp., Sunwayle, California, USA). NO2– was determined by using sodium nitrite as a standard. The cell-free medium alone contained 5 to 8 μM of NO2–. This value was determined in each experiment and subtracted from the value obtained from the medium with RAW264 cells.
Cytokine assay: The amount of TNF-α, IL-6, MCP-1 and adiponectin secreted by the cells was measured by a modified ELISA, as described previously21. First, a sandwich ELISA was conducted for TNF-α, IL-6, MCP-1 and adiponectin in duplicate in 96-well ELISA plates (Nunc, Denmark). Next each plate was coated with 100 μl aliquots of mouse anti-mouse TNF-α, IL-6, MCP-1 and adiponectin monoclonal antibodies at 1.0 μg/ml in PBS (pH 7.4). The plates were then incubated overnight at 4°C, washed with PBS containing 0.05 per cent Tween-20 (Sigma, St. Louis, MO, USA), and blocked for 1 h with PBS containing 1 per cent BSA, 5 per cent sucrose and 0.05 per cent NaN3. After washing the plates again, a sample or recombinant TNF-α, IL-6, MCP-1 and adiponectin standards was added and incubated the plates at 37°C for 2 h. After the second incubation, the plates were washed, added 0.2 μg/ml of biotinylated anti-mouse TNF-α, IL-6, MCP-1 and adiponectin, and incubated again at 37°C for 2 h. After washing the plates again, avidin-peroxidase was added and plates and incubated for a further 30 min at 37°C. Finally, ABTS substrate (Sigma, USA) was added. Absorbance was measured at 405 nm an automated microplate ELISA reader. Standard curve for each assay plate was made by using recombinant mouse TNF-α, IL-6, MCP-1 and adiponectin in serial dilutions.
RNA isolation and RT-PCR: Total RNA was extracted from culture cell samples using an easy-BLUE RNA extraction kit (iNtRON Biotech, Seoul, South Korea) and QIAzol (Qiagen, USA) as described previously21. The concentration of total RNA in the final elutes was determined by spectrophotometry. Total RNA (2 μg) were subjected to the RT reaction with oligo-dT in a final volume of 20 μl using a cDNA synthesis kit (AmershamPharmacia, NJ, USA). RT-PCR was carried out with 1 μl of a cDNA mixture, in 20 μl final volume with 2.5 mM MgCl2, 200 mM dNTPs, 25 pM of cytokine primers, and 2.5 U of Taq DNA polymerase in the reaction buffer (50 mM KCl, 10 mM Tris-HCl, pH 9, and 0.1% Triton X-100). PCR amplification was performed in a final volume of 20 μl with aliquots of cDNA (25 ng) and iTaq DNA polymerase (Bio-Rad Laboratories, USA) using a PTC-200 Peltier thermal cycler (MJ Research, Inc., Waltham, USA). The following primers were used (BIONEER Corp, Daejeon, Republic of Korea): iNOS (5’CTT GCC CCT GGA AGT TTC TCT T 3’; 5’GCT GGT AGG TTC CTG TTG TTT C 3’); COX-2(5’GGA GAG ACT ATC AAG ATA GT 3’; 5’ ATG GTC AGT AGA CTT TTA CA 3’); TNF-α(5’ TAC AGG CTT GTC ACT CGA ATT 3’; 5’ ATG AGC ACA GAA AGC ATG ATC 3’); IL-6(5’ CCC AAG CTT CTA CGT TTG CCG AGT AGA 3’; 5’ CGG GAT CCA TGT TCC CTA CTT CAC AA 3’); MCP-1(5’ CCC ACT CAC CTG CTG CTA CT 3’; 5’ GTC TGG ACC CAT TCC TTC TT 3’); adiponectin (5’ AAG GAC AAG GCC GTT CTC T 3’; 5’ TAT GGG TAG TTG CAG TCA GTT GG 3’); and Glycereldehyde 3-phosphate dehydrogenase (GAPDH) (5’ TTC ACC ACC ATG GAG AAG GC 3’; 5’ GGC ATG GAC TGT GGT CAT GA 3’).
Preparation of nuclear extract & Western blot analysis: Nuclear extract was prepared as described previously18. Nuclear extract were made by boiling cells in sample buffer [62.5 mM Tris-HCl, pH 6.8, 2 per cent sodium dodecyl sulphate (SDS), 20 % glycerol, and 10 % 2-mercaptoethanol]. Proteins in the nuclear extract were then separated by 10 per cent SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose paper. The membrane was then blocked with 5 per cent skim milk in PBS-tween-20 for 1 h at room temperature and then incubated with 1 mg/ml anti-NF-κB and anti-histone antibodies for 1 h. After washing in PBS-tween-20 three times, the blot was incubated with 1:3000 diluted anti-rabbit IgG, horseradsh peroxidase linked whole antibody for 30 min, and the antibody-specific proteins were visualized using the enhanced chemiluminesence detection kit (Amersham Co. Newark, NJ, USA) according to the recommended procedure.
Statistical analysis: Results were expressed as the mean ± SEM of independent experiments (n=3), and statistical analyses were performed by Mann-Whitney U test to express the difference between groups. All statistical analyses were performed using SPSS v11.0 statistical analysis software (SPSS Inc., IL, USA).
Results
Effect of SoSoSo or chrysophanol on cell viability: To determine the effect of SoSoSo or chrysophanol on the viability of RAW264 cells, MTT assay was performed. When RAW264 cells were treated with SoSoSo (0.01 to 1 mg/ml) or chrysophanol (1 to 100 μM)20, cytotoxicity by SoSoSo or chrysophanol was shown (Fig. 2A). Therefore, in this study, 0.01 to 1 mg/ml of SoSoSo or 1 to 100 μM of chrysophanol were used.
Inhibitory effect of SoSoSo or chrysophanol on the NO production in LPS-stimulated RAW264 cells and CM-treated 3T3-L1 cells: SoSoSo significantly (P<0.05) inhibited the NO production induced by LPS at concentrations of 0.1 and 1 mg/ml. Chrysophanol also significantly (P<0.5) inhibited the NO production induced by LPS at concentrations of 1 to 100 μM (Fig. 2B). SoSoSo or chrysophanol dose-dependently inhibited the NO production in CM-treated 3T3-L1 (Fig. 2C).

- Effect of SoSoSo or chrysophanol on viability of RAW264 cells and NO production both in LPS-stimulated RAW264 cells and in CM-treated 3T3-L1 cells. (A) RAW264 cells were pretreated with various concentrations of SoSoSo or chrysophanol and then stimulated with LPS for 24 h. Cell viability was determined with an MTT assay. (B) RAW264 cells were pretreated with various concentrations of SoSoSo or chrysophanol and then stimulated with LPS for 24 h. (C) 3T3-L1 adipocytes were treated with the CM of LPS-stimulated RAW264 cells for 24 h. RAW264 cells were pretreated with various concentrations of SoSoSo or chrysophanol and then stimulated with LPS for 24 h. NO release was measured as a form of nitrite by the Griess method. The results represent three independent experiments conducted in duplicated. NO (nitrite) released into the medium is presented as the mean ± SEM of three independent experiments.
Inhibitory effect of SoSoSo or chrysophanol on the production and mRNA expression of IL-6 and TNF-α in LPS-stimulated RAW264 cells: Chrysophanol significantly (P<0.05) inhibited the IL-6 production induced by LPS at the concentration of 100 μM. SoSoSo inhibited the TNF-α production induced by LPS at the concentrations of 0.1 and 1 mg/ml. Chrysophanol also inhibited the TNF-α production induced by LPS at concentrations of 1 to 100 μM (Fig. 3A, B). SoSoSo or chrysophanol significantly attenuated the expression of proinflammatory genes (IL-6 and TNF-α) induced by LPS. SoSoSo or chrysophanol dose-dependently inhibited the mRNA expression of IL-6 and TNF-α (Figs 3C and D).
Inhibitory effect of SoSoSo or chrysophanol on NF-κB activation in LPS-stimulated RAW264 cells: Next, we examined whether SoSoSo or chrysophanol could inhibit the translocation of NF-κB in RAW264 cells. As shown in Fig. 4, we showed that LPS treatment considerably increased the nuclear NF-κB protein level, which is an indication of nuclear translocation of NF-κB. Pretreatment of SoSoSo or chrysophanol inhibited the LPS-induced increase of the nuclear NF-κB levels.

- Inhibitory effect of SoSoSo or chrysophanol on the production and mRNA expression of IL-6 and TNF-α in LPS-stimulated RAW264 cells. RAW264 cells were pretreated with various concentrations of SoSoSo or chrysophanol and then stimulated with LPS for 24 h. (A) IL-6 and (B) TNF-α levels in the supernatant were measured with the ELISA method. Each value represents the mean ± SEM of three independent experiments. mRNA level was measured using RT-PCR. 1: unstimulated cell; 2: LPS; 3: LPS + SoSoSo 0.1 mg/ml; 4: LPS + SoSoSo 1 mg/ml; 5: LPS + chrysophanol 10 μM; 6: LPS + chrysophanol 100 μM. The experiment was repeated three times and similar results were obtained. (C) IL-6, and (D) TNF-α mRNA expression levels were quantitated with densitometry. The values from each treatment were expressed as a percentage relative to the control (LPS-stimulated cells; 100%).
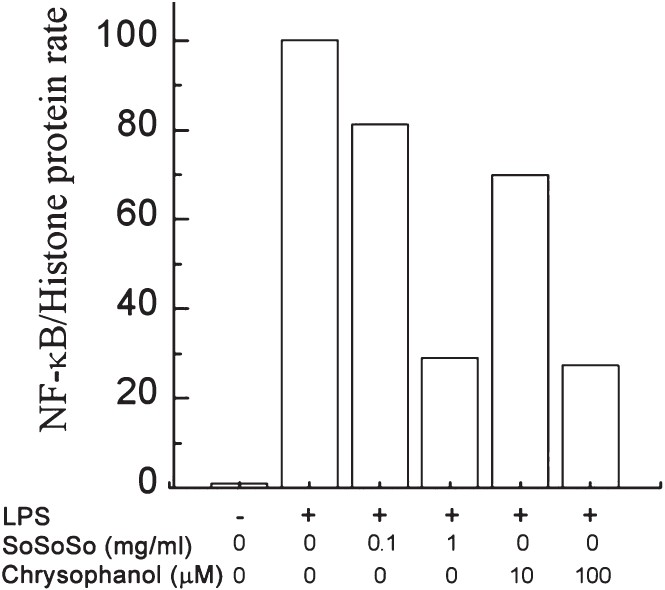
- SoSoSo or chrysophanol inhibited NF-κB activation induced by LPS in RAW264 cells. RAW264 cells were pretreated with various concentrations of SoSoSo or chrysophanol and then stimulated with LPS for 30 min. Nuclear proteins for NF-κB were analysed with Western blotting. 1: unstimulated cell; 2: LPS; 3: LPS + SoSoSo 0.1 mg/ml; 4: LPS + SoSoSo 1 mg/ml; 5: LPS + chrysophanol 10 μM; 6: LPS + chrysophanol 100 μM. The experiment was repeated three times and similar results were obtained. Protein level was quantitated by densitometry. The values from each treatment were expressed as a percentage relative to the control (LPS-stimulated cells; 100%).
Inhibitory effect of SoSoSo or chrysophanol on the production and mRNA expression of proinflammatory cytokines and adipokines in CM-treated 3T3-L1 cells: SoSoSo (1 mg/ml) or chrysophanol (100 μM) significantly inhibited the production of IL-6 in CM-treated 3T3-L1 cells and SoSoSo (0.1 mg/ml) or chrysophanol (10 and 100 μM) significantly (P<0.05) inhibited the production of TNF-α in CM-treated 3T3-L1 cells. Further, SoSoSo (1 mg/ml) or chrysophanol (100 μM) significantly (P<0.05) inhibited the production of MCP-1 in CM-treated 3T3-L1 cells. Finally, SoSoSo (0.1 and 1 mg/ml) or chrysophanol (100 μM) significantly (P<0.05) inhibited the reduction of adiponectin in CM-treated 3T3-L1 cells (Fig. 5A–D). SoSoSo dose-dependently inhibited the expression of IL-6 mRNA (Fig. 5E). SoSoSo or chrysophanol inhibited the expression of TNF-α mRNA (Fig. 5F), MCP-1 mRNA (Fig. 5G), and the reduction of adiponectin mRNA (Fig. 5H).
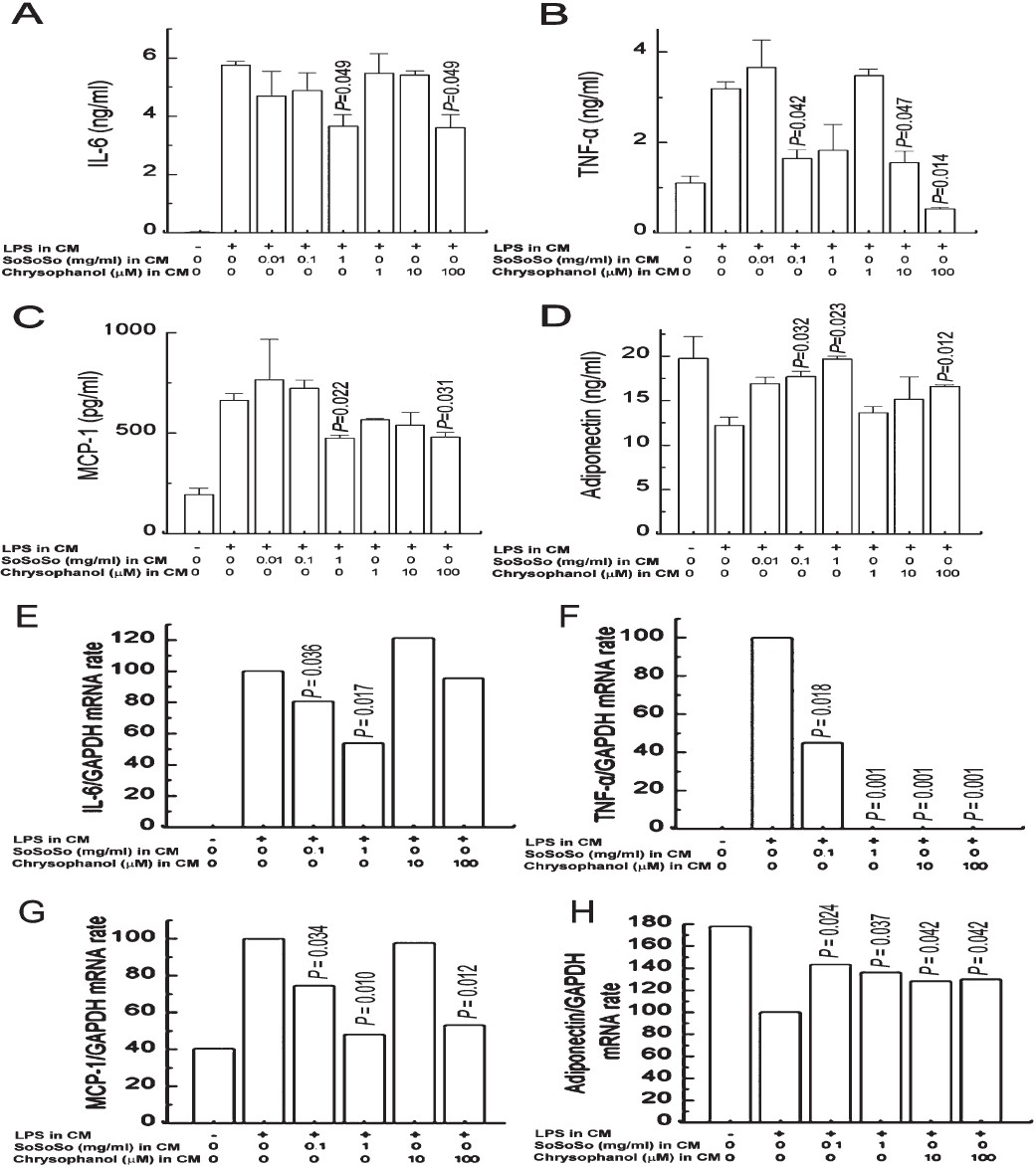
- Inhibitory effect of SoSoSo or chrysophanol on the production and mRNA expression of IL-6, TNF-α, MCP-1, and adiponectin in CM-treated 3T3-L1 cells. 3T3-L1 adipocytes were treated with the CM of LPS-stimulated RAW264 cells for 24 h. RAW264 cells were pretreated with various concentrations of SoSoSo or chrysophanol and then stimulated with LPS for 24 h. (A) IL-6, (B) TNF-α, (C) MCP-1, and (D) adiponectin levels in the supernatant were measured with the ELISA method. Each value represents the mean ± S.E.M. of three independent experiments. (E) IL-6, (F) TNF-α, (G) MCP-1, and (H) adiponectin mRNA expression levels were quantitated with densitometry. The values from each treatment were expressed as a percentage relative to the control (CM of LPS-stimulated RAW264 cells; 100%).
Discussion
Cassiae semen, the main component of SoSoSo, contains 0.61 ± 0.06 mg/g of chrysophanol in water extract of Cassiae semen22. In our study chrysophanol not only suppressed the production of proinflammatory cytokines in LPS-stimulated RAW264 cells, but also regulated the production of adipokine and proinflammatory cytokines in CM-treated 3T3-L1 cells. These results indicated that chrysophanol could inhibit the inflammatory reaction in obese adipose tissue. Basu et al23 reported that green tea decreased body weight and BMI in obese subjects with metabolic syndrome. (-)-Epigallocatechin gallate, active ingredient of green tea, reduced body weight gain in high fat-fed obese mice24. Allicin, active ingredient of garlic, also inhibited the increase in bodyweight in fructose-fed rats25.
Obesity is associated with chronic and mild inflammation. It has been demonstrated that obese adipose tissue is characterized by increased infiltration of macrophages, suggesting that these are important sources of inflammation in the adipose tissue1. Activated macrophages release proinflammatory cytokines and biologically active molecules such as NO, IL-6 and TNF-α26. Choi27 reported that subjects with increased serum cholesterol or triglyceride concentrations exhibited remarkably high nitrate and nitrites levels. Our results showed that SoSoSo or chrysophanol inhibited the NO production in LPS-stimulated RAW264 cells as well as in CM-treated 3T3-L1 cells, indicating that the inhibitory effect of SoSoSo or chrysophanol on the production of NO would be helpful to control increased lipids.
The analysis of gene expression profiles of adipocytes revealed that macrophages produce almost all of the TNF-α, whereas mature adipocytes secret the majority of leptin, and IL-6 is expressed roughly equally among adipocytes, macrophages, and others1. These observations suggest that the macrophages infiltrated in the adipose tissue contribute to the elevation in circulating inflammatory markers, including TNF-α and IL-6, that are common in obesity1. SoSoSo inhibited the production of TNF-α and chrysophanol inhibited the productions of IL-6 and TNF-α in LPS-stimulated RAW264 cells. The expression of IL-6 and TNF-α is regulated by transcription factor, NF-κB in LPS-stimulated RAW264 cells28. In this study, SoSoSo or chrysophanol inhibited the LPS-induced activation of NF-κB in RAW264 cells indicating that SoSoSo or chrysophanol has an anti-inflammatory potential through the inhibition of NF-κB activation in macrophages.
TNF-α is one of the cytokines whose expression and secretion by the adipose tissue is elevated in obese subjects10. IL-6 is another cytokine similar to TNF-α that is overexpressed in the adipose tissue of obesity10. MCP-1 plays a crucial role in the augmentation of inflammatory responses in obesity by enhancing the migration of monocytes into adipose tissue29. Adiponectin is an important anti-inflammatory molecule, and is produced by the adipose cells, and is also a marker of adipocyte differentiation. The secretion is reduced in obesity, insulin resistance, and type 2 diabetes30. Our results showed that SoSoSo or chrysophanol inhibited the productions of IL-6, TNF-α, and MCP-1 as well as the reduction of adiponectin production in CM-treated 3T3-L1 cells.
In conclusion, the present study showed that SoSoSo or chrysophanol regulates macrophage-mediated IL-6, TNF-α, MCP-1 and adiponectin production in adipocytes. These anti-inflammatory effects of SoSoSo or chrysophanol are dependent on the inhibition of NF-κB. Overall, our results suggest a potential of SoSoSo or chrysophanol as a source of anti-inflammatory agent for obesity, although further in vivo study would be required to confirm these conjectures.
Acknowledgment
This research was supported by Basic Science Research Programme through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0005591) and by a grant from the Kyung Hee University in 2010 (KHU- 20100120).
References
- Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796-808.
- [Google Scholar]
- Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J. 2008;29:2959-71.
- [Google Scholar]
- Early effects of gastric bypass on endothelial function, inflammation, and cardiovascular risk in obese patients. Surg Endosc. 2011;25:2650-9.
- [Google Scholar]
- Inhibition of TNF-alpha and IL-6 production by Aucubin through blockade of NF-kappaB activation RBL-2H3 mast cells. Cytokine. 2002;18:252-9.
- [Google Scholar]
- Dokhwaljihwang-tang inhibits LPS-induced inflammatory cytokine production in peripheral blood mononuclear cells. Neurol Res. 2010;32(Suppl 1):48-52.
- [Google Scholar]
- Increases in nitric oxide concentrations correlate strongly with body fat in obese humans. Clin Chem. 2001;47:1106-9.
- [Google Scholar]
- Protection from obesity-induced insulin resistance in mice lacking TNF-α function. Nature. 1997;389:610-4.
- [Google Scholar]
- Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280:E745-51.
- [Google Scholar]
- Modulatory effect of grape-seed procyanidins on local and systemic inflammation in diet-induced obesity rats. J Nutr Biochem. 2011;22:380-7.
- [Google Scholar]
- The implication of obesity and central fat on markers of chronic inflammation: The ATTICA study. Atherosclerosis. 2005;183:308-15.
- [Google Scholar]
- Adipokines and adipocyte targets in the future management of obesity and the metabolic syndrome. Mini Rev Med Chem. 2007;7:39-45.
- [Google Scholar]
- Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930-5.
- [Google Scholar]
- Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731-7.
- [Google Scholar]
- Influence of temperature on the chemical constituents and pharmacological effects of semen Cassiae. Zhongguo Zhong Yao Za Zhi. 1996;21:663-5. 703
- [Google Scholar]
- Anti-inflammatory activity of chrysophanol through the suppression of NF-kappaB/caspase-1 activation in vitro and in vivo. Molecules. 2010;15:6436-51.
- [Google Scholar]
- Use of scopoletin to inhibit the production of inflammatory cytokines through inhibition of the IkappaB/NF-kappaB signal cascade in the human mast cell line HMC-1. Eur J Pharmacol. 2007;555:218-25.
- [Google Scholar]
- Toxicity of dimethylsulfoxide as a solvent in bioassay system with HeLa cells evaluated colorimetrically with 3-(5,5-dimethylthiazole-zyl)-2,5-diphenyl-tetrazolium bromide. Agric Biol Chem. 1990;54:2961-6.
- [Google Scholar]
- Inhibitory effect of Gamibojungikgitang extract on mast cell-mediated allergic reaction in murine model. J Pharm Pharm Sci. 2005;8:94-101.
- [Google Scholar]
- Chemical constituents isolated from Polygala japonica leaves and their inhibitory effect on nitric oxide production in vitro. J Enzyme Inhib Med Chem. 2009;24:230-3.
- [Google Scholar]
- Antigenotoxic properties of Cassia tea (Cassia tora L.): mechanism of action and the influence of roasting process. Life Sci. 2004;76:85-101.
- [Google Scholar]
- Green tea supplementation affects body weight, lipids, and lipid peroxidation in obese subjects with metabolic syndrome. J Am Coll Nutr. 2010;29:31-40.
- [Google Scholar]
- (-)-Epigallocatechin-3-gallate inhibits pancreatic lipase and reduces body weight gain in high fat-fed obese mice. Obesity (Silver Spring). 2012;20:2311-3.
- [Google Scholar]
- The effects of allicin on weight in fructose-induced hyperinsulinemic, hyperlipidemic, hypertensive rats. Am J Hypertens. 2003;16:1053-6.
- [Google Scholar]
- Enhanced nitric oxide production is closely associated with serum lipid concentrations in adolescents. Clin Chim Acta. 2004;347:151-6.
- [Google Scholar]
- Biochanin-A, an isoflavon, showed anti-proliferative and anti-inflammatory activities through the inhibition of iNOS expression, p38-MAPK and ATF-2 phosphorylation and blocking NFκB nuclear translocation. Eur J Pharmacol. 2011;653:8-15.
- [Google Scholar]
- Mesenteric adipose tissue derived monocyte chemoattractant protein-1 plays a crucial role in adipose tissue macrophage migration and activation in obese mice. Obesity. 2006;14:1353-62.
- [Google Scholar]
- Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79-83.
- [Google Scholar]






