Translate this page into:
Simplified clinical algorithm for immediate antiretroviral therapy initiation: The HATI [HIV awal (early) Test & Treat in Indonesia] implementation research in Indonesia
For correspondence: Ms Nur Aini Kusmayanti, Center for Tropical Medicine, Faculty of Medicine Public Health & Nursing, Universitas Gadjah Mada, Gedung Penelitian dan Pengembangan FK-KMK UGM, Sayap Utara Lantai 2, Jl. Medika, Senolowo, Sinduadi, Mlati, Sleman, Yogyakarta 55281, Indonesia e-mail: nurainikusmayanti@gmail.com
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Although the World Health Organization recommends same day or rapid (< seven days) antiretroviral therapy (ART) initiation, delays in ART initiation remain common due to waiting for laboratory test results. This study employed a simplified clinical algorithm the HATI [HIV Awal (Early) Test & Treat Indonesia]-SAI (Simple ART Initiation) aimed to increase the proportion of ART uptake and decrease the time to ART initiation that can be used in various care settings.
Methods:
This study compared the percentage of ART uptake and retention, viral load (VL) suppression and time to ART initiation between the observation and intervention phases among newly diagnosed HIV patients from key populations. As part of the intervention, the newly diagnosed patients underwent screening using a simple form [consisting of data on age, height and weight (for body mass index calculation), questions on the presence of symptoms of HIV stages 1 and 2, tuberculosis, history of diabetes, hypertension and kidney disease], to determine eligibility for immediate ART initiation. Those who met the pre-defined criteria immediately received a combination of tenofovir lamivudine and efavirenz for two weeks. The baseline laboratory examination due to this was moved up to two weeks post ART. Factors significantly associated with ART uptake were also determined and their odds ratios were measured using logistic regression analysis.
Results:
A total of 2173 people newly diagnosed with HIV were recruited, with 1579 and 594 in the observation and intervention phases, respectively. In both phases, the majority were men who have sex with men, who were young (<30 yr old) and employed, with high levels of education. The intervention phase significantly increased the proportion of ART initiation [91%, 95% confidence interval (CI): 89-93% vs. 78%, 95% CI: 76-80%] but did not have any impact on the proportion of six months retention and VL suppression. The intervention also significantly decreased the time to ART initiation from median ± interquartile range: 9±20 days to 2±10 days.
Interpretation & conclusions:
The findings of this study suggest that the HATI-SAI intervention increased the uptake and decreased the time for immediate ART initiation. The HATI-SAI provides a simple and safe clinical approach that can readily be adopted in different settings without a costly investment in technology.
Keywords
ART
Early HIV Awal Test and Treat Indonesia
HIV-AIDS
HATI SAI
The Joint United Nations Programme on Acquired Immunodeficiency Syndrome (UNAIDS) launched the (95-95-95) target for controlling the human immunodeficiency virus (HIV) by 2030, i.e. 95 per cent of people at risk of HIV will be tested and diagnosed, 95 per cent of HIV-positive patients will commence anti-retroviral therapy (ART) and 95 per cent of those starting will achieve viral suppression1. Many countries, including Indonesia have shown that the level of HIV-testing is high, but the coverage of ART initiation, particularly the immediate initiation, remains poor, which could be increasing the risk of disease progression and ongoing transmission in the population2-10. Moreover, a HATI [HIV Awal (Early) Test and Treat Indonesia] study11 reported that, at diagnosis, the majority of people with HIV were in stages 1 and 2 (86%) and had CD4 levels of more than 200 cells/µl (65%), indicating relatively early diagnosis11. Readiness and initiation to ART are influenced by several individual, social and structural factors and their combination12-16.
In the 2017 revised guidelines for HIV care and treatment17, the WHO recommended rapid same-day initiation of ART for people testing positive for HIV. Here rapid initiation was defined as the starting of ART within seven days of HIV diagnosis. Rapid treatment initiation is viewed as a key intervention to uptake of ART increase by newly diagnosed patients, particularly by reducing the loss to care between diagnosis of HIV and treatment initiation.
In many settings, standard procedures for starting ART require laboratory testing that are expensive and requires attendance at tertiary-level hospitals5,18,19. The procedures can be lengthy and burdensome, with multiple clinic visits and long waiting times for patients, preventing some patients from starting ART entirely or leading to long delays for others. There is a need for a simple clinical procedure for ART initiation that eliminates pre-ART laboratory test requirements, so ART initiation can be safely delivered in a range of healthcare facilities, including primary healthcare.
Several studies have been conducted on rapid or same-day initiation of ART by developing or modifying the clinical algorithm18-22. A study on a simplified regime of ART initiation was conducted in China, in which HIV-positive patients underwent CD4 testing and ART initiation on the same day. Furthermore, irrespective of CD4 counts and the mortality rate was measured as an outcome18. The RapIT trial showed that offering same-day initiation could increase the uptake of ART and viral suppression20, but they used point-of-care test instruments to determine the CD4 counts for patient eligibility, which was not feasible for routine care21. The SLATE (Simplified Algorithm for Treatment Eligibility) study19 used a clinical algorithm that allows healthcare workers to determine eligibility for same-day treatment and initiate ART and it was shown to increase ART uptake within 28 days. An improved version of the algorithm was then tested in a randomized trial (SLATE II study), in which they administered checks with, medical history, physical examination, symptom reports readiness assessment and lipoarabinomannan tuberculosis (LAM-TB) examination for those with symptoms22.
The HATI study11 aimed to develop and evaluate four packages of interventions to improve the cascade of HIV care in Indonesia under the 95-95-95 UNAIDS target by examining their finding in the observational phase and qualitative study. Furthermore, interventions including oral fluid testing to enhance HIV testing, SMS reminders and motivational interviewing to increase the proportion of ART retention and viral load (VL) suppression were also reported earlier23. The present study aimed to implement the HATI - simplified anti-retroviral initiation-simple ART initiation (HATI-SAI) intervention to increase the proportion of ART initiation and shorten the time between HIV diagnosis to treatment.
Material & Methods
The present study was conducted at the Center for Tropical Medicine, Gadjah Mada University; Yayasan Kerti Praja, Bali & Research Center for Care and Control of Infectious Disease, Padjajaran University, Bandung, Indonesia.
Ethical approval was obtained from Health Research Ethics Committees (HREC) of National Institute of Health Research and Development & Gadjah Mada University, Indonesia; University of New South Wales, Australia. The study was also registered with ClinicalTrials.gov (NCT03429842). All eligible participants who screened HIV-positive and gave a written informed consent for the use of their de-personalized data in future epidemiological analysis were included in this study.
Study design: This study was based on an implementation research format. Through this study the HATI-SAI procedure was implemented in order to address WHOs recommendation for rapid HIV treatment initiation in HIV clinics in Indonesia.
To corroborate the effect of implementing the HATI-SAI procedure for immediate ART initiation, data were collected using a prospective longitudinal cohort design, consisting of an observation phase (November 2015-November 2017) and an intervention phase (November 2017-December 2018). In the observational phase data pertaining the standard HIV care, particularly for the initiation of ART were recorded. Intervention packages were then designed following the findings in the observation phase.
The basic methodology of the HATI process was published previously from the data recorded from the observational phase11. Briefly, participants who met the inclusion criteria were offered study enrolment by the clinic staff. Clinical monitoring at follow up clinic visits and six-month routine CD4 and VL testing were administered to those who agreed to join. The HATI standardized data collection forms were applied at eligibility screening, registration to the study, first visit to an ART treatment site and follow up visit.
The same methodology was applied to the participants in the intervention phase. In addition, for the HATI-SAI study, a simple ART-initiation eligibility form was used.
Study setting: The HATI study was conducted in four major cities in Indonesia: Jakarta, Bandung, Yogyakarta and Denpasar. The study sites were chosen purposively based on the resident key populations with a high prevalence of HIV in their respective settings. Moreover, in each location, the HIV testing and treatment services were well-established and had coordinating unit-affiliated to a university with long-standing experience in research among key on HIV/AIDS populations. The coordinating units in each location selected the satellite clinics on the basis of their documented high levels of HIV testing11.
In this study the HATI-SAI was particularly implemented in Bandung, Yogyakarta and Denpasar. Overall, there were nine healthcare facilities administrating the HATI-SAI (5 in Bandung, 3 in Yogyakarta and 1 in Denpasar), including primary care facilities as well as hospitals.
The primary care facilities were government-run public health centres or ‘Puskesmas’ and community clinics run by private (or partially government-supported) institutions. There were five primary care facilities included in this study, three being Puskesmas (Puskesmas Ibrahim Adjie in Bandung; Puskesmas Gedongtengen and Umbulharjo Satu in Yogyakarta) and two community clinics [Amarta Clinic (Kerti Praja Foundation) in Denpasar, Bali; and Mawar Clinic in Bandung]. There were four hospitals involved in the study: two were secondary (Ujungberung and Bungsu Hospital in Bandung) and two were tertiary (Hasan Sadikin Hospital in Bandung and Dr Sardjito Hospital in Yogyakarta) referral centres.
Primary care facilities, both Puskesmas and community clinics run by private institutions, generally have a low infrastructure as compared to hospitals, with only simple laboratory facilities. Before undertaking this study, the five participating primary care facilities were trained in the processes of ART initiation and ongoing management but had not yet begun operations. The hospitals in this study were already established ART centers. The clinic staff across all the study sites and facilities were trained on the simplified intervention package.
Study participants: The HATI study participants have been previously described in detail11. In both the observation and intervention phases, the study participants included were members of HIV key populations [female sex workers (FSW), men who have sex with men (MSM) and transgender (waria)], aged 16 yr or older, HIV positive for the first time (or who had not previously tested positive for HIV), and were able to provide informed consent11.
‘HIV positive for the first time’ refers to HATI participants recruited to the study on the day they were diagnosed with HIV infection. Clinic staff checked that no prior diagnosis was recorded in the clinic medical records or the HATI database across health facilities.
The study aimed to recruit 780 participants in the intervention phase i.e. 80 per cent power to detect at least a six per cent increase in ART initiation compared to the 72 per cent recorded in the standard of care phase.
Operational definitions for study participants: (i) Retention in care was defined as retention in care up to six months after ART initiation. (ii) Participants who were lost to follow up or transferred out within six months of ART initiation were assumed to have stopped treatment or continued ART but in non-HATI sites. (iii) People with HIV viral suppression were defined as <40 copies/ml in those with VL tests after ART initiation (within a window from three to nine months after ART initiation).
Observation phase: During the observation phase, all HIV-positive individuals were given ART under Indonesia’s standard national treatment guidelines as described previously11 after enrolment.
Intervention phase: In the intervention phase, all participants attending the health facilities at the start of this phase, whether HATI subjects or non-HATI subjects were included. All were given the option and those who consented were treated using the intervention package, but data from only those meeting the HATI study eligibility criteria were recorded and further analyzed.
Newly diagnosed HIV patients underwent a screening process for determining the eligibility to rapidly initiate ART by using the screening form designed specifically for this study (Appendix). The eligibility criteria under the HATI-SAI algorithm in under: <50 yr age, body mass index (BMI) >18.5 kg/m2, a symptomatic for TB (i.e., any night sweats fever, weight loss, or cough) or other opportunistic infections indicating HIV stage 3 or 4, no history of or current status of diabetes, hypertension or kidney disease. Patients who met the criteria were dispensed anti-retrovirals (ARVs) immediately on the same day and place of the clinic visit. Patients who failed to meet the criteria were referred for further investigation, care, tests or other services before being dispensed ARVs.
The available ART combination during the period of the study was a tenofovir-based regiment, i.e. tenofovir, lamivudine and efavirenz (TDF + 3TC + EFV). Patients were then given two weeks of drug supply; at which time they were examined for their creatinine level to check for kidney function abnormality after being exposed to tenofovir. If the creatinine level was within the normal limits, the participant could continue the treatment with the same regimen. For those who showed creatinine levels above the upper normal limit, were referred for further clinical workup (Fig. 1 and Supplementary Table).
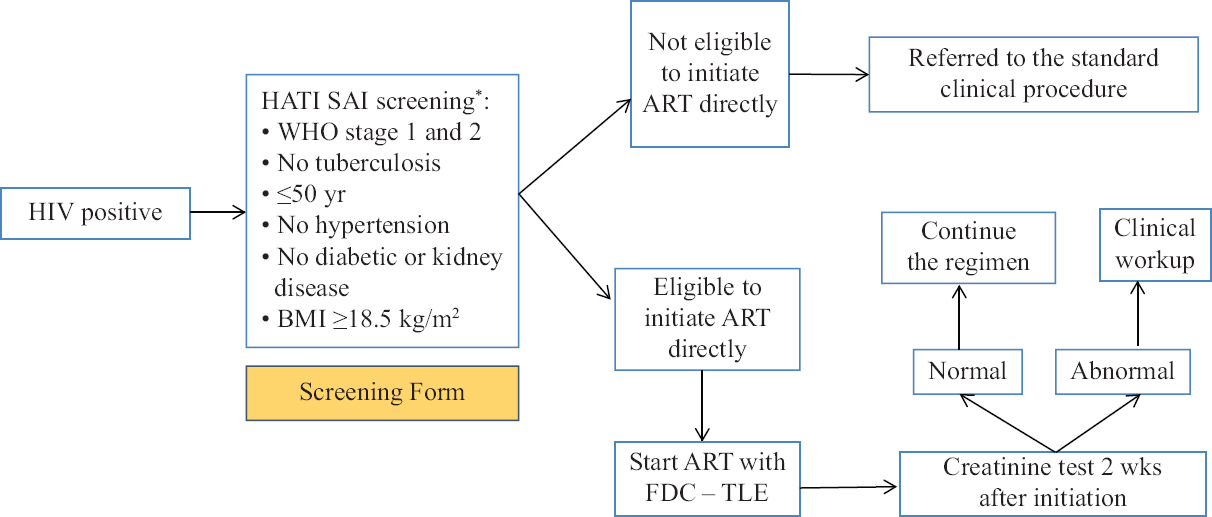
- The HATI SAI procedure (intervention phase). *Screening form is provided in Supplementary Table.
| Variables | Result | |
|---|---|---|
| Yes | No | |
| Is the patient above 50 yr old | ||
| Is the patient’s BMI | ||
| <18.5 kg/m2 (for >18 yr) or | ||
| <16 kg/m2 (for 16-18 yr) | ||
| Does the patient have TB symptoms* | ||
| Weakness or feeling sick | ||
| Cough (more than two or three weeks) | ||
| Fever | ||
| Night sweat | ||
| Weight loss | ||
| Does the patient currently or have a history of diabetes mellitus | ||
| Does the patient currently or have a history of hypertension (or systolic pressure of >140 mmHg) | ||
| Does the patient currently or have a history of kidney disease | ||
| Is the patient HIV clinical stage III (except for oral candidiasis) or IV | ||
| Is the patient eligible for rapid ART initiation? | ||
*If one or more is YES, the patient would be interpreted as YES and referred for further TB diagnosis, such as sputum AFB using the GeneXpert and/or chest X-ray. HIV, human immunodeficiency virus; BMI, body mass index; ART, antiretroviral therapy; TB, tuberculosis; AFB, acid-fast bacillus
The existing clinical and laboratory procedures to determine the treatment eligibility were dropped under the HATI-SAI intervention, following negotiation with provincial health offices to ensure their support for this trial. The intervention in the three cities was started at different time points, depending on the approval time points from the local health offices.
Statistical analysis: Patient baseline characteristics were summarized for all participants in the observation and intervention phases. Comparisons were made between participants recruited in the observation phase and those in the intervention phase, with regard to proportions of ART initiation, retention in care and HIV viral suppression. Additional comparison was for the proportion of initiating ART within seven, 14 and 28 days, including the comparison of those proportions in primary healthcare and in hospitals. The significance from all comparisons was tested by a likelihood ratio Chi-square test.
The overall time (median, IQR) to initiate ART was compared between the observation and intervention phases. Kaplan-Meier methods were used to plot the time to ART initiation in the observation and intervention phases. Participants’ follow up were censored either when they were lost to follow up, transferred out from the clinic or died before the end of the study period. Log-rank test was performed to compare two survival curves produced from the observation and intervention phases with the assumption that the survival times were continuous and the risk of an event in both groups do not change with time.
The impact of our intervention on the primary endpoint was measured based on the odd’s ratio (OR) and the 95 per cent confidence interval (CI) resulting from the logistic regression that was performed based on the assumption that our outcome was binary, no repeated measurement in our observation and no multicollinearity. The primary endpoint was rapid ART initiation, defined as initiating ART ≤ seven days after HIV positivity was diagnosed.
The logistic regression was also performed to assess the impact of our intervention on the long-term outcomes, i.e. remained on ART and had VL suppression six months after initiation. Additional covariates included in these analyses that were measured as the part of ART initiation included, SAI eligibility criteria, HIV stage, TB symptoms and CD4 and VL level at baseline.
All statistical analyses were performed using the STATA 17 (StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC, USA). P<0.05 was considered as significant
Results
There were a total of 2173 subjects recruited in the study, including 1579 recruited in the observation phase and 594 in the intervention phase. In both phases, the majority of subjects were MSM, young (<30 yr old), with high levels of education, and employed (Table I).
| Variables | Total enrolment (n=2173) | Observation phase, n (%) | Intervention phase, n (%) |
|---|---|---|---|
| Age (yr) (median±IQR) | 27±10 | 26±9 | 27±9 |
| Key population | |||
| Transgender | 50 | 43 (3) | 7 (1) |
| MSM | 1841 | 1326 (84) | 515 (87) |
| FSW | 282 | 210 (13) | 72 (12) |
| Type of healthcare facilities | |||
| Primary healthcare | 867 | 658 (42) | 209 (35)** |
| Hospital-based Education | 1306 | 921 (58) | 385 (65) |
| Education | |||
| Junior high school or less | 449 | 347 (22) | 102 (17)* |
| Senior high school or higher | 1723 | 1232 (78) | 491 (83) |
| Employment status | |||
| Unemployed or student | 500 | 365 (23) | 135 (23) |
| Employed | 1672 | 1214 (77) | 458 (77) |
P *<0.05, **<0.01. IQR, interquartile range; FSW, female sex worker; MSM, men who have sex with men
Cascade of HIV care: Overall, until the end of the study period, out of 2173 participants, as many as 1771 (82%, 95% CI: 80-83%) initiated ART, of whom 1053 (48%, 95% CI: 46-51) stayed on treatment at six months, 934 (43%, 95% CI: 41-45) had a VL test and 748 (34%, 95% CI: 32-36) were virologically suppressed at six months. The proportion of ART initiation coverage was significantly higher in the intervention phase (91%, 95% CI: 89-93) compared to the observation phase (78%, 95% CI: 76-80) (P=0.002). The proportion of staying on six months of treatment was similar between the two phases, with 47 per cent (95% CI: 44-49) in the observation phase and 53 per cent (95% CI: 49-57) in the intervention phase (P=0.511). The proportion of VL suppression in patients who remained on ART at six months also remained relatively same (P=0.894) in the observation phase (34%, 95% CI; 31-36) and intervention phase (37%, 95% CI: 33-41) (Fig. 2).
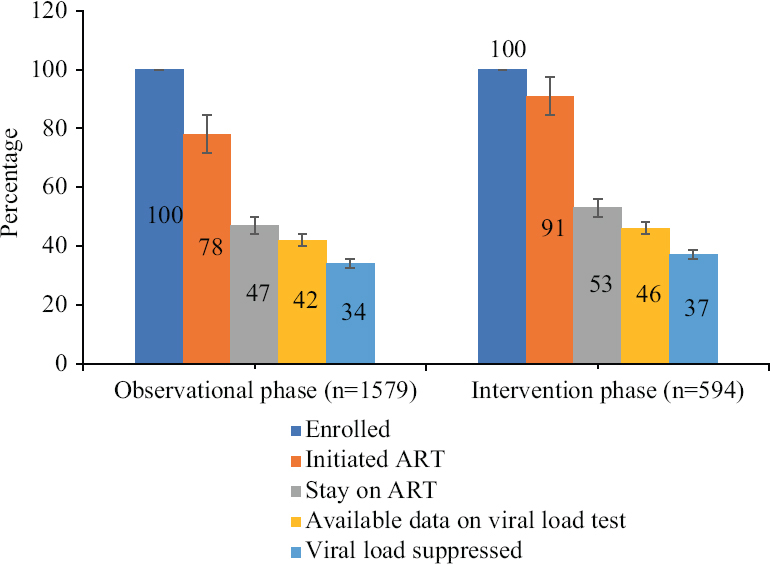
- HIV treatment cascade in the observation and intervention phases.
The proportion initiating ART increased in both primary care settings as well as hospitals. The improvement was slightly higher in primary care facilities [observation (65%) vs. intervention (92%)] than in hospitals [observation (87%) vs. intervention (91%)] (Fig. 3).
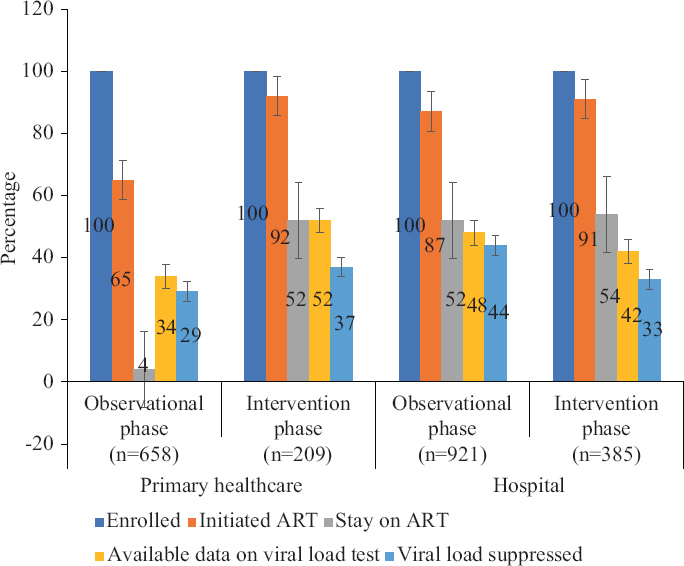
- HIV treatment cascade based on study phases and type of health facilities.
The HATI-simple ART initiation (SAI) clinical algorithm for identification of eligible participants for rapid antiretroviral therapy (ART) initiation: Among 594 people in the intervention phase, 424 (74%) participants were eligible for rapid ART initiation, 142 (24%) were not eligible and 28 (2%) did not have data on the ART eligibility criteria. The screening form questions enabled identification of those who were suspected of TB (90 patients), WHO clinical stage 3 or 4 (76 patients), low BMI (49 patients), those over 50 yr old (9 patients), having diabetes or kidney disease (4 patients), or high blood pressure (4 patients) or a combination of those conditions, and were referred for further investigation or management before they can start ART. Among those following the HATI-SAI clinical algorithm, all had normal creatinine levels at two weeks of ART using tenofovir.
Time to ART initiation: The time to initiate ART from HIV diagnosis was significantly shorter in the intervention phase (median±IQR, 2±10 days) compared to the observation phase (9±20 days) (Fig. 4).
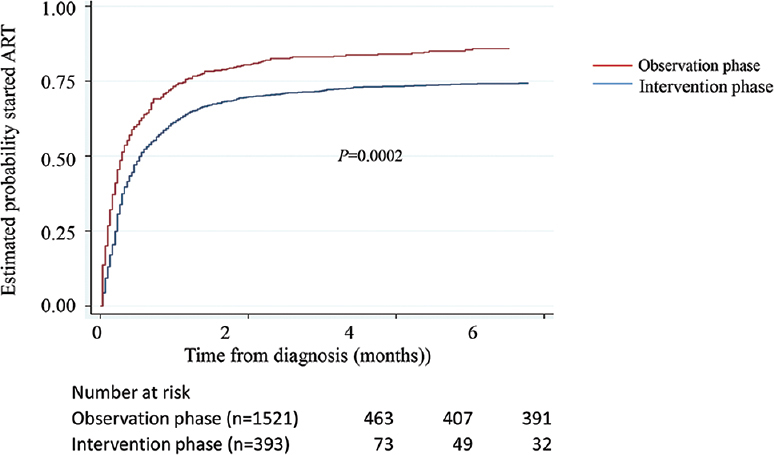
- Time to ART initiation based on the study phase.
A significant decrease in the number of days to start ART was seen in the primary care facilities [13 days (IQR 6-32) to 0 days (0-4)], whereas a smaller reduction was seen in the hospitals [8 days (4-20) to 4 days (1-16)]. The overall number of those initiating ART within seven days was 41 per cent (95% CI: 40-44) (904/2173). Our intervention resulted in an increased number of those initiating ART within seven days, i.e. from 33 per cent (95% CI: 31-36) in the observation phase to 65 per cent (95% CI: 60-68) in the intervention phase. The increased number of rapid ART was seen both in primary care [24% (95% CI: 21-27) to 78% (95% CI: 71-83)] and in hospital [40% (95% CI: 37-43) to 57% (95% CI: 51-62)] facilities (Table II).
| Study setting | Total enrollment | Median±IQR (days) | Initiating ART within seven days, n [%, (95% CI)] | Initiating ART within 14 days, n [%, (95% CI)] | Initiating ART within 28 days, n [%, (95% CI)] |
|---|---|---|---|---|---|
| Primary healthcare | |||||
| Observational phase | 658 | 13 (6-32) | 158 [24, (21-27)] | 229 [35, (31-39)] | 311 [47, (43-51)] |
| Intervention phase | 209 | 0 (0-4) | 162 [78, (71-83)]*** | 178 [85, (80-90)]*** | 186 [89, (84-93)]*** |
| Hospital-based | |||||
| Observational phase | 921 | 8 (4-20) | 366 [40, (37-43)] | 546 [59, (56-62)] | 775 [49, (47-52)] |
| Intervention phase | 385 | 4 (1-16) | 218 [57, (51-62)]*** | 258 [67, (62-72)]* | 436 [73, (70-77)]*** |
| Overall | |||||
| Observational phase | 1579 | 9 (4-24) | 524 [33, (31-36)] | 775 [49, (47-52)] | 971 [61, (59-64)] |
| Intervention phase | 594 | 2 (0-10) | 380 [65, (60-68)]*** | 436 [73, (70-77)]*** | 483 [81, (78-84)]*** |
P *<0.05, ***<0.001
Factors associated with rapid ART initiation: These included being a part of the intervention phase as compared to the observational phase (OR 3.16, 95% CI: 2.54-3.92); being FSW compared to other key populations (OR 2.01, 95% CI: 1.01-3.98), and being employed (OR 1.28, 95% CI: 1.03-1.60) based on univariate analysis. The intervention phase was the only factor that remained significantly associated with rapid ART initiation in the multivariate analysis [adjusted odds ratio (aOR) 3.23, 95% CI: 1.05-1.35] (Table III).
| Variables | Total enrolment (n) | Rapid ART initiation, n (%) | Univariate analysis, OR (95% CI) | Multivariate analysis, aOR (95% CI) |
|---|---|---|---|---|
| Study phase | ||||
| Observation phase | 1579 | 524 (33) | 1 | 1 |
| Intervention phase | 594 | 380 (91) | 3.16 (2.54-3.92)*** | 3.23 (1.05-1.35)** |
| Age (yr) (median±IQR)## | 27±10 | 27±9 | 1.01 (0.99-1.01) | 0.99 (0.99-1.01) |
| Key population | ||||
| MSM | 1841 | 741 (40) | 1 | 1 |
| FSW | 282 | 146 (52) | 1.57 (1.19-2.08)*** | 1.69 (1.18-2.41)** |
| Transgender | 50 | 17 (34) | 0.78 (0.41-1.49) | 0.92 (0.47-1.79) |
| Type of healthcare facilities | ||||
| Hospital | 1306 | 584 (45) | 1 | 1 |
| Primary healthcare | 867 | 320 (37) | 1.05 (0.86-1.27) | 1.06 (0.87-1.30) |
| Education | ||||
| Junior high school or less | 449 | 197 (44) | 1 | 1 |
| Senior high school or higher | 1723 | 706 (41) | 0.83 (0.66-1.05) | 0.99 (0.73-1.34) |
| Employment status | ||||
| Unemployed or student | 500 | 190 (38) | 1 | 1 |
| Employed | 1672 | 713 (43) | 1.28 (1.03-1.60)* | 1.24 (0.98-1.58) |
P *<0.05, **<0.01, ***<0.001. ##Risk per five years increase. Rapid ART initiation was defined as initiated more than or equal to seven days. OR, odds ratio; aOR, adjusted OR; CI, confidence interval; FSW, female sex worker; MSM, men who have sex with men
Furthermore, the increase was higher in the primary care facilities (aOR 9.57, 95% CI: 6.14-14.93) compared to the hospitals (aOR 2.06, 95% CI: 1.59-2.68) (Table IV).
| Variables | Primary healthcare, aOR (95% CI; P) | Hospital-based, aOR (95% CI; P) |
|---|---|---|
| Study phase | ||
| Observation phase | 1 | 1 |
| Intervention phase | 9.57 (6.14-14.93)*** | 2.06 (1.59-2.68)*** |
| Age (yr) (median±IQR)## | 0.99 (0.99-1.02) | 0.99 (0.98-1.01) |
| Key population | ||
| MSM | 1 | 1 |
| FSW | 1.79 (0.90-3.57) | 1.64 (1.07-2.50)* |
| Transgender | 0.35 (0.10-1.26) | 1.24 (0.54-2.83) |
| Education | ||
| Junior high school or less | 1 | 1 |
| Senior high school or higher | 1.43 (0.78-2.62) | 0.87 (0.61-1.24) |
| Employment status | ||
| Unemployed or student | 1 | 1 |
| Employed | 0.79 (0.52-1.19) | 1.52 (1.12-2.06)** |
P *<0.05, **<0.01, ***<0.001; ##Risk per five years increase. The proportion of rapid ART initiation in primary healthcare was 24 per cent in the observation phase and 78 per cent in the intervention phase (Table II); The proportion of rapid ART initiation in hospital was 40 per cent in the observation phase and 57 per cent in the intervention phase (Table II).
Long-term outcomes: In multivariate analyses (Table V), earlier ART initiation (≤7 days; (P=0.857) and being in the intervention phase (P=0.953) did not significantly increase the risk of not retaining ART. The following covariates were, however, associated with a greater likelihood of retention: older age [aOR 1.13, 95% CI (1.05-1.20)] and higher education level [aOR 1.55, 95% CI (1.15-2.08)].
| Variables | Initiated ART (n) | Retention on ART, n (%) | Bivariate analysis, OR (95% CI; P) | Multivariate analysis, aOR (95% CI; P) |
|---|---|---|---|---|
| Time to initiate ART (days) | ||||
| ≤7 | 904 | 518 (57) | 1 | 1 |
| >7 | 1269 | 535 (42) | 1.02 (0.85-1.24) | 1.02 (0.83-1.26) |
| Study phase | ||||
| Observation phase | 1229 | 737 (60) | 1 | 1 |
| Intervention phase | 542 | 316 (58) | 0.93 (0.76-1.15) | 1.01 (0.80-1.27) |
| Age (yr) (median±IQR)## | 27±9 | 27±9 | 1.10 (1.03-1.17)** | 1.13 (1.05-1.20)*** |
| Key population | ||||
| Transgender | 39 | 21 (54) | 1 | 1 |
| MSM | 1492 | 921 (62) | 1.38 (0.73-2.62) | 1.41 (0.73-2.74) |
| FSW | 240 | 111 (46) | 0.74 (0.37-1.45) | 0.89 (0.44-1.79) |
| Type of healthcare facilities | ||||
| Hospital | 626 | 376 (60) | 1 | 1 |
| Primary healthcare | 1145 | 677 (59) | 0.96 (0.79-1.17) | 1.01 (0.80-1.26) |
| Education | ||||
| Junior high school or less | 360 | 175 (49) | 1 | 1 |
| Senior high school or higher | 1410 | 878 (62) | 1.75 (1.38-2.20)*** | 1.55 (1.15-2.08)** |
| Employment status | ||||
| Unemployed or student | 411 | 234 (57) | 1 | 1 |
| Employed | 1359 | 819 (60) | 1.15 (0.92-1.43) | 1.14 (0.89-1.45) |
| WHO clinical stages | ||||
| 3 and 4 | 216 | 124 (57) | 1 | 1 |
| 1 and 2 | 1554 | 928 (60) | 1.10 (0.82-1.47) | 1.09 (0.76-1.56) |
| TB co-infection | ||||
| Yes | 60 | 29 (48) | 1 | 1 |
| No | 1709 | 1022 (60) | 1.59 (0.95-2.66) | 1.54 (0.85-2.81) |
| CD4 baseline (cell/µl) | ||||
| >350 | 377 | 211 (56) | 1 | 1 |
| ≤350 | 1147 | 714 (62) | 1.30 (1.02-1.64)* | 1.21 (0.94-1.55) |
| Missing value | 247 | 128 (48) | 0.85 (0.61-1.17) | 0.81 (0.57-1.17) |
| VL baseline (copies/ml) | ||||
| <10,000 | 189 | 112 (59) | 1 | 1 |
| 10,001-100,000 | 633 | 392 (62) | 1.12 (0.80-1.56) | 1.11 (0.78-1.56) |
| ≥100,001 | 827 | 488 (59) | 0.99 (0.72-1.37) | 0.90 (0.64-1.26) |
| Missing value | 122 | 61 (50) | 0.69 (0.43-1.09) | 0.74 (0.45-1.20) |
P *<0.05, **<0.01, ***<0.001. ##OR for per five years increase
Significant covariates that can inhibit VL suppression after six months of ART in multivariate analysis included being employed [aOR 0.59, 95% CI (0.37-0.93)], baseline CD4 <350 cell/µl [aOR 0.63, 95% CI (0.38-1.02)] and baseline VL >100.000 copies/ml [aOR 0.33, 95% CI (0.17-0.64)] (Table VI).
| Variables | On ART (n) | Availability data, n (%) | VL suppressed, n (%) | Bivariate analysis, OR (95% CI) | Multivariate analysis, aOR (95% CI) |
|---|---|---|---|---|---|
| Time to initiate ART (days) | |||||
| ≤7 | 518 | 485 (94) | 384 (79) | 1 | 1 |
| >7 | 535 | 449 (84) | 364 (81) | 1.09 (0.79-1.51) | 1.11 (0.77-1.59) |
| Study period | |||||
| Observation phase | 737 | 663 (90) | 531 (80) | 1 | 1 |
| Intervention phase | 316 | 271 (86) | 217 (80) | 0.98 (0.68-1.39) | 0.99 (0.66-1.48) |
| Age (yr) (median±IQR)## | 27±9 | 27±9 | 27±9 | 0.93 (0.85-1.03) | 1.00 (0.91-1.12) |
| Key population | |||||
| Transgender | 21 | 18 (86) | 14 (78) | 1 | 1 |
| MSM | 921 | 818 (89) | 658 (80) | 1.20 (0.39-3.69) | 1.20 (0.36-4.04) |
| FSW | 111 | 98 (88) | 76 (78) | 0.99 (0.29-3.30) | 0.97 (0.27-3.53) |
| Type of healthcare facilities | |||||
| Hospital | 370 | 329 (89) | 281 (85) | 1 | 1 |
| Primary healthcare | 683 | 605 (89) | 467 (78) | 0.59 (0.41-0.85)** | 0.69 (0.47-1.01) |
| Education | |||||
| Junior high school or less | 175 | 155 (89) | 120 (77) | 1 | 1 |
| Senior high school or higher | 878 | 779 (88) | 628 (81) | 1.24 (0.82-1.88) | 1.18 (0.69-1.99) |
| Employment status | |||||
| Unemployed or student | 234 | 212 (91) | 183 (86) | 1 | 1 |
| Employed | 819 | 722 (88) | 565 (78) | 0.58 (0.38-0.89)* | 0.59 (0.37-0.93)* |
| WHO clinical stages | |||||
| 3 and 4 | 124 | 95 (77) | 67 (71) | 1 | 1 |
| 1 and 2 | 928 | 839 (90) | 681 (81) | 1.84 (1.14-2.95)* | 1.36 (0.77-2.43) |
| TB co-infection | |||||
| Yes | 29 | 22 (76) | 14 (64) | 1 | 1 |
| No | 1022 | 911 (89) | 734 (81) | 2.41 (0.99-5.84) | 1.69 (0.60-4.71) |
| CD4 baseline (cell/µl) | |||||
| >350 | 211 | 193 (91) | 169 (88) | 1 | 1 |
| ≤350 | 714 | 640 (90) | 495 (77) | 0.50 (0.31-0.79)** | 0.63 (0.38-1.02)* |
| Missing data | 128 | 101 (79) | 84 (69) | 0.70 (0.36-1.38) | 0.85 (0.39-1.82) |
| VL baseline (copies/ml) | |||||
| <10,000 | 112 | 106 (95) | 95 (90) | 1 | 1 |
| 10,001-100,000 | 392 | 349 (89) | 307 (88) | 0.89 (0.44-1.80) | 0.95 (0.46-1.94) |
| ≥100,001 | 488 | 433 (89) | 306 (71) | 0.28 (0.15-0.54)*** | 0.33 (0.17-0.64)*** |
| Missing data | 61 | 46 (75) | 40 (87) | 0.77 (0.27-2.23) | 0.77 (0.25-2.36) |
P *<0.05, **<0.01, ***<0.001. ##OR for per five years increase. On ART was defined as retained on ART during six months of ART initiation; Availability data were defined as the availability of the result of the VL test at six months of ART initiation; VL suppressed was defined as 40 copies per ml or less
Discussion
This study showed that the HATI-SAI intervention was associated with an improvement in the cascade of HIV care, with a substantial increase in ART coverage and reduction in time to initiation of ART, but it did not impact the number of those retained in care at six months nor those with VL suppression. Immediate ART initiation is one of the keys to controlling the HIV epidemic by reducing the morbidity and mortality of patients and minimizing the risk of transmission in the community. Various conditions, however, influence and can be barriers to ART uptake, such as readiness to start life-long treatment24,25, the concern of adverse effects26, the experience of criminalization among PWID27, the presence of informal peer network, community-based organization, outreach workers and fear of stigma14,15. Other than these clinical or structural factors may also influence ART uptake, such as inefficient multi-step process12, geography, type of healthcare facility (primary vs. hospital) and place of HIV diagnosis and treatment (e.g. one-stop service)16. Adherence to life-long treatment and subsequent viral suppression are other major problems in HIV control, which are influenced by many factors. The present study findings, along with previous studies, countered the concern that rapid ART could lead to lower levels of treatment retention and VL suppression28,29. Time (rapid or delayed) to ART initiation and type of population can have a varied impact on adherence28-31, and one study in pregnant mothers found that rapid ART was not associated with the level of adherence and VL suppression32. Additional interventions may be needed to improve ART retention and viral suppression in key populations.
Methods and strategies for rapid ART have been evaluated elsewhere5,16,18,21,33. However, the need for examination of CD4 counts and LAM-TB, pre-initiation multi-step counselling and referral to higher-level health facilities were still found in those studies. Our HATI-SAI clinical algorithm is simpler and does not need additional laboratory or radiographic examinations. Primary care clinicians would only have to perform a simple anamnesis and physical examination with a short screening form to identify those eligible for immediate ART, thereby allowing more patients to start their medication on the same day of diagnosis or first-time visits to ART centers. Supportive laboratory testing, such as baseline complete blood count, liver and renal function tests and CD4 counts, can be taken at the subsequent scheduled visit in 2-4 wk time. The argument is that ART can and should be initiated regardless of CD4 and VL counts17, the requirement to screen for TB can be conducted using a simple form and that laboratory and/or radiologic examination for TB is only conducted on those who have one or more signs and symptoms of TB19,22. Furthermore, the ART regimen [tenofovir, lamivudine and efavirenz (TDF + 3TC/FTC + EFV)] is safe enough without prior or baseline laboratory data. Only TDF should be used with caution in several situations, e.g. BMI under 18.5 (kg/m2), known to have uncontrolled and long-standing diabetes or hypertension, and older age (>50 yr), all of which are already asked for and screened for in the HATI-SAI form.
Our simple screening method to determine eligibility for SAI can be safely given in same-day service and at the same health facilities regardless of the laboratory’s capability. This has the potential to reduce delays in ART initiation and improve treatment uptake. Prescribing ART on the day of HIV diagnosis ensures that people with HIV infection are linked to care and that health facilities do not lose the opportunity to initiate ART. This study found that the majority of newly diagnosed HIV patients in Indonesia were eligible to initiate ART rapidly. While it is difficult to say whether baseline CD4 or VL differed between the two study periods, there was no evidence for differences in HIV-related stages, so this factor is unlikely to have confounded the uptake of treatment.
This clinical algorithm for rapid ART initiation and the results of our study add to the body of information supporting the use of rapid ART in health facilities with limited resources and facilities. There is an urgent need to expand treatment services to the primary care level and community clinics, which may provide more ‘friendly’ services to socially stigmatized ‘key populations’. Readiness to initiate treatment at primary and community levels may also be the need for hospital referrals for ART, one of the major obstacles to rapid ART initiation11,14,16,28.
Strengths of our study include the prospective nature of recruitment and follow up as well as the comparison between three key populations and two types of healthcare facilities. The limitation of the study was its non-randomized, before and after design. The standard care and intervention phases were in different time periods, which may have led to the confounding of outcomes due to changes in other conditions, such as policy and general awareness of the importance of early treatment among clinicians or patients. We adopted this design as a pragmatic means of assessing the impact of the simplified approach.
Another limitation was the representativeness of our study participants to the overall HIV-positive individuals in Indonesia because four big cities were involved and only HIV key populations. Nevertheless, the finding that the HATI-SAI was proven feasible and effective in improving rapid initiation coverage in big cities, optimism can be shown similar towards other cities. The HATI-SAI is able to resolve the hampers to initiating ART in limited resource sttings by eliminating advanced laboratory testing procedures to initiate ART. Moreover, implementing the HATI-SAI procedure in the general population might be easier after the achievement in HIV key populations who are more difficult to reach. However, as the majority of our participants were MSM and only a few participants from FSW and transgender sub-populations, the findings of this study may be generalizable to MSM.
Overall, this study showed that our simplification of the ART initiation procedure was feasible, effective and clinically safe (no one experienced a significant increment in creatinine level). It was associated with a reduced delay time to initiate ART and increased rapid treatment uptake and can be used in any type of health facility. As per the author’s knowledge so fare there are no technical guidelines covering clear step-by-step patient intake to do rapid ART. This simplified procedure therefore should be scaled up at the national level and in other countries where relevant to enable rapid treatment initiation and reach the HIV test and treatment goals as directed per the WHO guidelines.
Acknowledgment:
We dedicate this manuscript to our beloved mentors, Prof. David Cooper, who passed away in March 2018, and Prof. Nyoman Dewa Wirawan, who passed away in September 2020. Authors acknowledge L. Septiani and D. N. Widyanthini, who coordinated data management in Jogjakarta and Bali, the Provincial and District Health Offices involved as HATI sites for their technical and regulatory support, HATI’s assigned healthcare facilities (Puskesmas, Clinics and hospitals), the outreach workers and others for their help with recruitment and follow up procedure, and CBO who worked on the HIV issue, for their advice on study design and result interpretation
Financial support & sponsorship: This study was funded by the Australian Government Department of Foreign Affairs and Trade, WHO and the Indonesian Government. The cost of the VL and CD4 tests for those participants who were not covered by the national insurance scheme were covered under this study.
Conflicts of Interest: None.
References
- Achieving the 95 95 95 targets for all: A pathway to ending AIDS. PLoS One. 2022;17:e0272405.
- [Google Scholar]
- Joint United Nations Programme on HIV/AIDS. Fact sheet –World AIDS Day 2019. 2019. Global HIV statistics. Available from: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf
- [Google Scholar]
- Joint United Nations Programme on HIV/AIDS. 90–90–90: Good progress, but the world is off-track for hitting the 2020 targets. Available from: https://www.unaids.org/en/resources/presscentre/featurestories/2020/septem ber/20200921_90-90-90
- [Google Scholar]
- 2022. Joint United Nations Programme on HIV/AIDS. HIV AIDS Asia Pacific research statistical data information resources –AIDS data hub. Available from: http://aphub.unaids.org/
- Short-term outcomes of rapid initiation of antiretroviral therapy among HIV-positive patients: Real-world experience from a single-centre retrospective cohort in Taiwan. BMJ Open. 2019;9:e033246.
- [Google Scholar]
- Undetectable equals untransmittable: A game changer for HIV prevention. Clin Chem. 2020;66:406-7.
- [Google Scholar]
- Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive partner taking suppressive antiretroviral therapy (PARTNER): Final results of a multicentre, prospective, observational study. Lancet. 2019;393:2428-38.
- [Google Scholar]
- World Health Organization. Consolidated guidelines on HIV prevention, diagnosis, treatment and care for key populations. Available from: http://apps.who.int/iris/bitstream/10665/128048/1/9789241507431_eng.pdf?ua=1
- 2018. World Health Organization. WHO ARV as prevention transmission. Available from: http://www.who.int accessed February 13, 2020
- 2015. World Health Organization. Policy brief consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection what's new. Available from: https://www.who.int/publications/i/item/9789241549684
- The cascade of HIV care among key populations in Indonesia: A prospective cohort study. Lancet HIV. 2018;5:e560-8.
- [Google Scholar]
- Stumbling blocks at the clinic: Experiences of seeking HIV treatment and care in South Africa. AIDS Behav. 2018;22:765-73.
- [Google Scholar]
- Exploring treatment needs and expectations for people living with HIV in South Africa: A qualitative study. AIDS Behav. 2018;22:2543-52.
- [Google Scholar]
- Increasing HIV treatment access, uptake and use among men who have sex with men in urban Indonesia: Evidence from a qualitative study in three cities. Health Policy Plan. 2020;35:16-25.
- [Google Scholar]
- Understanding the social influences on engaging key populations with HIV prevention: A qualitative study with men who have sex with men in three Indonesian cities. AIDS Educ Prev. 2019;31:206-23.
- [Google Scholar]
- Same-day antiretroviral treatment (ART) initiation and associated factors among HIV positive people in Northwest Ethiopia: Baseline characteristics of prospective cohort. Arch Public Health. 2020;78:87.
- [Google Scholar]
- World Health Organization. Managing advanced HIV disease and rapid initiation of antiretroviral therapy. Geneva: WHO; 2017.
- Simplified HIV testing and treatment in China: Analysis of mortality rates before and after a structural intervention. PLoS Med. 2015;12:e1001874.
- [Google Scholar]
- Simplified clinical algorithm for identifying patients eligible for same-day HIV treatment initiation (SLATE): Results from an individually randomized trial in South Africa and Kenya. PLoS Med. 2019;16:e1002912.
- [Google Scholar]
- Initiating antiretroviral therapy for HIV at a patient's first clinic visit: The RapIT randomized controlled trial. PLoS Med. 2016;13:e1002015.
- [Google Scholar]
- The evolving role of CD4 cell counts in HIV care. Curr Opin HIV AIDS. 2017;12:123-8.
- [Google Scholar]
- Improved simplified clinical algorithm for identifying patients eligible for immediate initiation of antiretroviral therapy for HIV (SLATE II): Protocol for a randomized evaluation. Trials. 2018;19:548.
- [Google Scholar]
- HIV self-testing for men who have sex with men: An implementation trial in Indonesia. AIDS Care. 2022;34:527-34.
- [Google Scholar]
- Factors associated with the lack of antiretroviral therapy initiation among eligible HIV-positive pregnant women in Swaziland. S Afr J Obstet Gynaecol. 2017;23:63-8.
- [Google Scholar]
- Readiness for antiretroviral therapy: Implications for linking HIV-infected individuals to care and treatment. AIDS Behav. 2016;27:138-44.
- [Google Scholar]
- A qualitative study to identify perceptual barriers to antiretroviral therapy (ART) uptake and adherence in HIV positive people from UK Black African and Caribbean communities. AIDS Behav. 2019;23:2514-21.
- [Google Scholar]
- HIV and the criminalisation of drug use among people who inject drugs: A systematic review. Lancet HIV. 2017;4:e357-74.
- [Google Scholar]
- Effectiveness of same-day antiretroviral therapy initiation in retention outcomes among people living with human immunodeficiency virus in Ethiopia: Empirical evidence. BMC Public Health. 2020;20:1802.
- [Google Scholar]
- Predictors of lost to follow-up in a “test and treat” programme among adult women with high-risk sexual behavior in Kampala, Uganda. BMC Public Health. 2020;20:353.
- [Google Scholar]
- Relationship between time to initiation of antiretroviral therapy and treatment outcomes: A cohort analysis of ART eligible adolescents in Zimbabwe. J Acquir Immune Defic Syndr. 2017;74:390-8.
- [Google Scholar]
- Factors influencing retention in care after starting antiretroviral therapy in a rural South African programme. PLoS One. 2011;6:e19201.
- [Google Scholar]
- Same-day antiretroviral therapy (ART) initiation in pregnancy is not associated with viral suppression or engagement in care: A cohort study. J Int AIDS Soc. 2018;21:e25133.
- [Google Scholar]
- Factors associated with initiation of antiretroviral therapy among HIV-positive people who use injection drugs in a Canadian setting. AIDS. 2016;30:925-32.
- [Google Scholar]






