Translate this page into:
Serum procalcitonin as a biomarker of bloodstream infection & focal bacterial infection in febrile patients
For correspondence: Dr Janjam Harikrishna, Department of Medicine, Sri Venkateswara Institute of Medical Sciences, Tirupati 517 507, Andhra Pradesh, India e-mail: hari_janjam@yahoo.co.in
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Bacteraemia is a serious form of infection in patients presenting with fever, thus, there is a necessity for a biomarker for rapid diagnosis of bacteraemia in such patients to make better therapeutic decisions. This study was conducted to measure the serum procalcitonin (PCT) levels at the time of initial presentation as a biomarker for identifying bacteraemia and as a predictor of mortality in patients admitted with acute fever.
Methods:
Four hundred and eighty patients, who presented with acute fever requiring admission to a tertiary care teaching hospital in south India, were prospectively studied. All patients were evaluated with a detailed history, physical examination, laboratory and imaging studies. Baseline serum PCT was measured for each patient within six hours of admission.
Results:
Among patients with single infectious cause (n=275), significantly higher median serum PCT levels were evident in bacteraemia compared to leptospirosis (P=0.002), dengue (P<0.001), scrub typhus (P<0.001) and evident focus of infection without bacteraemia (P=0.036). By receiver-operator characteristic curve analysis, at a cut-off value of >3.2 ng/ml, the sensitivity and specificity of serum PCT levels in predicting bacteraemia were 81.1 and 63.3 per cent, respectively. As per the worst-case scenario analysis, 91 (18.9%) patients had a poor outcome and these had significantly higher median serum PCT levels compared to survivors (n=389) [9.46 (2.03-44.4) vs. 1.23 (0.34-7.645); P<0.001]. At a cut-off value of >3.74 ng/ml, serum PCT levels at initial presentation predicted in-hospital mortality with a sensitivity and specificity of 67 and 67.5 per cent, respectively.
Interpretation & conclusions:
Our observations suggest that serum PCT level may be a useful biomarker for identifying bacteraemia as well as predicting mortality in patients with acute fever requiring admission to hospital.
Keywords
Acute fever
bacteraemia
biomarker
focal bacterial infection
mortality
serum procalcitonin
Fever with or without accompanying systemic symptoms or signs is a common presentation encountered by physicians. The aetiology of fever in the majority of cases is microbial infections in addition to other conditions such as malignancy, autoimmune disorders and trauma among others1. In the case of microbial infection, it is desirable to distinguish bacterial infections from other microbial causes. Thus, there is a need for a biomarker which is highly specific and sensitive for bacterial infections with a minimum turnaround time. Further, it should be capable of reliably distinguishing between bacterial infections from other kinds of viral, fungal or protozoan infections and non-infectious causes. Such a biomarker should be able to determine the infection severity and response to treatment, thus acting as an effective prognostic indicator2.
Traditional markers of sepsis, like C-reactive protein (CRP) and leucocyte count are widely used worldwide but lack specificity. Novel biomarkers which hold promise include procalcitonin (PCT), interleukins, eosinophil count (eosinopenia), adrenomedullin, interferon-γ, resistin, natriuretic peptides and copeptin, and the list is ever expanding3. PCT is a precursor of calcitonin, produced by the C-cells of the thyroid under the control of the CALC-1 gene4. Normally, the expression of the gene is found in the neuroendocrine cells of the thyroid and the lung. However, during microbial infections, there is an increased CALC-1 gene expression in various extra-thyroid tissues and cells including kidneys, liver, pancreas, leucocytes and adipose tissues5. Serum PCT has been tested as a biomarker in patients with conditions such as sepsis6, pneumonia7, urosepsis8 and febrile neutropenia9 and in selected settings such as emergency department10 and intensive care unit (ICU)11. Limited published data are available on the utility of serum PCT in predicting bacteraemia in patients admitted with fever1213141516 with varying results and cut-off points of serum PCT for predicting bacteraemia and mortality have not been reported in these studies12131415. Hence, the present study was conducted in patients with acute fever admitted to the medical wards and medical ICU (MICU) of a tertiary care hospital to study the utility of serum PCT measurement at the time of initial presentation as a biomarker for identifying bacteraemia and bacterial infection as the aetiological cause.
Material & Methods
All consecutive patients presenting with acute fever (oral body temperature ≥38°C) requiring admission to the medical wards and MICU in Sri Venkateswara Institute of Medical Sciences, Tirupati, a tertiary care referral hospital in south India, during January 2014 to July 2015, were included. These also included patients already admitted to the medical wards and MICU who developed new-onset fever during in-hospital stay. Pregnant women, patients with human immunodeficiency virus (HIV) infection and those unwilling to participate in the study were excluded. The study was approved by the Institutional Ethics Committee. Written informed consent was obtained from all patients participating in the study. In case the patient was unconscious, consent was obtained from the next responsible attendant.
A detailed history was obtained, and a thorough physical examination was carried out. Laboratory testing was done to ascertain the cause of fever as appropriate including complete haemogram, blood for malaria parasite by peripheral blood smear and/or quantitative buffy coat method (Becton Dickinson, USA), serum biochemistry, urinalysis, urine culture, serological testing for dengue fever, scrub typhus, leptospirosis, HIV, anti-nuclear antibodies; blood cultures and imaging studies such as chest radiograph, ultrasonography of abdomen, chest; computed tomography (CT), magnetic resonance imaging and positron emission tomography (PET)-CT and any other relevant investigation as considered appropriate. Blood cultures were obtained in two pairs of bottles and were performed by incubating in aerobic conditions using the BacT/ALERT 3D, an automated microbial detection system (bioMerieux, USA). From the positive samples, a small volume was inoculated onto blood agar and MacConkey agar plates and incubated overnight at 37°C for identifying the bacterial species. Serum PCT levels were measured using QDx Instacheck™ PCT (Boditech Med Inc., Gangwon-do, South Korea), as per the manufacturer's instructions within six hours of presentation. In patients with hospital-acquired infections (HAIs) (defined as new-onset fever while in hospital irrespective of primary admission diagnosis), appropriate laboratory investigations as described above were done within six hours of onset of fever.
Statistical analysis: Data were recorded on a predesigned proforma and managed using Microsoft Excel 2007 (Microsoft Corp., USA). All the entries were double-checked for any possible error. Patients were divided into eight groups, namely group 1 (bacteraemia), group 2 (evident focus of infection without bacteraemia), group 3 (leptospirosis), group 4 (scrub typhus), group 5 (malaria), group 6 (dengue), group 7 (non-infectious causes) and group 8 (undiagnosed). Descriptive statistics for the categorical variables were performed by computing the frequencies (percentages) in each category. For the quantitative variables, approximate normality of the distribution was assessed by the Kolmogorov-Smirnov test. Continuous variables following normal distribution were summarized by mean and standard deviation; the remaining variables were summarized as median [interquartile range (IQR)]. Median serum PCT levels were compared between patients with community-acquired infections and HAIs, survivors and non-survivors using the Mann-Whitney U-test. The comparison of group differences for continuous variables was performed using the Kruskal-Wallis test; post hoc analysis was carried out by pairwise comparisons and adjusting the significance level16. Worst-case scenario analysis17 was undertaken where patients who were discharged against medical advice (DAMA) were considered to have the worst outcome i.e., were considered to be non-survivors.
A receiver-operator characteristic (ROC) curve was plotted for serum PCT level to predict bacteraemia and to predict mortality. Stratified ROC analysis for defining the serum PCT cut-off for predicting bacteraemia was carried out for variables age (≥65 and <65 yr) and gender (male and female) and for only community-acquired infections. Further, multivariable ROC analysis was carried out after adjusting for age and gender.
The sample size for detecting bacteraemia was calculated assuming type I error of 0.05, power of the study of 90 per cent, area under the ROC curve of 0.65 and ratio of sample in negative to positive groups to be 10. It was required to study at least 44 patients with bacteraemia and 440 without bacteraemia. The sample size for mortality was calculated assuming type I error of 0.05, power of the study of 90 per cent, area under the ROC curve of 0.65 and ratio of sample in survivor and non-survivor groups to be 5. It was required to study at least 47 non-survivors and 235 survivors18.
Statistical software IBM SPSS version 20 (IBM SPSS Statistics, Somers, NY, USA); Stata/IC 12 for Windows (StataCorp LP, Texas, USA); MedCalc version 11.3.0 for Windows 2000/XP/Vista/7 (MedCalc Software bvba, Belgium) and R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria) were used for statistical analysis.
Results
During the study period, 501 patients were admitted in medical wards and MICU, of whom 480 met the inclusion criteria. Their mean age was 51.4±18.2 yr, and the male-to-female ratio was 1.3:1. Among these, in 286 (59.6%) patients, an infectious or non-infectious cause could be found, whereas in the rest 40.4 per cent of the cases, the aetiology of fever could not be ascertained (Table I). One hundred and fifty one patients had some focal infection without bacteraemia, whereas 53 (11.0%) patients had bacteraemia. Among the focal infections, the majority of the patients had pneumonia (33.1%), urinary tract infection (25.2%) and tuberculosis (9.4%) (Table II). Among the bacteraemia patients (n=53), 41 (77.4%) had Gram-negative bacteraemia compared to the rest 22.6 per cent with Gram-positive bacteraemia. Among Gram-negative organisms, Escherichia coli (n=21) and Pseudomonas (n=11) were the predominant isolates followed by Klebsiella, aerobic non-fermentative Gram-negative bacteria (3 each), Enterobacter spp. (n=2) and Salmonella Typhi (n=1). Gram-positive bacteraemia isolated was Staphylococcus aureus (n=12).
| Diagnostic category | n (%) |
|---|---|
| Aetiological cause evident | 286 (59.6) |
| Infection | 278* |
| Evident focus of infection without bacteraemia† | 151 (31.5) |
| Bacteraemia | 53 (11.0) |
| Leptospirosis | 23 (4.8) |
| Scrub typhus | 22 (4.6) |
| Dengue | 14 (2.9) |
| Malaria | 12 (2.5) |
| Other causes (non-infectious) | 8 (1.7) |
| Undiagnosed | 194 (40.4) |
*275 patients had single infection as aetiological cause; 3 patients had leptospirosis+scrub typhus co-infection; †Described in Table II
| Site | n (%) |
|---|---|
| Pneumonia | 50 (33.1) |
| UTI | 38 (25.2) |
| Cellulitis/abscess | 24 (15.9) |
| TB (pulmonary and extrapulmonary) | 14 (9.3) |
| Pyelonephritis | 10 (6.6) |
| Acute meningitis | 6 (4.0) |
| Viral | 5 |
| Bacterial | 1 |
| Acute gastroenteritis | 5 (3.3) |
| Puerperal sepsis | 2 (1.3) |
| Swine flu | 2 (1.3) |
UTI, urinary tract infection; TB, tuberculosis
The comparison of serum PCT levels in patients presenting with acute febrile illness in whom an aetiological diagnosis could be established is shown in Table III. The highest median serum PCT levels at initial presentation were observed in patients with bacteraemia, followed by focal infection without bacteraemia. Figure 1 shows the comparison of serum PCT levels in patients presenting with acute febrile illness in whom an aetiological diagnosis could be established. On pairwise comparison, statistically significantly higher median serum PCT levels were evident in patients with bacteraemia compared to leptospirosis (P=0.002), dengue (P <0.001), scrub typhus (P <0.001) and evident focus of infection without bacteraemia (P=0.036) (Fig. 1B). Significantly higher median serum PCT levels were found in patients with evident focus of infection compared to dengue (P=0.005) and scrub typhus (P=0.035) (Fig. 1A).
| Bacteraemia (n=53) |
Malaria (n=12) |
Evident cause of infection (n=151) |
Leptospirosis (n=23) |
Dengue (n=14) |
Scrub typhus (n=22) |
|---|---|---|---|---|---|
| 8.8 (3.5-29.5) | 5.9 (2.6-25.5) | 3.3 (0.6-17.4) | 0.8 (0.3-8.6) | 0.4 (0.3-0.9) | 0.6 (0.3-2.6) |
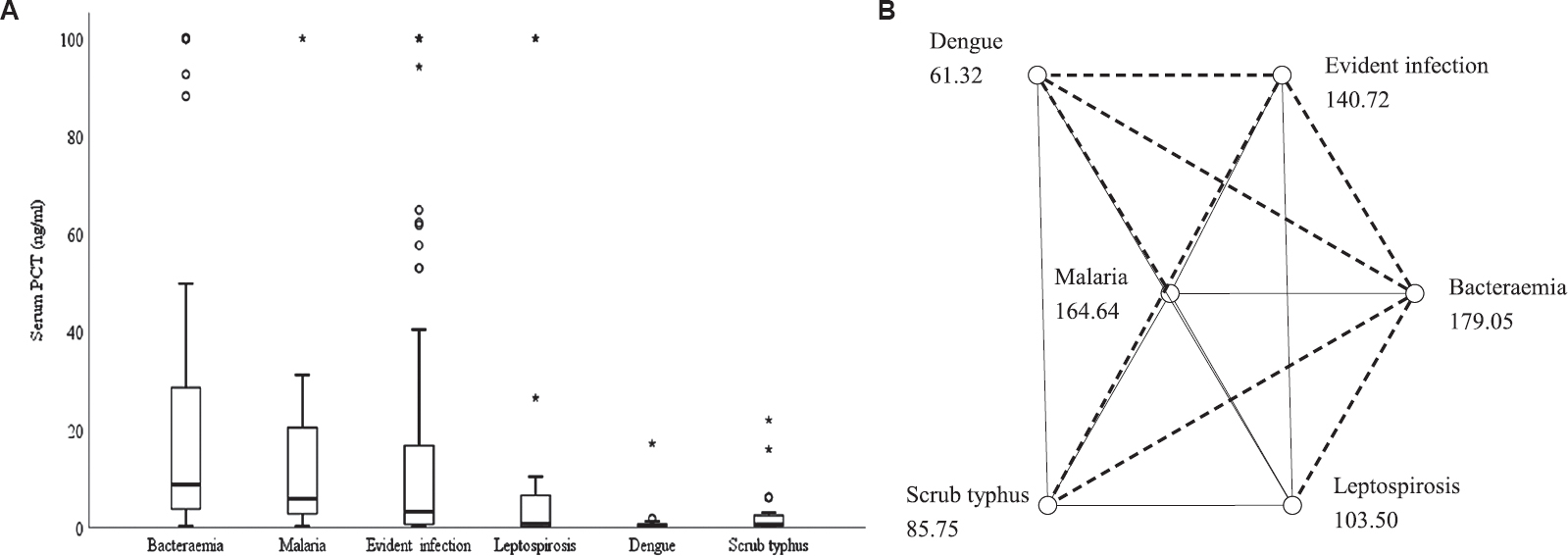
- (A) Box-whisker plot showing comparison of serum procalcitonin levels in patients presenting with acute febrile illness in whom an aetiological diagnosis could be established (n=278). (B) Post hoc analysis, pairwise comparison. Each node shows the sample average rank of aetiological group. The dashed line indicates significant pairwise comparison. oOutliers, *extreme values.
The ROC curve for calculating the optimal cut-off value of serum PCT for predicting bacteraemia at the time of initial presentation and the interactive dot diagram for serum PCT levels are shown in Figure 2. At a cut-off value of >3.2 ng/ml, the sensitivity and specificity of serum PCT levels in predicting bacteraemia was calculated to be 81.1 per cent [95% confidence interval (CI): 68.0-90.6%] and 63.3 per cent (95% CI: 58.8-68.2%), respectively. The area under the ROC curve was 0.724. Positive and negative predictive values of serum PCT at a cut-off value of >3.2 ng/ml for predicting bacteraemia were calculated to be 20 and 96 per cent, respectively. Positive and negative likelihood ratios (LRs) of serum PCT at a cut-off value of >3.2 ng/ml for predicting bacteraemia were calculated to be 2.18 and 0.3, respectively.
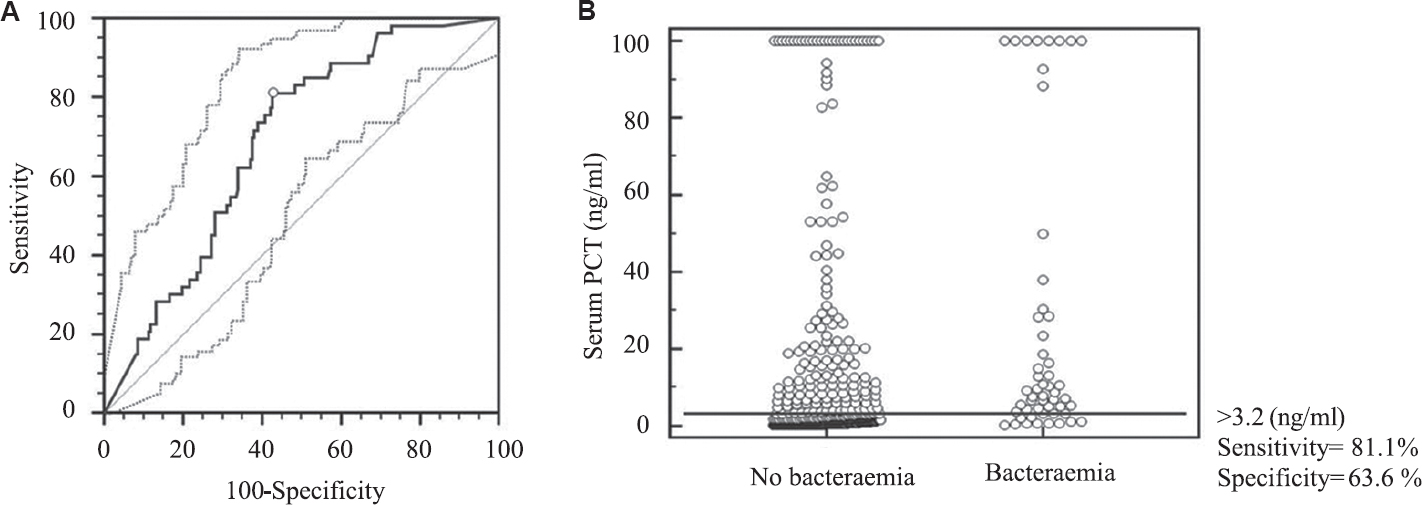
- (A) Receiver-operator characteristic (ROC) curve along with 95% confidence bounds for calculating the cut-off value for serum procalcitonin (PCT) at initial presentation with acute fever to predict bacteraemia. The area under the ROC curve=0.724; standard error=0.0309; 95% confidence interval=0.681-0.763; P (area=0.5)=0.001. (B) Interactive dot diagram for serum PCT levels at initial presentation with acute fever. The horizontal line depicts the cut-off value.
Thirty patients developed HAI. Ventilator-associated pneumonia (n=20, 66.7%) was the most common cause of HAI, followed by bacteraemia (n=9, 30%) and hospital-acquired pneumonia (n=1). It was seen that patients with a HAI causing fever (n=30) had significantly higher median serum PCT (ng/ml) levels compared to those with community-acquired infection (n=450) [7.1 (0.9-24.5) vs. 1.8 (0.4-10.4); P=0.011].
In the present study, 75 patients died and 16 patients were DAMA. As per the worst-case scenario analysis, overall mortality (75 dead +16 DAMA=91) was calculated to be 18.9 per cent. Non-survivors (n=91) had significantly higher median serum PCT (ng/ml) levels compared to survivors (n=389) [9.46 (2.03-44.4) vs. 1.23 (0.34-7.645); P <0.001]. ROC analysis revealed that at a cut-off value of >3.74 ng/ml, serum PCT levels at the time of initial presentation predicted mortality with a sensitivity and specificity of 67 per cent (95% CI: 56.5-76.5%) and 67.5 per cent (62.6-72.6%), respectively. Positive and negative predictive values of serum PCT at a cut-off value of >3.74 ng/ml for predicting mortality were calculated to be 32 and 89 per cent, respectively. Positive and negative LRs of serum PCT at a cut-off value of >3.74 ng/ml for predicting mortality were calculated to be 2.06 and 0.48, respectively. The stratified ROC analysis for defining serum PCT cut-off for predicting bacteraemia is shown in Table IV. In the stratified ROC analysis, serum PCT cut-off (>3.2 ng/ml) for predicting bacteraemia remained consistent even though minor changes in sensitivity, specificity and 95 per cent CI were observed.
| Disease group | Serum PCT (ng/ml) cut-off for predicting bacteraemia | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) |
|---|---|---|---|
| Age >65 yr (n=121) | >3.2 | 88.9 (51.8-99.7) | 66.1 (56.5-74.7) |
| Age <65 yr (n=359) | >3.17 | 79.6 (64.7-90.2) | 62.7 (57.1-68.1) |
| Males (n=268) | >3.2 | 82.1 (63.1-93.9) | 60.4 (53.9-66.6) |
| Females (n=212) | >3.17 | 80.0 (59.3-93.2) | 67.7 (60.5-74.4) |
| Patients with community acquired infection (n=450) | >3.2 | 81.8 (67.3-91.8) | 65.0 (60.2-69.7) |
| Overall (n=480) | >3.2 | 81.1 (68.0-90.6) | 63.6 (58.8-68.2) |
On multivariable ROC analysis, the cut-off level of serum PCT for predicting bacteraemia was found to be >4.4 ng/ml and the sensitivity, specificity and area under the curve were 69.8, 56.9 and 0.639 per cent, respectively. On multivariable ROC analysis, the cut-off level of serum PCT for predicting mortality was found to be >11.6 ng/ml and the sensitivity, specificity and area under the curve were 44, 81.1 and 0.650 per cent, respectively.
Discussion
Infection is the most common cause of pyrexia in medical ward and ICU1 setting. Identification of bacterial cause of fever is of paramount importance as a delay in diagnosis can result in the disease progressing to sepsis and the associated high mortality. The dearth of data on utility of serum PCT in patients admitted to medical wards and ICU with acute fever prompted us to investigate this issue in a tertiary care referral hospital in south India. The median (IQR) age of patients with bacteraemia in the present study was 53 (32.5-65.0) yr. Similar observations were observed in two other studies from India1920. In the present study, an aetiological cause of fever could be established in 59.6 per cent patients with 11 per cent presenting with bacteraemia. In a study21 from China, of the 326 patients admitted to a medical ward with fever, bacteraemia was confirmed in 17.8 per cent. Gram-negative sepsis was predominant in our study (77.4%) which was in agreement with the earlier Indian study19. In a report from Switzerland in which of the 16,682 isolates from bloodstream infections over a one year period from a 1000-bed hospital, 62 per cent were Gram-negative bacilli, whereas Gram-positive cocci constituted only 35.4 per cent of the isolates22.
In the present study, patients with bacteraemia as the cause of acute fever had higher median (IQR) serum PCT levels at the time of initial presentation, and at a cut-off value of >3.2 ng/ml, serum PCT had a sensitivity and specificity in predicting bacteraemia. On the stratified ROC analysis, the PCT cut-off of >3.2 ng/ml for predicting bacteraemia remained consistent even though minor changes in sensitivity, specificity and 95 per cent CI were noted, suggesting that confounders such as age, gender and type of infections (community acquired vs. hospital acquired) had not influenced the cut-off. In a study from Spain23 which evaluated the usefulness of PCT and CRP for predicting bacteraemia in urinary tract infections in the emergency department at a cut-off ≥1.16 ng/ml, serum PCT had a sensitivity of 100 per cent, a specificity of 97 per cent, a positive predictive value of 84 per cent and a negative predictive value of 100 per cent in detecting bacteraemia. A meta-analysis also concluded that serum PCT was fairly accurate in predicting bacteraemia in adult patients with suspicion of infection or sepsis24. In a study from China21, the median serum PCT level at the time of initial presentation with fever was 3.19 ng/ml (0.43-10.33) which was lower than the present findings. This difference may be due to the differences in the bacterial population type responsible for the fever since Gram-negative bacteraemias cause a higher elevation of PCT than those caused by Gram-positive pathogens25, and in our study, there was a predominance of Gram-negative pathogen. For non-bacteraemic patients having a focal bacterial infection in our study, ROC analysis revealed that at a cut-off value of >3 ng/ml, serum PCT had a sensitivity and specificity of 53.8 and 68 per cent, respectively, in detecting bacterial infection. This finding was in accordance with the observations that there was a low rise in PCT levels in localized infections and in infections caused by intracellular bacteria2627. However, caution should be exercised in excluding bacterial infections based on a low PCT level. If the clinical evaluation suggests a possible diagnosis of bacterial sepsis, but serum PCT levels are not elevated at the time of initial presentation, patients should still be treated for sepsis initially. Clinical monitoring over the next 48 h with serial measurements of PCT can assist in further decision-making28.
In the present study, non-survivors had significantly higher median serum PCT levels at the time of initial presentation with fever compared to survivors. In an earlier study from New Delhi, India19, the levels of serum PCT (ng/ml) were significantly higher in non-survivors compared to that of survivors. In this Indian study19, a serum PCT level of ≥7 ng/ml on day 1 predicted mortality [Hazard ratio (HR): 2.5 (1.1-6.2); P=0.02]. In the present study, at a cut-off value of >3.74 ng/ml, serum PCT levels at the time of initial presentation predicted mortality with a sensitivity and specificity of 67 and 67.5 per cent, respectively. PCT has been found to be a predictor of mortality in a study involving 472 critical care patients29. PCT measurement was done daily for these patients, and it was seen that both high maximum PCT level and the increase in the levels following the first reading of >1.0 ng/ml were independent predictors of 90-day mortality. White cell count and CRP were not found to be predictors of mortality in this study.
The present study showed that compared with measurements in patients with bacteraemia, the median serum PCT levels were significantly lower in patients with leptospirosis, dengue fever, scrub typhus and swine flu. However, patients with malaria had higher median serum PCT levels compared to other non-bacterial causes. Whether this reflects the scenario of malarial fever or whether the elevated levels of serum PCT are the consequence of occult bacterial sepsis often reported in patients with severe complicated malaria30 needs to be studied further. In malaria-endemic areas, in patients with fever and elevated serum PCT, malaria should also be considered in the differential diagnosis and should be ruled out.
The strength of the present study was that the study population were patients with fever admitted to medical wards and ICU, rather than a specific set of patients or setting, which was close to the real scenario. Due to high cost serial measurement of PCT could not be done, which would have given us more useful information. As most of the studies regarding the utility of PCT have been done in Western countries, the infection spectrum (like malaria and tuberculosis) and the pathogen profile (Gram-positive and Gram-negative sepsis) may not correspond to the situation prevalent in the Indian subcontinent. This calls for wider nationwide studies at different centres, so that the cumulative findings can be translated to a more useful guideline regarding the use of PCT in the healthcare settings.
To conclude, our observations suggested that serum PCT level might be a useful biomarker for identifying bacteraemia as well as predicting mortality in patients with acute fever requiring admission to hospital. Serum PCT in patients presenting with fever had a high negative predictive value for bacteraemia, and in malaria-endemic areas, in patients with fever and elevated serum PCT, malaria should be considered in the differential diagnosis.
Acknowledgment
Authors thank Dr L. Jeyaseelan, Department of Biostatistics, Christian Medical College, Vellore, for help and guidance with stratified analysis and multivariable ROC analysis.
Financial support & sponsorship: This study was funded by Sri Balaji Arogya Vara Prasadini scheme of Sri Venkateswara Institute of Medical Sciences and Tirumala Tirupati Devasthanams, Tirupati
Conflicts of Interest: None.
References
- Biomarker-guided antibiotic therapy in adult critically ill patients: A critical review. Ann Intensive Care. 2012;2:32.
- [Google Scholar]
- Diagnostic and prognostic biomarkers of sepsis in critical care. J Antimicrob Chemother. 2011;66(Suppl 2):ii33-40.
- [Google Scholar]
- Calcitonin gene family of peptides. In: Becker K, ed. Principles and practice of endocrinology and metabolism. Philadelphia: Lippincott; 2001. p. :520-34.
- [Google Scholar]
- Ubiquitous expression of the calcitonin-i gene in multiple tissues in response to sepsis. J Clin Endocrinol Metab. 2001;86:396-404.
- [Google Scholar]
- Procalcitonin is a marker of gram-negative bacteremia in patients with sepsis. Am J Med Sci. 2015;349:499-504.
- [Google Scholar]
- Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med. 2018;379:236-49.
- [Google Scholar]
- Procalcitonin reflects bacteremia and bacterial load in urosepsis syndrome: A prospective observational study. Crit Care. 2010;14:R206.
- [Google Scholar]
- Assessment of procalcitonin as a diagnostic marker of underlying infection in patients with febrile neutropenia. Clin Infect Dis. 2001;32:1718-25.
- [Google Scholar]
- Comparison between white blood cell count, procalcitonin and C reactive protein as diagnostic and prognostic biomarkers of infection or sepsis in patients presenting to emergency department. Clin Chem Lab Med. 2014;52:1465-72.
- [Google Scholar]
- Efficacy of procalcitonin in the early diagnosis of bacterial infections in a critical care unit. Shock. 2009;31:586-91.
- [Google Scholar]
- Evaluation of procalcitonin as a marker of infection in a nonselected sample of febrile hospitalized patients. Diagn Microbiol Infect Dis. 2004;49:237-41.
- [Google Scholar]
- Utility of procalcitonin as an early diagnostic marker of bacteremia in patients with acute fever. Yonsei Med J. 2011;52:276-81.
- [Google Scholar]
- Low serum procalcitonin level accurately predicts the absence of bacteremia in adult patients with acute fever. Clin Infect Dis. 2002;35:156-61.
- [Google Scholar]
- Prediction of microbial infection and mortality in medical patients with fever: Plasma procalcitonin, neutrophilic elastase-alpha1-antitrypsin, and lactoferrin compared with clinical variables. Clin Infect Dis. 1999;29:398-407.
- [Google Scholar]
- Nonparametric pairwise multiple comparisons in independent groups using Dunn's test. Stata J. 2015;15:292-300.
- [Google Scholar]
- Cleistanthus collinus poisoning: Experience at a medical intensive care unit in a tertiary care hospital in South India. Indian J Med Res. 2016;143:793-7.
- [Google Scholar]
- The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29-36.
- [Google Scholar]
- Procalcitonin as a prognostic marker for sepsis: A prospective observational study. BMC Res Notes. 2014;7:458.
- [Google Scholar]
- Procalcitonin as a rapid diagnostic biomarker to differentiate between culture-negative bacterial sepsis and systemic inflammatory response syndrome: A prospective, observational, cohort study. J Crit Care. 2015;30:218e7-12.
- [Google Scholar]
- Evaluation of procalcitonin, C-reactive protein, interleukin-6 & serum amyloid A as diagnostic biomarkers of bacterial infection in febrile patients. Indian J Med Res. 2015;141:315-21.
- [Google Scholar]
- Blood culture-based diagnosis of bacteraemia: State of the art. Clin Microbiol Infect. 2015;21:313-22.
- [Google Scholar]
- Usefulness of procalcitonin and C-reactive protein for predicting bacteremia in urinary tract infections in the emergency department. Actas Urol Esp. 2015;39:502-10.
- [Google Scholar]
- The diagnostic accuracy of procalcitonin for bacteraemia: A systematic review and meta-analysis. Clin Microbiol Infect. 2015;21:474-81.
- [Google Scholar]
- Serum procalcitonin elevation in critically ill patients at the onset of bacteremia caused by either gram negative or gram positive bacteria. BMC Infect Dis. 2008;8:38.
- [Google Scholar]
- Pro/Con debate: Is procalcitonin useful for guiding antibiotic decision making in critically ill patients? Crit Care. 2008;12:211.
- [Google Scholar]
- Diagnostic and prognostic role of procalcitonin in infections. ScientificWorldJournal. 2010;10:1941-6.
- [Google Scholar]
- Biomarkers for the diagnosis of bacterial infections: In pursuit of the 'holy grail' Indian J Med Res. 2015;141:271-3.
- [Google Scholar]
- Procalcitonin increase in early identification of critically ill patients at high risk of mortality. Crit Care Med. 2006;34:2596-602.
- [Google Scholar]
- Procalcitonin levels in severe Plasmodium falciparum malaria: Predictor of outcome or reflection of pathomechanisms? J Infect Dis. 2001;184:1091-2.
- [Google Scholar]






