Translate this page into:
Seroprevalence of transplacentally acquired measles antibodies in HIV-exposed versus HIV-unexposed infants at six months of age
Reprint requests: Dr. Anju Seth, Department of Pediatrics, Lady Hardinge Medical College, New Delhi 110 092, India e-mail: anjuseth.peds@gmail.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Measles infection is reported to be more severe, prolonged and associated with a higher complication rate in children with HIV infection. Reports indicate that infants born to HIV-infected women [HIV exposed infants (HEI)] may be more vulnerable to measles. The World Health Organization recommends measles vaccination starting at six months of age in these infants who may be HIV-infected themselves. However, in India, they are given measles vaccination at nine months of age like all other infants. In this study, the seroprevalence of transplacentally acquired measles antibodies was compared in HEI and unexposed infants (HUnI) at six months of age and the proportion of HEI undergoing seroconversion after immunization with measles vaccine was assessed.
Methods:
In this prospective longitudinal study, measles IgG antibodies were estimated in serum of 49 HEI and 50 HUnI aged 6-7 months. Measles vaccine was then administered to HEI. Assessment for measles IgG antibodies was repeated 8-12 wk post-immunization.
Results:
Measles IgG antibodies were detected in two of 49 (4.1%) HEI and 16 of 50 (32%) HUnI. HEI were 11 times more likely to lack measles antibodies as compared to HUnI (odds ratio=11.05, 95% confidence interval=2.989-40.908). Post-vaccination, seroprevalence of measles antibodies increased to 38.5 per cent (P< 0.001) in HEI compared to 4 per cent at baseline.
Interpretation & conclusions:
Most HEI lacked measles antibodies at six months age and were, therefore, more vulnerable to measles than HUnI. Seroconversion in response to a single dose of measles vaccine administered at six months age was low in these infants, signifying the need of additional dose(s) of measles/measles-containing vaccine.
Keywords
HIV-exposed infants
measles antibodies
measles immunization
Measles, one of the leading vaccine-preventable diseases in the world, still accounts for a large proportion of childhood mortality and morbidity1. HIV-infected children constitute a significant proportion of children hospitalized with measles in countries with high HIV prevalence23. The mortality rates in children co-infected with HIV and measles are reported to be as high as 50 per cent especially in resource constrained circumstances4. In these children, measles is also reported to be unusual in its presentation456. The distinguishing clinical feature of measles, the morbiliform rash, is a manifestation of host cellular immune response to measles virus. Children with HIV may, therefore, lack the characteristic clinical signs of measles and the infection may remain unrecognised7. Further, these children may continue to shed the measles virus for a prolonged period8, thereby increasing the risk for widespread transmission in both community and healthcare settings.
Infants born to women with HIV infection [HIV-exposed infants (HEI)] are more vulnerable to acquire measles infection as compared to those who are born to HIV-uninfected women irrespective of their own HIV status for several reasons. A few reports suggest that these infants may have lower levels of transplacentally acquired measles antibodies and may, therefore, be susceptible to measles at a younger age as compared to HIV-unexposed infants (HUnI)91011. Those HEI who are HIV-infected themselves may lose protective antibody titres by 2-3 yr of age following immunization even if they mount an adequate primary antibody responses to measles vaccine121314. If immunized when already immune-compromised, the response to measles vaccination may be poor15. Thus, HIV-exposed children remain vulnerable to measles even if immunized during infancy or early childhood at the usual recommended age.
The World Health Organization (WHO) recommends that HIV-infected children should be immunized against measles as soon as possible (i.e., at six months and again at nine months) except those infants who are severely immune-compromised with CD4 count <15 per cent or an absolute CD4 count lower than normal for age, any history of AIDS-defining illness or those with clinical manifestations of symptomatic HIV16. The Indian Academy of Paediatrics and the National AIDS Control Organization (NACO) currently recommend measles immunization at nine months of age in HEI1718. There is a paucity of literature from India to decide the optimum age of measles vaccination in these vulnerable children. The present study was planned to determine the seroprevalence of transplacentally acquired measles antibodies in HEI and their short-term response to measles vaccination at six months of age.
Material & Methods
This was a hospital-based, prospective longitudinal study conducted from November 2012 to March 2014 in Kalawati Saran Children's Hospital, Lady Hardinge Medical College, New Delhi, India. The cases included 49 consecutive HEI in the age group 6-7 months who presented to the Paediatric Antiretroviral Therapy (ART) centre during the study. The control group included 50 age- and sex-matched infants born to HIV-uninfected mothers (HUnI) admitted consecutively in a single ward of the hospital for acute lower respiratory tract infection. The sample size was determined based on the work by Scott et al9 where 15 per cent of HEI versus 42 per cent of those unexposed had detectable levels of measles antibodies at six months of age. The requisite sample size to detect this difference with 95 per cent confidence interval and 80 per cent power was 45 in each group. Infants with a history of measles/known exposure to measles in the past 14 days were excluded from the study. Additional exclusion criteria for the control group were a history of recurrent infections or a chronic systemic disease in the past.
The enrolled infants were evaluated as per a pre-structured proforma which included information on age, sex, birth, HIV status, feeding and immunization details, maternal details such as clinical stage, ART/anti-retroviral (ARV) prophylaxis duration and regimen to mother and infants. Weight and length of the infants were recorded. Weight for age (WFA), length for age (LFA) and weight for length (WFL) z-scores were calculated as per the WHO growth reference standards19. WFA <-2 standard deviation (SD) was defined as undernutrition and <-3SD severe undernutrition, LFA <-2SD stunting and <-3SD severe stunting, WFL <-2SD wasting and <-3SD severe wasting. Infants were evaluated for the presence of any acute infection, signs of any nutritional deficiencies and anaemia. The HEI was followed up and evaluated for their HIV infection status as per the current NACO guidelines by a DNA PCR test performed at the ages of six weeks, six months, and six weeks after stopping breastfeeding18. The test was first carried out on a dry blood spot (DBS), followed by confirmation on whole blood sample if the result on DBS was found positive. The infants diagnosed to be infected with HIV were assessed for the WHO clinical staging and were started on ART as per the National guidelines given by NACO18.
Venous blood sample (3 ml) was drawn for assessment of measles IgG antibodies for all HEI and HUnI. The HEI were then immunized with a single dose of measles vaccine available in the Hospital [Serum Institute of India Ltd., each 0.5 ml containing 1000 CCID50 of measles virus (Edmonston-Zagreb strain)]. Batch number, expiration date and proper cold chain maintenance were verified. All vaccines used were from the same lot carrying identical batch number. A single subcutaneous dose of 0.5 ml of the vaccine was given in the upper arm or the anterolateral thigh. The caregivers were asked to maintain a diary of events at home and were asked to report back in case of development of any new symptoms during one month period after vaccination. They were also actively followed up by telephone on days 1, 3, 7, 14 and 28, for any side effects associated with vaccination or development of any other illness including measles. Venous sample was collected again after a time interval of 8-12 wk for estimation of measles IgG antibodies. All enrolled HEI further received MMR vaccine at 12-15 months age. Infants confirmed to be HIV infected having severe symptoms/belonging to clinical stage 3 or 4, with intercurrent illness or opportunistic infections, or with CD4 count <15 per cent of the total lymphocyte counts were excluded for vaccine administration.
A written informed consent from each selected child's caregiver was obtained. The study protocol was approved by the Institutional Ethics Committee.
Sample processing and assessment of measles antibodies: Serum was separated and stored at -20°C in sterile plastic aliquots until serologic testing was performed. Levels of measles antibodies (IgG) were measured qualitatively using NovaLisa™ ELISA kits (NovaTec Immundiagnostica GmbH, Germany) in accordance with the manufacturer's instructions. This assay had a diagnostic specificity and sensitivity of >95 per cent, while the inter- and intra-assay coefficients of variation were <10 per cent for each. The cut-off was defined as the mean absorbance value of the cut-off control determinants. The results were positive if absorbance value was more than 10 per cent over the cut-off and were calculated in Nova Tec units (NTU) as follows:
Patient (mean) absorbance value ×10÷cut-off=NTU. Using a cut-off value of 10 NTU, levels >11 NTU were considered as positive, <9 NTU to be negative and between 9 and 11 NTU, to be equivocal for the presence of measles antibodies.
Outcome variables & statistical analysis: The primary outcome variables were proportions of HEI and HUnI detected positive for measles antibodies. Secondary outcome variables included proportion of HEI (i) testing positive for measles antibodies 8-12 wk after a single dose of measles vaccine given at six months, and (ii) suffering from adverse effects following measles vaccination.
The data were analyzed using SPSS program version 15.0 (SPSS Inc., Chicago, IL, USA). For comparing qualitative variables between the two groups Chi-square/Fischer exact test/McNemar's test was used, while for the quantitative variable unpaired t test was used. Data were checked for normality before statistical analysis.
Results
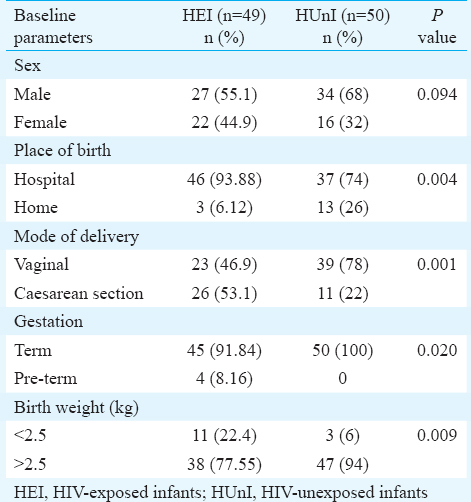
Baseline characteristics of the infants including sex, place and mode of delivery, gestation, birth weight, immunization details at the start of study are shown in Table I. HEI were more likely to be low birth weight (P=0.009), preterm (P=0.02), have a hospital delivery (P=0.004) through caesarean section (P=0.001) as compared to HUnI. Of the 49 HEI, 10 were subsequently confirmed to be HIV-positive while 39 were HIV negative.
Maternal details: Of the 49 HIV-positive mothers, 46 were in WHO clinical stage T1 and two in T2. Clinical stage of one mother was not determined as she had expired before enrolment of infant. Twenty one mothers (42.86%) had CD4 count <350/μl and remaining above this. Thirty seven mothers were on ART. In 32, ART was started before delivery, and in the remaining five it was started after delivery.
Of the 17 mothers who were not on ART at the time of delivery, eight received single-dose nevirapine during delivery. The remaining nine mothers did not receive ART or ARV prophylaxis during delivery. Among infants born to HIV-positive mothers 43 of 49 received single-dose nevirapine at birth.
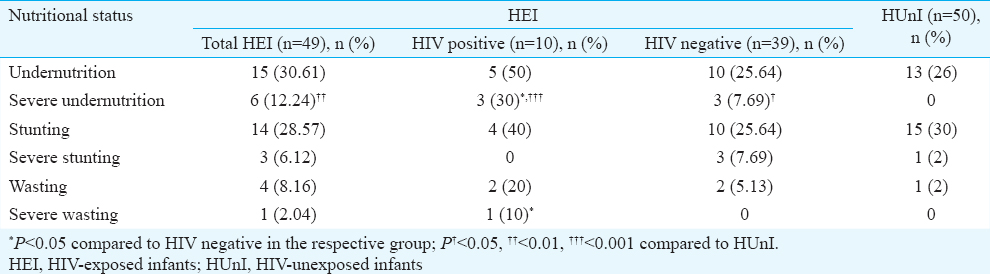
Nutritional status of infants: At the time of enrolment, the status of undernutrition was not different among HEI and HUnI, or among HIV positive and negative HEI. However, severe undernutrition was more prevalent among the HEI as compared to HUnI (P=0.01). Severe undernutrition was also more common in HIV-positive as compared to HIV negative HEI (P=0.05) and HIV positive or negative HEI in comparison to HUnI (P< 0.001 and 0.05, respectively). There was no difference in other parameters between HEI and HUnI or any of the sub-groups. However, HIV-positive HEI had higher percentage of severe wasting as compared to HUnI (P=0.05) and HIV-negative HEI (P=0.05) (Table II).
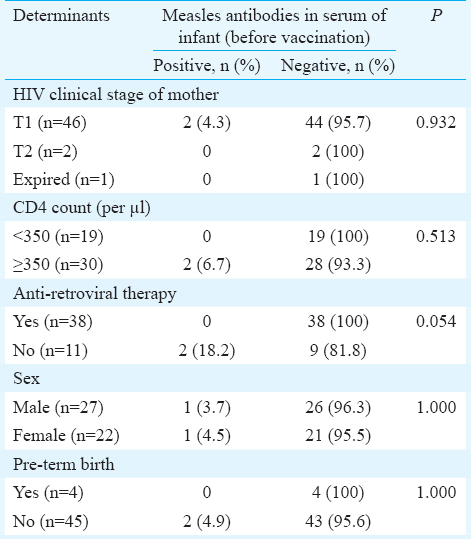
Proportion of infants testing positive for measles antibodies at six months of age: Two of the 49 HEI (4%) were detected to be positive for measles antibodies before immunization at six months. Both of them were HIV positive [2/10 (20%)]. One case with equivocal result was taken to be negative for measles antibodies for computation of results. In contrast, 16 of 50 (32%) HUnI tested positive for measles antibodies (P< 0.001). The HEI were 11 times more likely to lack detectable measles antibodies as compared to HUnI (odds ratio=11.05, 95% confidence interval=2.99-40.91). Various maternal and infant variables like maternal clinical stage, her CD4 count, whether on ART or not, infant sex and gestation at birth among the HEI with and without detectable measles antibodies, were compared. None of these factors were significantly different among the two groups (Table III).
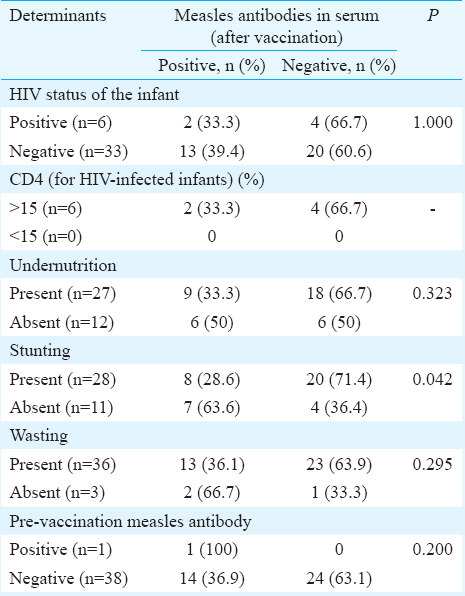
Seroconversion following measles immunization in HIE: Among the 49 HEI, 46 (7 HIV positive and 39 HIV negative) received measles immunization at six months of age. Three HIV-positive infants did not receive immunization since one infant had CD4 count <15 per cent and two were severely symptomatic in clinical stage 3 of HIV. Thirty nine of these 46 (6 HIV-infected and 33 HIV-uninfected) infants could be tested for the prevalence of measles antibodies 8-12 wk following measles immunization. Proportion of infants testing positive for measles antibodies increased from baseline value of 4 per cent (2/49) to post-vaccination value of 38.5 per cent (15/39, P< 0.001). Within this group, HIV-infected infants showed an increase in the prevalence from 20 per cent (2/10) to 33.3 per cent (2/6), while HIV-uninfected infants showed an increase from 0 per cent (0/39) to 39.4 per cent (13/33), (Figure). Various factors such as HIV status of infant, CD4%, nutritional status and presence of measles antibodies were compared before vaccination among those who showed seroconversion and those who did not. None of these factors showed a significant difference except infants with stunting who had better seroconversion (Table IV).
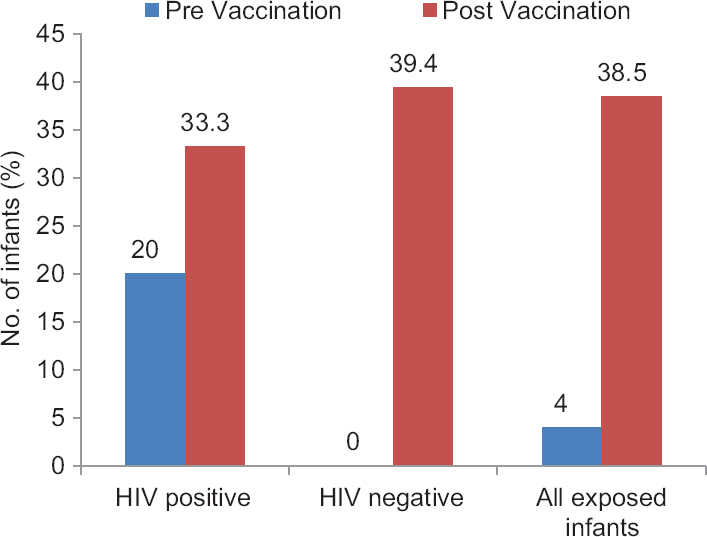
- Seroprevalence of measles IgG antibodies in HIV-exposed infants before and after measles vaccination.
Adverse events following measles immunization: Of the 46 HEI who received measles vaccine, seven infants (2 HIV infected and 5 uninfected) developed fever on 7-14 days after vaccination. Three of these (1 HIV infected and 2 uninfected) also developed a mild rash along with fever. These symptoms were self-limiting and resolved spontaneously without any treatment. No serious adverse effect was seen attributable to measles vaccination.
Discussion
In this study, 32 per cent of HUnI had demonstrable measles antibodies at six months of age. A prevalence of measles antibodies ranging between 0 and 42 per cent in the healthy/HUnI has been shown by various workers920212223.
In our study a lower proportion of HEI tested positive for maternally acquired measles antibodies as compared to HUnI. A few other studies have shown similarly low prevalence of measles antibodies in HEI. Lepage et al22 from Rwanda found that 10.7 per cent of HIV-exposed as against 25.3 per cent of HUnI had measles antibodies at six months of age. Scott et al9 from Zambia reported a prevalence of 15 per cent in HIV-exposed vs. 42 per cent in HUnI. Possible reasons for lower levels of antibodies to measles virus in infants born to HIV-infected mothers may be low levels of measles antibodies in the mother and/or impaired placental transfer of measles antibodies to infants born to HIV-infected mothers911. In addition, infants born to HIV-infected mothers, whether themselves infected or not, may lose their passively acquired maternal antibodies earlier than children of HIV seronegative mothers22.
Our study also demonstrated a higher seroprevalence of measles antibodies in HIV infected as compared to HIV-uninfected exposed infants. This observation was in concordance with reports by Cutts et al24 (11.8% of HIV-infected and 6.5% of HIV-exposed uninfected infants), Rudy et al25 (16.7% of HIV-infected and 0% of HIV-exposed uninfected infants) and Lepage et al22 (15% of HIV-infected and 10% of HIV-exposed uninfected infants). The possible explanation for apparent longer persistence of maternally acquired measles antibodies among HIV-infected infants may be the presence of polyclonal B-cell activation and hypergammaglobulinemia in these infants giving rise to false positive test results26.
Despite absence of measles antibodies in most infants before immunization, a low seroconversion was observed in the HEI after immunization with measles vaccine, seroconversion was present in 33.4 per cent of HIV-infected and 39.4 per cent of HIV-exposed uninfected cases. A better seroconversion in both HIV infected and uninfected infants has been shown by other workers - 69.2 per cent in HIV-infected and 77.3 per cent in HIV-uninfected infants by Rudy et al25, 59 per cent in HIV-infected and 68 per cent in HIV-uninfected infants by Helfand et al27, and 100 per cent in HIV-infected and 80 per cent in HIV-uninfected infants by Chandwani et al28. The possible factors that may have contributed towards a lower seroconversion rate in our infants include: (i) the immune system of six months old infants may not be capable of mounting a response to measles vaccine as measured by ELISA; (ii) due to relatively lower sensitivity of ELISA, the presence of trace amount of undetectable maternally acquired measles antibodies might interfere with the seroconversion of these infants; and (iii) 30 per cent of our infants were undernourished, 12.3 per cent being severely undernourished. Associated malnutrition of these children may have contributed. However, on statistical analysis, none of the anthropometric parameters was found to be associated with lower seroconversion.
We did not find any variable like HIV status of infants, their CD4%, WFA/WFL z-scores and presence/absence of measles antibodies before vaccination to be associated with seroconversion. However, we found stunting to be associated with better seroconversion in these infants. This apparently unexpected finding is likely to be because of the small number of subjects enrolled for the study with only two infants having demonstrable measles antibodies before immunization.
Despite measles being a live-attenuated vaccine, none of our infants had any serious adverse effects associated with vaccination. Some other studies2224 where a high titre measles vaccine was used also reported similar low incidence of adverse effects with no serious or life-threatening adverse effect to be associated with measles vaccine. In a systematic review and meta-analysis on safety and immunogenicity of measles vaccination in HIV-infected children, among 39 studies involving >1200 HIV-infected children, none of the study reported death in relation to measles vaccine in HIV-infected children29.
Our study had certain limitations. ELISA, being a qualitative method, was used to measure measles antibodies, since the gold standard, plaque reduction technique that can measure titre of measles antibodies was not available. ELISA has been used in other studies as also in our previous published work30 due to its technical ease. Second, since the number of enrolled infants was small, interpretation of determinants of the presence of demonstrable measles antibodies at baseline as well as post-vaccination seroconversion has limited significance.
In conclusion, the majority of HEI lacked measles antibodies at six months of age. Thus, earlier immunization than at the currently recommended age of nine months in the national schedule may be beneficial for them. Further, a large proportion of these infants remained unprotected following measles vaccination at six months of age signifying a need for additional doses of measles/measles containing vaccine.
Conflicts of Interest: None.
References
- WHO/UNICEF. Joint Statement, Global Plan for Reducing Measles Mortality 2006-2010. Available from: http://www.who.int/vaccines
- Prospective study of measles in hospitalized, human immunodeficiency virus (HIV)-infected and HIV-uninfected children in Zambia. Clin Infect Dis. 2002;35:189-96.
- [Google Scholar]
- HIV type 1 infection is a risk factor for mortality in hospitalized Zambian children with measles. Clin Infect Dis. 2008;46:523-7.
- [Google Scholar]
- Measles giant cell pneumonia in a child with human immunodeficiency virus infection. Pediatr Infect Dis J. 1991;10:542-4.
- [Google Scholar]
- Population-based study of measles and measles immunization in human immunodeficiency virus-infected children. Pediatr Infect Dis J. 1992;11:1008-14.
- [Google Scholar]
- Measles in a Dutch hospital introduced by an immuno-compromised infant from Indonesia infected with a new virus genotype. Lancet. 2000;355:201-2.
- [Google Scholar]
- Prolonged measles virus shedding in human immunodeficiency virus-infected children, detected by reverse transcriptase-polymerase chain reaction. J Infect Dis. 2001;183:532-8.
- [Google Scholar]
- The influence of HIV-1 exposure and infection on levels of passively acquired antibodies to measles virus in Zambian infants. Clin Infect Dis. 2007;45:1417-24.
- [Google Scholar]
- Neonatal measles immunity in rural Kenya: The influence of HIV and placental malaria infections on placental transfer of antibodies and levels of antibody in maternal and cord serum samples. J Infect Dis. 2005;191:1854-60.
- [Google Scholar]
- Factors determining prevalence of maternal antibody to measles virus throughout infancy: A review. Clin Infect Dis. 2000;31:110-9.
- [Google Scholar]
- Immunogenicity of standard-titer measles vaccine in HIV-1-infected and uninfected Zambian children: An observational study. J Infect Dis. 2007;196:347-55.
- [Google Scholar]
- Measles antibody in vaccinated human immunodeficiency virus type 1-infected children. Pediatrics. 1996;97:653-7.
- [Google Scholar]
- Decline of measles antibody titers after immunization in human immunodeficiency virus-infected children. Pediatr Infect Dis J. 1995;14:149-51.
- [Google Scholar]
- Abnormalities of measles antibody response in human immunodeficiency virus type 1 (HIV-1) infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10:540-8.
- [Google Scholar]
- IAP guide book on immunization 2013-2014. Gwalior: National Publication House, Indian Academy of Pediatrics; 2014. p. :366-7.
- 2007. National AIDS Control Organisation (NACO). Guidelines for HIV Care and Treatment in Infants and Children. Available from: http://naco.gov.in/care-support-treatment
- The WHO Child Growth Standards. Available from: http://www.who.int/childgrowth/standards/en/
- Immune response to measles vaccine in 6 month old infants in Papua New Guinea. Trop Med Int Health. 2009;14:167-73.
- [Google Scholar]
- Kinetics of decline of maternal measles virus-neutralizing antibodies in sera of infants in France in 2006. Clin Vaccine Immunol. 2008;15:1845-50.
- [Google Scholar]
- Safety and immunogenicity of high-dose Edmonston-Zagreb measles vaccine in children with HIV-1 infection. A cohort study in Kigali, Rwanda. Am J Dis Child. 1992;146:550-5.
- [Google Scholar]
- Antibody response to measles immunization in India. Bull World Health Organ. 1984;62:737-41.
- [Google Scholar]
- Immunogenicity of high-titer Edmonston-Zagreb measles vaccine in human immunodeficiency virus-infected children in Kinshasa, Zaire. J Infect Dis. 1993;167:1418-21.
- [Google Scholar]
- Responses to measles immunization in children infected with human immunodeficiency virus. J Pediatr. 1994;125:72-4.
- [Google Scholar]
- Direct polyclonal activation of human B lymphocytes by the acquired immune deficiency syndrome virus. Science. 1986;233:1084-6.
- [Google Scholar]
- Evaluation of the immune response to a 2-dose measles vaccination schedule administered at 6 and 9 months of age to HIV-infected and HIV-uninfected children in Malawi. J Infect Dis. 2008;198:1457-65.
- [Google Scholar]
- Safety and immunogenicity of early measles vaccination in children born to HIV-infected mothers in the United States: Results of Pediatric AIDS Clinical Trials Group (PACTG) protocol 225. J Infect Dis. 2011;204(Suppl 1):S179-89.
- [Google Scholar]
- Measles vaccination in HIV-infected children: Systematic review and meta-analysis of safety and immunogenicity. J Infect Dis. 2011;204(Suppl 1):S164-78.
- [Google Scholar]
- Evaluation of immune response to measles component of MMR vaccine in children with HIV infection receiving antiretroviral therapy. Pediatr Infect Dis J. 2016;35:e8-11.
- [Google Scholar]






