Translate this page into:
Seroprevalence of Toxoplasma gondii in newly diagnosed HIV seropositive patients
For correspondence: Dr Nayana Ingole, Department of Microbiology, 5th Floor, New Building, Seth G.S. Medical College & K.E.M. Hospital, Parel, Mumbai 400 012, Maharashtra, India e-mail: nayanaingole@gmail.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Immunocompromised individuals mainly HIV infected patients are at a great risk for developing toxoplasmosis. The presence of toxoplasmosis among HIV-infected patients directly correlates with the prevalence of anti- Toxoplasma gondii antibodies and the degree of immunosuppression (measured by CD4 counts). The data regarding the seroprevalence of toxoplasmosis in HIV-infected patients are scarce in India. Therefore, this study was initiated to find out the seroprevalence of toxoplasmosis in treatment-naïve HIV seropositive patients and to determine its association with CD4 counts, if any.
Methods:
Four hundred newly diagnosed antiretroviral therapy (ART) naïve adult HIV positive patients coming for CD4 count estimation were tested for the presence of anti-Toxoplasma IgG antibodies. Risk factors for acquisition of toxoplasmosis as well as the age, gender and CD4 counts of the patient were noted down.
Results:
Toxoplasma IgG was positive in 292 (73%) patients, and the positivity was not related to their CD4 counts. The proportion of anti-Toxoplasma IgG positivity showed no significant association with age, gender and risk factors of the patients.
Interpretation & conclusions:
In the absence of any specific vaccine or prophylaxis for toxoplasmosis, it is pertinent to screen all HIV-positive patients for Toxoplasma IgG at diagnosis, irrespective of their CD4 counts, and sensitize them about the means to prevent either acquisition or activation of infection to avert the development of toxoplasmic encephalitis.
Keywords
ART
CD4 count
HIV
immunocompromised
seroprevalence
Toxoplasma gondii
toxoplasmosis
The global seroprevalence of toxoplasmosis has been reported to be 46.1 per cent, and in India, it has been found to be between 16.3 and 30.8 per cent12. In immunocompetent patients, 80-90 per cent of toxoplasmosis is asymptomatic3. However, in the immunocompromised patients, the disease may be severe. Acute toxoplasmosis in patients with HIV infection principally affects the central nervous system4.
Immune response to Toxoplasma gondii is highly heterogeneous and complex due to heterogeneity in the genetic background of hosts. Despite the robust immune response, tissue cysts develop leading to latent infection. This latent infection may reactivate under immunocompromised status5. Prolonged immune activation leads to activation-induced cell death of CD4+ T lymphocytes which are the primary immune cells for protection against toxoplasmosis6. CD8+ T lymphocytes, another important component of defence against toxoplasmosis is also affected by HIV6. Human CD4+ and CD8+ T lymphocytes are both cytotoxic to T. gondii infected cells therefore, HIV infection increases susceptibility to T. gondii infection [not necessarily with toxoplasmic encephalitis (TE)]. Studies have shown that the risk of developing TE increases to 30 per cent when CD4 count is less than 100 cells/μl7.
In immunocompromised patients, reactivation of chronic infection is the most common manifestation of toxoplasmosis8; therefore, the British HIV Association recommends an initial assessment of anti-Toxoplasma IgG antibodies in these patients9. Majority of patients with AIDS have IgG antibody to T. gondii in serum8. Although IgG titres do not correlate with active infection, serologic evidence of infection always precedes the development of TE9. The HIV Medicine Association of the Infectious Disease Society of America also recommends that when and if the anti-Toxoplasma IgG levels are known and a patient comes with the signs and symptoms of TE, empirical treatment can be started10.
Singh et al11 reported the association between HIV infection and toxoplasmosis in 1996. Due to the need to identify the people at risk of toxoplasmosis and initiate necessary steps to decrease the morbidity and mortality associated with it, the present study was conducted in antiretroviral therapy (ART) naïve HIV-positive patients to determine the seroprevalence of toxoplasmosis and its association with their CD4 counts, if any.
Material & Methods
This cross-sectional study was initiated in the department of Microbiology of Seth G.S. Medical College & K.E.M. Hospital in Mumbai, India, from March 2015 to July 2016. Four hundred consecutive newly diagnosed ART naïve adult HIV positive patients coming for CD4 count estimation for the first time were enrolled for participation in the study after taking their written informed consent. The study was approved by the Institutional Review Board. Patients receiving primary prophylaxis for toxoplasmosis or pneumocystis were excluded from the study. Risk factors for the acquisition of toxoplasmosis were noted along with other demographic factors such as age and gender of the patient along with their CD4 counts.
The sample size was derived by using the earlier reported seroprevalence of 67 per cent from the same institute with a precision of 0.05 and level of confidence 95 per cent2. Using the formula, 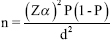 , where, n=sample size, (1−α) per cent=level of confidence, Zα=value of standard normal variant, P=expected prevalence, and d=absolute margin of error.
, where, n=sample size, (1−α) per cent=level of confidence, Zα=value of standard normal variant, P=expected prevalence, and d=absolute margin of error.
Four millilitres of blood sample was collected aseptically from each patient. The sample was collected along with the blood sample for CD4 count estimation. The serum was separated and the aliquot was stored in a sterile storage vial at −20°C till testing. Samples were evaluated for anti-Toxoplasma IgG levels using E-TXG-K18 Toxoplasma IgG ELISA kit (Ratio Diagnostics, Germany).
Statistical analysis: The statistical analysis was done doing Chi-square test and Karl-Pearson correlation test.
Results & Discussion
Of the 400 patients enrolled, 292 (73%) were positive for Toxoplasma IgG. Only 39 of the 292 (13.35%) Toxoplasma IgG-positive patients provided a definite history of risk factor exposure (blood transfusion, n=20, raw meat intake, n=11, contact with cats n=8). In the remaining 253 patients, exposure to risk factor remained obscure. The sex-specific proportions of Toxoplasma IgG in males and females were 74.6 and 71.56 per cent (Table I), respectively, and there was no significant difference between the two. The age-specific proportions of Toxoplasma IgG in the different age groups ranged from 68.35 per cent (≥45 yr) to 76.58 per cent (18-34 yr) (Table II) suggesting no significant difference of anti-Toxoplasma IgG antibodies between the different age groups.
| Gender | Positive | Negative | Total | Per cent proportion positive |
|---|---|---|---|---|
| Males | 151 | 60 | 211 | 71.56 |
| Females | 141 | 48 | 189 | 74.60 |
| Total | 292 | 108 | 400 | 73.00 |
| Age (yr) | Positive | Negative | Total | Per cent proportion positive |
|---|---|---|---|---|
| 18-34 | 157 | 48 | 205 | 76.58 |
| 35-44 | 81 | 35 | 116 | 69.82 |
| ≥45 | 54 | 25 | 79 | 68.35 |
| Total | 292 | 108 | 400 | 73.00 |
Though the Toxoplasma IgG positivity increased as CD4 counts decreased (Table III), Karl-Pearson correlation coefficient (r) was −0.0305077, indicating that Toxoplasma IgG levels and CD4 counts were not related. The regression line's equation (y=482.401−0.116313x and the P=0.55) also indicated that Toxoplasma IgG levels and CD4 counts were not related. Eleven patients had CD4 <200 cells/μl with Toxoplasma IgG levels >150 IU/ml.
| CD4 counts (no. of cells/µl) | Total | Positive | Per cent proportion |
|---|---|---|---|
| Normal ≥500 | 169 | 112 | 66 |
| Immunocompromised (≤499) | 231 | 180 | 77.92 |
Toxoplasmosis is a ubiquitous disease, caused by the coccidian parasite, T. gondii. Seroprevalence of Toxoplasma IgG in HIV-positive patients varies geographically from 7 to 80 per cent, such as 27 per cent reported by Holliman12 in the United Kingdom, 44.8 per cent by Nissapatorn et al13 in Malaysia, 15.43 per cent by Sucilathangam et al14 in Tamil Nadu, 34.78 per cent by Anuradha and Preethi15 in Telangana, India. Some studies have reported a high prevalence such as 67.8 per cent (Mumbai, India) by Meisheri et al2, 58 per cent by Osunkalu et al16 (Malaysia), and 76.5 per cent by Muluye et al17 (Ethiopia).
In the present study, the seroprevalence was found to be 73 per cent, which was higher when compared to other parts of India like Tamil Nadu and Telangana1415. This could be due to the difference in the geographical distribution of the agent and infection being more common in warm climates and at lowers altitudes than in cold and mountainous regions.
Toxoplasmosis mainly spreads through the ingestion of raw and undercooked meat, contact with cats (whose faeces are infected with oocysts of Toxoplasma), and blood transfusion18. In our study, only 13.3 per cent (39/292) positive cases were found to have associated risk factors, the most common being blood transfusion followed by a history of intake of raw meat and history of contact with cats. In a study by Walle et al18 majority reported having contact with cats and the habit of eating raw or undercooked meat. Yohanes et al19 also found a significant association with eating of raw meat and history of contact with infected cats with positivity. Both the above studies reported little association with blood transfusion1819.
Bhattacharyya et al20 have reported the level of anti-Toxoplasma IgG to be inversely correlated with CD4+ levels, which was not seen in the present study. Deroiun et al7 showed that incidence of TE was significantly higher in patients with IgG titres ≥150 IU/ml than in patients with titres <150 IU/ml (with a relative risk, 3.1) when the CD4 counts fall below 200 cells/μl. Hellerbrand et al21 also noted that high titres of Toxoplasma IgG were observed several months prior to the first clinical and radiological signs of TE. In the present study, 11 of 48 (22.9%) patients who had CD4 <200 cells/μl were found to have Toxoplasma IgG levels ≥150 IU/ml.
The present study had several limitations. This being a cross-sectional study, the patients were not followed up for the development of TE and there was no control group. Only 13.3 per cent patients could give a history of exposure to risk factors. Since the cases were not followed up, further questioning of exposure to risk factors could not be done which otherwise would have added value to the study. Further, the positive cases could not be confirmed by other tests such as enzyme-linked fluorescence assay or immunofluorescence assay.
In India, ART is started as soon as the patient is diagnosed with HIV/AIDS, and trimethoprim-sulphamethoxazole (TMP-SMX) is given as a prophylaxis for Pneumocystis jiroveci pneumonia when CD4 counts are less than 200 cells/μl22. TMP-SMX has been shown to offer some benefit against toxoplasmosis also, but it may not be protective in all cases22. Furthermore, often opportunistic disease is the first manifestation of HIV infection5. Such patients will be ART-naïve and will not be receiving TMP-SMX prophylaxis. Therefore, in the absence of any specific vaccine or prophylaxis for toxoplasmosis, it is pertinent to screen all HIV-positive patients for Toxoplasma IgG at diagnosis, irrespective of their CD4 status to prevent either acquisition or activation of infection, to limit the development of TE.
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- Epidemiology of toxoplasmosis in Switzerland: National study of seroprevalence monitored in pregnant women 1990-1991. Schweiz Med Wochenschr Suppl. 1995;65:29S-38S.
- [Google Scholar]
- A prospective study of seroprevalence of toxoplasmosis in general population, and in HIV/AIDS patients in Bombay, India. J Postgrad Med. 1997;43:93-7.
- [Google Scholar]
- 2019. Toxoplasmosis: Background, Etiology and Pathophysiology, Epidemiology. Available from: https://emedicine.medscape.com/article/229969-overview
- Kasper DL, Stephen LH, Jameson GL, Fauci AS, Longo DL, Loscalzo J, eds. Toxoplasma infections: Harrison's principles of internal medicine (19th ed). New York: McGraw- Hill; 2015.
- Predictive value of Toxoplasma gondii antibody titres on the occurrence of toxoplasmic encephalitis in HIV-infected patients. AIDS. 1996;10:1521-7.
- [Google Scholar]
- Principles and practice of Infectious diseases (8th ed). Philadelphia: Elsevier Saunders; 2014.
- British HIV Association guidelines for the treatment of opportunistic infection in HIV-positive individuals. London: British HIV Association; 2010.
- [Google Scholar]
- Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: Recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009;58:1-207.
- [Google Scholar]
- AIDS associated toxoplasmosis in India and its correlation with serum tumour necrosis factor-alpha. J Parasit Dis. 1996;20:49-52.
- [Google Scholar]
- Serological study of the prevalence of toxoplasmosis in asymptomatic patients infected with human immunodeficiency virus. Epidemiol Infect. 1990;105:415-8.
- [Google Scholar]
- Toxoplasmosis-serological evidence and associated risk factors among pregnant women in southern Thailand. Am J Trop Med Hyg. 2011;85:243-7.
- [Google Scholar]
- Seroprevalence of Toxoplasma gondii in southern districts of Tamil Nadu using IgG-ELISA. J Parasit Dis. 2012;36:159-64.
- [Google Scholar]
- Seroprevalence of toxoplasma igg antibodies in hiv positive patients in and around Khammam, Telangana state. J Clin Diagn Res. 2014;8:DL01-2.
- [Google Scholar]
- Seroprevalence of Toxoplasma gondii IgG antibody in HIV-infected patients at the Lagos University teaching hospital. HIV AIDS (Auckl). 2011;3:101-5.
- [Google Scholar]
- Prevalence of Toxoplasma gondii and associated risk factors among people living with HIV at Gondar University Hospital Northwest Ethiopia. ISRN Trop Med. 2013;2013:1-5.
- [Google Scholar]
- Seroprevalence and risk factors for toxoplasmosis in HIV infected and non-infected individuals in Bahir Dar, Northwest Ethiopia. Parasit Vectors. 2013;6:15.
- [Google Scholar]
- Latent Toxoplasma gondii infection and associated risk factors among HIV-infected individuals at Arba Minch hospital, South Ethiopia. AIDS Res Treat. 2014;2014:652941.
- [Google Scholar]
- Anti- Toxoplasma gondii antibody detection in serum and urine samples by enzyme-linked immunosorbent assay in HIV-infected patients. Indian J Pathol Microbiol. 2013;56:20-3.
- [Google Scholar]
- High predictive value of Toxoplasma gondii IgG antibody levels in HIV-infected patients for diagnosis of cerebral toxoplasmosis. Eur J Clin Microbiol Infect Dis. 1996;15:869-72.
- [Google Scholar]
- Guidelines for prevention and management of common opportunistic infections/ malignancies among HIV-infected adults and adolescents. Available from: http://www.indiahivinfo.naco.gov.in/naco/resource/guidelines-prevention-and-management-commonopportunistic-infections-malignancies-among-hiv






