Translate this page into:
Serological tests for the diagnosis of active tuberculosis: relevance for India
Reprint requests: Dr. Karen R Steingart, Affiliate Assistant Professor, Department of Health Services, University of Washington School of Public Health, Seattle, WA 98195-7660, USA e-mail: karenst@uw.edu
-
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Diagnostic tests for active tuberculosis (TB) based on the detection of antibodies (serological tests) have been commercially available for decades, although no international guidelines have recommended their use. An estimated 1.5 million serological TB tests, mainly enzyme-linked immunosorbent assays, are performed in India alone every year, mostly in the private sector. The cost of serological tests in India is conservatively estimated at US $15 million ( 825 million) per year. Findings from systematic reviews on the diagnostic accuracy of serological tests for both pulmonary and extra-pulmonary TB suggest that these tests are inaccurate and imprecise. A cost-effectiveness modelling study suggests that, if used as a replacement test for sputum microscopy, serology would increase costs to the Indian TB control sector approximately 4-fold and result in fewer disability-adjusted life years averted and more false-positive diagnoses. After considering all available evidence, the World Health Organization issued a strong recommendation against the use of currently available commercial serological tests for the diagnosis of TB disease. The expanding evidence base continues to demonstrate that the harms/risks of serological tests far outweigh the benefits. Greater engagement of the private sector is needed to discontinue the use of serological tests and to replace these tests with WHO-endorsed new diagnostics in India. The recent ban on import or sale of TB serological tests by the Indian health ministry is a welcome step in the right direction.
825 million) per year. Findings from systematic reviews on the diagnostic accuracy of serological tests for both pulmonary and extra-pulmonary TB suggest that these tests are inaccurate and imprecise. A cost-effectiveness modelling study suggests that, if used as a replacement test for sputum microscopy, serology would increase costs to the Indian TB control sector approximately 4-fold and result in fewer disability-adjusted life years averted and more false-positive diagnoses. After considering all available evidence, the World Health Organization issued a strong recommendation against the use of currently available commercial serological tests for the diagnosis of TB disease. The expanding evidence base continues to demonstrate that the harms/risks of serological tests far outweigh the benefits. Greater engagement of the private sector is needed to discontinue the use of serological tests and to replace these tests with WHO-endorsed new diagnostics in India. The recent ban on import or sale of TB serological tests by the Indian health ministry is a welcome step in the right direction.
Keywords
Antibody
diagnosis
sensitivity and specificity
serology
tuberculosis
For the vast majority of patients, tuberculosis (TB) diagnosis still depends primarily on sputum smear microscopy, with chest radiography and tuberculin skin tests being available in some settings. These tests have limitations in sensitivity and specificity, especially in people living with HIV1–3. Mycobacterial cultures on solid media may take weeks to become positive. Improved diagnostic tests, such as liquid culture of Mycobacterium tuberculosis (M. tuberculosis) and nucleic acid amplification tests are often too expensive and complex to be used in routine by TB control programmes in low-income settings. The Xpert® MTB/RIF assay (Cepheid Inc, Sunnyvale, USA), a major advance in TB diagnostics that was recently endorsed by the World Health Organization (WHO), provides high sensitivity for detection of TB and rifampicin resistance; however, high cost is a barrier for scaling-up this new technology in many areas where the epidemic is most severe4.
Serological tests have a long history and have been used successfully for the diagnosis of many infectious diseases (e.g., HIV, syphilis, and viral hepatitis). In this review, serological tests refer to tests that detect humoral immune responses (antibodies) to M. tuberculosis antigens. In comparison to microscopy, serological TB tests could potentially enable rapid diagnosis as these tests have the advantages of speed (results can be available within hours or minutes), technological simplicity, and modest training requirements. In addition, these tests could be adapted to point-of-care formats and performed at peripheral health facilities without onsite microscopy services.
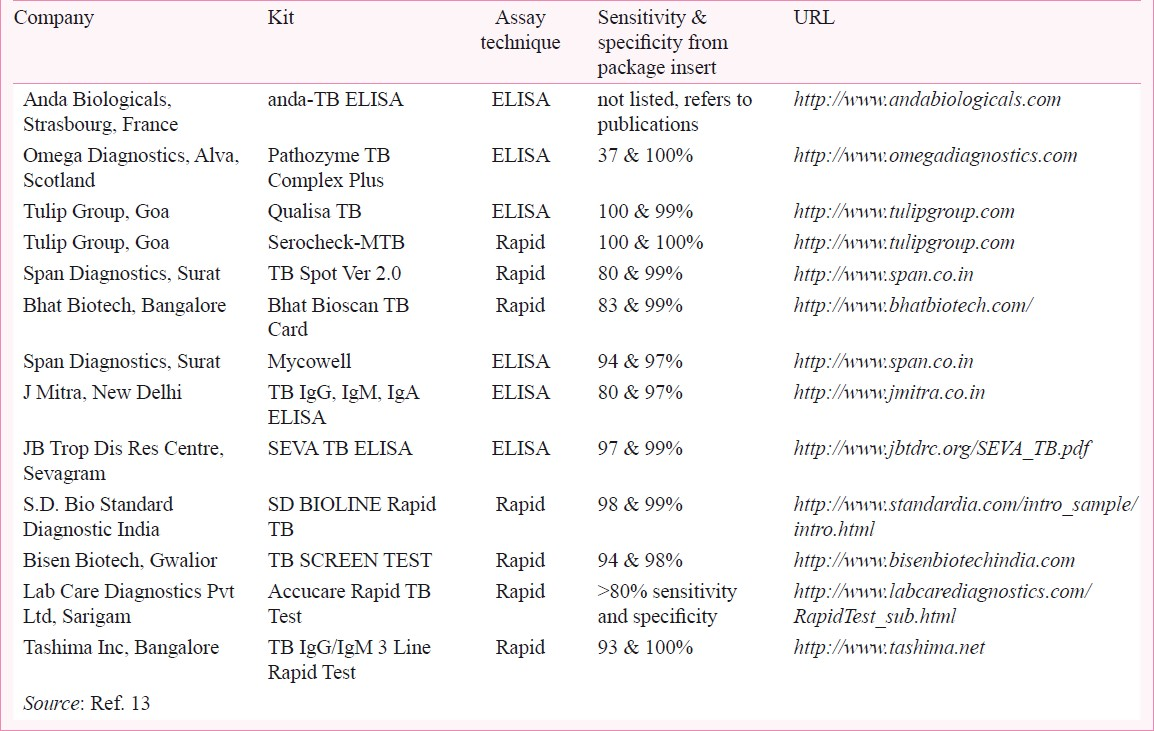
Although currently, the International Standards for TB Care discourage the use of serological tests in routine practice5 and no international guidelines recommend their use, dozens of commercial serological tests for TB diagnosis are offered for sale in many parts of the world6. According to a recent survey, serological tests are widely used in high-burden countries, including Afghanistan, Bangladesh, Brazil, Cambodia, China, India, Kenya, Myanmar, Nigeria, Pakistan, Russia, South Africa, Thailand and Vietnam7. India alone has a market for TB serological tests that exceeds the market for conventional microbiological tests (e.g., smears and culture). An estimated 1.5 million TB serological (ELISA) tests are performed in India every year78. The majority of these tests are performed by the private sector, the primary source of care for a large percentage of TB patients in India. The test kits, which are usually imported, account for an expenditure conservatively estimated at US $ 15 million ( 825 million)7 per year. Claims of high accuracy in package inserts are common (Table I). Recent media reports have highlighted the potential for TB misdiagnosis using serological tests and the fact that these tests are not used in developed countries, but instead marketed in resource-constrained countries with weak regulatory systems8–10.
825 million)7 per year. Claims of high accuracy in package inserts are common (Table I). Recent media reports have highlighted the potential for TB misdiagnosis using serological tests and the fact that these tests are not used in developed countries, but instead marketed in resource-constrained countries with weak regulatory systems8–10.
Serological tests for TB diagnosis, review
This article reviews the evidence on the diagnostic accuracy of serological tests for the diagnosis of active TB (pulmonary and extra-pulmonary TB). The evidence is derived largely from systematic reviews using standard guidelines and methods appropriate for diagnostic test accuracy reviews (Table II)1112. An expanding evidence base continues to document concerns about serological tests. In addition to the survey of high burden countries mentioned above7, we present key findings from two studies with particular relevance to India: a cost-effectiveness analysis of TB serological testing in India13 and a root-cause analysis of why serological testing is used widely by private Indian providers14. Finally, we discuss the translation of evidence into policy and the way forward for India.
Systematic reviews of serological TB tests
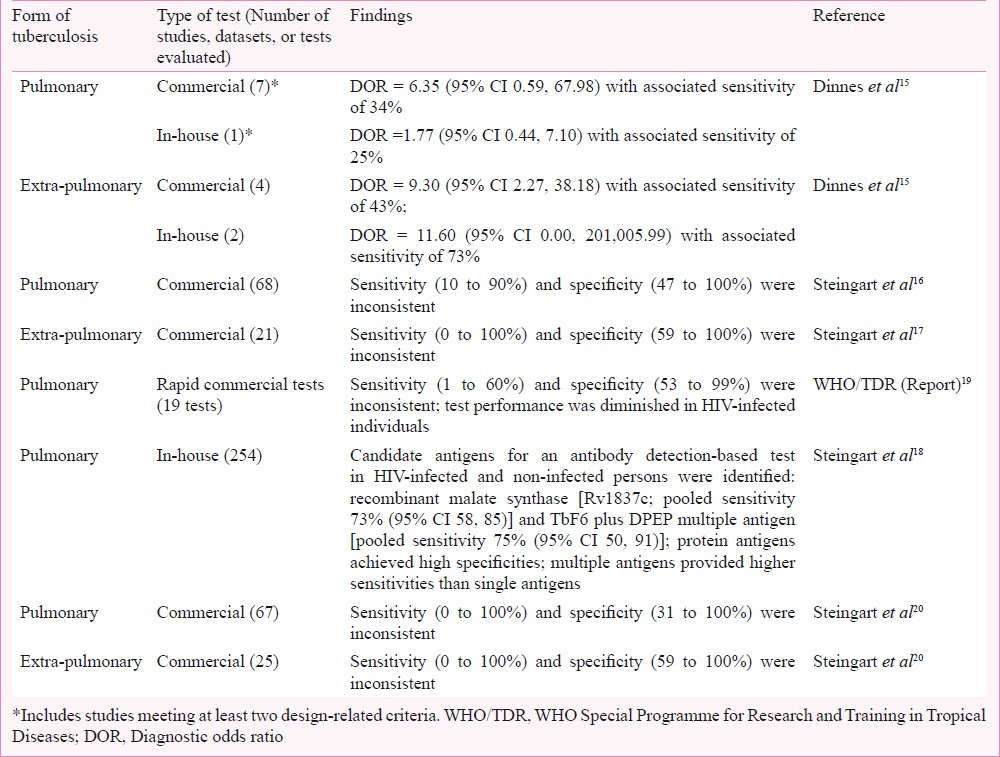
The performance of serological tests was evaluated in a comprehensive review of rapid TB diagnostics15. Studies with a cohort or case series type design were eligible for inclusion. The reference standards were culture and clinical diagnosis. For pulmonary TB (8 test evaluations), commercial serological tests showed modest performance [diagnostic odds ratio (DOR) = 7.30 (95% CI 1.95, 27.24)] with a pooled sensitivity of 88 per cent and pooled specificity of 50 per cent. When only studies meeting at least two methodological quality criteria (e.g., representative patient population and blinding of the serological test result) were considered (7 evaluations), the DOR decreased to 6.35 (95% CI 0.59, 67.98) and the sensitivity decreased to 34 per cent. For extra-pulmonary TB (4 test evaluations) pooled sensitivity was less than 50 per cent and pooled specificity was 93 per cent.
Three systematic reviews were commissioned by the WHO Special Programme for Research and Training in Tropical Diseases (TDR). Two reviews evaluated the performance of commercial serological tests for the diagnosis of pulmonary TB16 and extra-pulmonary TB17, and one review evaluated the performance of noncommercial (in-house) serological tests for pulmonary TB18. For all three reviews, studies with cross-sectional or case-control study designs were eligible for inclusion. The reference standards were culture and/or smear microscopy or, in addition, for extra-pulmonary TB, histopathological examination. The reviews of commercial serological tests for the diagnosis of pulmonary TB (68 studies)16 and extrapulmonary TB (21 studies)17 found highly variable sensitivity and specificity estimates.
For the review of non-commercial (in-house) tests for pulmonary TB, only studies evaluating tests using purified antigens were included; studies that used purified protein derivative, culture filtrates, or sonicated antigens were excluded18. The review yielded 254 test evaluations (including 51 distinct single antigens and 30 distinct multiple-antigen combinations) and found potential candidate antigens for inclusion in a serological test in both HIV-uninfected and -infected individuals. Multiple antigens provided higher sensitivities than single antigens. However, no antigen achieved sufficient sensitivity to replace smear microscopy18.
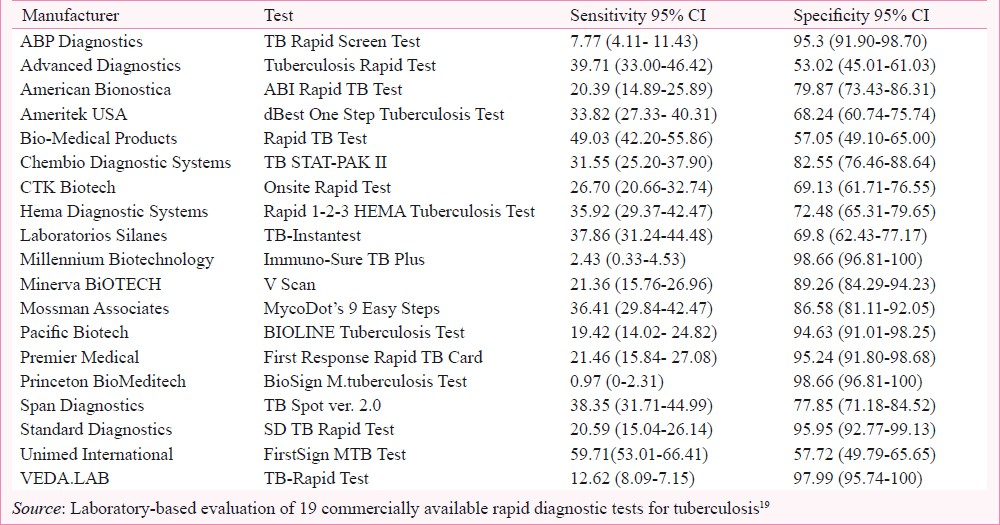
The performance of commercially available rapid (test result available in less than 15 min) TB diagnostic tests was assessed in a laboratory-based evaluation19. The reference standard was culture plus clinical follow-up. Serum samples were obtained from the WHO/TDR TB Bank and included specimens from Uganda, The Gambia, Canada, Tanzania, Brazil, and Spain. Test manufacturers were identified via the internet, conferences and contact with experts and TB programmes. Nineteen companies agreed to participate, seven companies declined and one withdrew. Sensitivity of the serological tests ranged from 1 to 60 per cent and specificity from 53 to 99 per cent (Table III). Test performance was diminished in HIV-infected individuals. An evaluation of smear plus serology yielded a gain equivalent to the detection of 57 per cent of smear negative, culture positive TB cases. However, there was a corresponding unacceptable decrease in specificity to 58 per cent19.
In order to develop policy guidance concerning commercial serological TB tests, WHO commissioned an updated systematic review20. For this review, the reference standards were culture for pulmonary TB and culture, smear, or histopathological examination for extra-pulmonary TB. Assessment of study quality was carried out using QUADAS (Quality Assessment of Diagnostic Accuracy Studies), a validated tool to evaluate the risk of bias in diagnostic accuracy studies21. Test performance was summarized using bivariate meta-analyses that jointly modelled sensitivity and specificity22. The review included 14 new papers (approximately 30% of the included papers) identified since the previous reviews1617. In total, 67 studies (5,147 participants) were included in the pulmonary TB group and 25 studies (1,809 participants) in the extra-pulmonary TB group. The results demonstrated that serological tests for both pulmonary and extra-pulmonary TB provided inconsistent and imprecise sensitivity and specificity estimates (Table II). Anda-TB IgG (Anda Biologicals, Strasbourg, France), the most commonly evaluated test, yielded pooled sensitivities of 76 per cent (95% CI 63, 87) in studies of smear-positive and 59 per cent (95% CI 10, 96) in studies of smear-negative patients; corresponding pooled specificities were 92 per cent (95% CI 74, 98) and 91 per cent (95% CI 79, 96), respectively20.
In the only two studies identified that evaluated HIV-infected TB patients, test sensitivities were poor. SDHO MTB test (SDHO Laboratories Inc., Canada) for pulmonary TB yielded sensitivity 16 per cent (95% CI 5, 34)23 and MycoDot (Mossman Associates, Milford) for extra-pulmonary TB yielded sensitivity 33 per cent (95% CI 19, 39)24.
The Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) approach was used to assign a grade to the quality of evidence in the updated systematic review25. In the GRADE system, quality of evidence reflects our confidence that an estimate of effect is correct. Quality may be compromised by five factors: risk of bias, directness of evidence, heterogeneity, precision of effect estimates, and risk of publication bias26. The quality of studies in the updated review was graded as ‘very low’ based on all five factors suggesting that “the true effect is likely to be substantially different from the estimate of effect”26.
Relevance for India
Dowdy and colleagues13 used a hypothetical patient cohort of 1.5 million TB suspects, a number conservatively equal to the annual volume of serological tests in India, in a decision analysis to estimate the costs and effectiveness of serological testing compared with other diagnostic tools. In this cohort, using output from the updated systematic review, serological testing for TB disease in adult Indian TB suspects was found to be more costly and even less effective than sputum smear microscopy. Specifically, the researchers found that, if used as a replacement test for sputum microscopy, serology would increase costs to the Indian TB control sector approximately 4-fold and result in 121,000 more false-positive diagnoses, 102,000 fewer disability-adjusted life years averted and 32,000 more secondary infections, despite an estimated 14,000 more TB diagnoses13.
In a root-cause analysis, Jaroslawski and Pai14 sought to understand the reasons why TB serological tests are so popular in the Indian private sector. One of the key findings in their analysis was that, given the absence of an accurate, validated, point-of-care test for TB, serological tests meet a perceived need among private providers and patients. Physicians consider smear microscopy to be insensitive and antiquated. In addition, sputum-based tests are unsuitable for the diagnosis of extra-pulmonary, smear-negative, and paediatric TB. From an economic perspective, imported molecular or liquid culture tests are too expensive, leaving serological tests as the main alternative. Although serological tests are inaccurate, various players along the value chain profit from their use, and this sustains a market for these tests. In India a large number of serological kits are available in the market. Private healthcare, in general, is poorly regulated, and doctors in the private sector do not necessarily follow standard guidelines. Reflecting upon lessons learned from serology, the authors described key characteristics for a successful new TB test: be a rapid test; be perceived by doctors as more sensitive and sophisticated than sputum smears; be suitable for the detection of extra-pulmonary TB; not require laboratories to make big investments in infrastructure/equipment; and not be too inexpensive or too expensive, but be in the middle range of approximately US $10 ( 550)14.
550)14.
Moving from evidence to recommendations
WHO has adopted a systematic and transparent process for moving evidence to recommendations27. In July 2010, WHO convened an Expert Group to review all available evidence on serological tests with the GRADE approach. As recommended by GRADE, the Expert Group was asked to draw conclusions based not only on the quality of evidence (considered very low), but in addition, the balance between benefits and harms, values and preferences, and estimated costs28. After considering the evidence, the Expert Group recommended against the use of currently available serological tests.
In July 2011, WHO issued a policy stating that commercial serological tests provide inconsistent and imprecise estimates of sensitivity and specificity. There is no evidence that existing commercial serological assays improve patient-important outcomes, and high proportions of false-positive and false-negative results adversely impact patient safety. Overall data quality was graded as very low, with harms/risks far outweighing any potential benefits. It is, therefore, recommended that these tests should not be used in individuals suspected of active pulmonary or extra-pulmonary TB, irrespective of their HIV status. The WHO policy strongly encourages targeted further research to identify new/alternative point-of-care tests for TB diagnosis and/or serological tests with improved accuracy29. Immediately following the WHO policy, the RNTCP published an advisory statement against the use of TB serological tests in India30. More recently, an expert committee convened by the Drug Controller General of India has recommended a ban on import and sale of TB serological tests in India31 and this ban has been endorsed by the Indian health ministry.
Conclusions
Findings from several systematic reviews and an independent evaluation of rapid tests suggest that serological tests for both pulmonary and extra-pulmonary TB are inaccurate. No test performs well enough to replace smear microscopy. Currently available commercial serological TB tests appear to do more harm than good because of the harm caused to patients through false-positive or false-negative tests results. Furthermore, considerable resources are required for the serological tests themselves (approx. US $ 10/ 550/antibody or US $ 30/
550/antibody or US $ 30/ 1650 per full investigation i.e., three tests for specific antibody classes (IgG, IgM and IgA).
1650 per full investigation i.e., three tests for specific antibody classes (IgG, IgM and IgA).
Echoing these concerns, in a recent editorial Singh and Katoch wrote, ‘Unfortunately, unethical medical practices provided major boost to these kits in recent years, without bothering much on quality of tests and implications of false-positive and false negative results…. This editorial is not the obituary for serology. Immunological detection with appropriate sensitivity and specificity will remain an attractive research option for developing immunodiagnosis of tuberculosis”32.
Serological tests: The way forward
Despite the impressive scale-up of the DOTS programme by the RNTCP, India continues to report more than two million TB cases every year and undiagnosed and mismanaged TB is partly responsible for this33. Recognizing these challenges, the Government of India has set an ambitious goal of providing universal access to quality diagnosis and treatment for all TB patients in the country (National Strategic Plan, 2012-2017). To improve TB diagnosis in India, several efforts are needed in parallel. India recently made TB a notifiable disease to ensure all cases in the private sector are reported to RNTCP. India must adopt new tools that are accurate, validated and WHO-endorsed, and replace suboptimal tests with good tests that can impact patient outcomes and reduce TB transmission in the community. Innovative tools and innovative delivery systems that engage both public and private sectors are essential for reaching this goal. Recently, the Indian Academy of Pediatrics discouraged the use of serological tests34, and other medical associations like the Indian Association of Medical Microbiologists, Indian Chest Society, and Indian Medical Association must follow suit. The Drug Controller General of India must tighten regulation of all in vitro diagnostics in the country and ensure that diagnostic tests undergo through validation before approval. India's RNTCP must set clear specifications for all TB diagnostics used in the country. Furthermore, increased attention must be paid to quality assurance in laboratories in India. Greater engagement of the private sector is also needed to reduce the misdiagnosis of TB and to replace bad tools with newer validated technologies.
Conflict of interests: All authors were involved in the WHO policy on serological assays. KRS serves as Co-ordinator of the Evidence Synthesis and Policy subgroup of Stop TB Partnership's New Diagnostics Working Group (NDWG). AR served as the secretary of the NDWG and is employed by WHO/TDR, the agency administering the USAID grant that funded several of the systematic reviews. AR contributed to critical revision of and decision to publish the manuscript. MP is a recipient of a New Investigator Award from the Canadian Institutes of Health Research (CIHR). This funding source had no role in the preparation of this manuscript, nor the decision to submit the manuscript for publication. MP serves as an external consultant for the Bill & Melinda Gates Foundation. The authors have no financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
- Sputum processing methods to improve the sensitivity of smear microscopy for tuberculosis: a systematic review. Lancet Infect Dis. 2006;6:664-74.
- [Google Scholar]
- Facing the crisis: improving the diagnosis of tuberculosis in the HIV era. J Infect Dis. 2007;196(Suppl 1):S15-27.
- [Google Scholar]
- How does the diagnosis of tuberculosis in persons infected with HIV differ from diagnosis in persons not infected with HIV? In: Frieden T, ed. Toman's tuberculosis: case detection, treatment, and monitoring - questions and answers (2nd ed). Geneva: World Health Organization; 2004. p. :80-3.
- [Google Scholar]
- Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005-15.
- [Google Scholar]
- Tuberculosis Coalition for Technical Assistance. In: International standards for tuberculosis care (ISTC) (2nd ed). The Hague: Tuberculosis Coalition for Technical Assistance; 2009.
- [Google Scholar]
- World Health Organization Special Programme for Research and Training in Tropical Diseases, Foundation for Innovative New Diagnostics. 2006. Diagnostics for tuberculosis: global demand and market potential. Geneva: World Health Organization; :49-72. Available from: http://www.who.int/tdr/publications/en/
- [Google Scholar]
- Widespread use of serological tests for tuberculosis: data from 22 high-burden countries. Eur Respir J. 2012;39:502-5.
- [Google Scholar]
- A deadly misdiagnosis. 2010. The New Yorker. :48-53. Available from: http://www.newyorker.com/reporting/2010/11/15/101115fa_fact_specter
- [Google Scholar]
- The scandal of TB misdiagnosis. 2010. Tropical diseases research to foster innovation & knowledge application. Available from: http://www.tropika.net/svc/news/20101113/Chinnock-20101113-News-TB-India
- [Google Scholar]
- Analysing and presenting results. In: Deeks JJ, Bossuyt PM, Gatsonis C, eds. Cochrane handbook for systematic reviews of diagnostic test accuracy, version 1. London: The Cochrane Collaboration; 2010. Available from: http://srdta.cochrane.org/
- [Google Scholar]
- Serological testing versus other strategies for diagnosis of active tuberculosis in India: a cost-effectiveness analysis. PLoS Med. 2011;8:e1001074.
- [Google Scholar]
- Why are inaccurate tuberculosis serological tests widely used in the Indian private healthcare sector.A root-cause analysis? J Epi Global Health. 2012;2:39-50.
- [Google Scholar]
- A systematic review of rapid diagnostic tests for the detection of tuberculosis infection. Health Technol Assess. 2007;11:1-196.
- [Google Scholar]
- Commercial serological antibody detection tests for the diagnosis of pulmonary tuberculosis: a systematic review. PLoS Med. 2007;4:e202.
- [Google Scholar]
- A systematic review of commercial serological antibody detection tests for the diagnosis of extrapulmonary tuberculosis. Thorax. 2007;62:911-8.
- [Google Scholar]
- Performance of purified antigens for serodiagnosis of pulmonary tuberculosis: a meta-analysis. Clin Vaccine Immunol. 2009;16:260-76.
- [Google Scholar]
- World Health Organization on behalf of the Special Programme for Research & Training in Tropical Diseases. 2008. Laboratory-based evaluation of 19 commercially available rapid diagnostic tests for tuberculosis. Geneva: World Health Organization; Available from: http://www.who.int/tdr/publications/documents/diagnostic-evaluation-2.pdf
- [Google Scholar]
- Commercial serological tests for the diagnosis of active pulmonary and extrapulmonary tuberculosis: an updated systematic review and meta-analysis. PLoS Med. 2011;8:e1001062.
- [Google Scholar]
- The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3:25.
- [Google Scholar]
- A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Stat Med. 2001;20:2865-84.
- [Google Scholar]
- Poor performance of a novel serological test for diagnosis of pulmonary tuberculosis in Bangui, Central African Republic. Clin Vaccine Immunol. 2006;13:702-3.
- [Google Scholar]
- Evaluation of the MycoDot test for the diagnosis of tuberculosis in HIV seropositive and seronegative patients. Int J Tuberc Lung Dis. 1997;1:259-64.
- [Google Scholar]
- GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924-6.
- [Google Scholar]
- GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401-6.
- [Google Scholar]
- New and improved tuberculosis diagnostics: evidence, policy, practice, and impact. Curr Opin Pulm Med. 2010;16:271-84.
- [Google Scholar]
- Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008;336:1106-10.
- [Google Scholar]
- World Health Organization. 2011. Commercial serodiagnostic tests for diagnosis of tuberculosis. Policy Statement. Geneva: World Health Organization; WHO/HTM/TB/2011.5. Available from: http://www.tbevidence.org/documents/policies/WHO%20Policy%20Statement%20on%20Commercial%20TB%20Serodiagnostic%20Tests%202011.pdf
- [Google Scholar]
- Directorate General of Health Services. 2011. Advisory against commercial serological tests. New Delhi: Central TB Division, Ministry of Health and Family Welfare; Available from: http://www.tbcindia.nic.in/pdfs/Letter_Serodiagnosis.pdf
- [Google Scholar]
- Health Ministry set to ban commonly used TB test. 2012. Indian Express. Available from: http://www.indianexpress.com/news/health-ministry-set-to-ban-commonlyused-tb-test/925754/
- [Google Scholar]
- Commercial serological tests for the diagnosis of active tuberculosis in India: time for introspection. Indian J Med Res. 2011;134:583-87.
- [Google Scholar]
- World Health Organization. 2011. Global tuberculosis control: WHO report. Geneva: World Health Organization; Available from: http://www.who.int/tb/publications/global_report/2011/gtbr11_full.pdf
- [Google Scholar]
- Indian Academy of Pediatrics. Consensus statement on childhood tuberculosis. Indian Pediatr. 2010;47:41-55.
- [Google Scholar]






