Translate this page into:
Serological survey of toxoplasmosis in a district in Tamil Nadu: Hospital-based study
+For correspondence: drgsucila@rediffmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Sir,
Toxoplasmosis is a parasitic disease that affects millions of people worldwide. Although it remained asymptomatic in the majority of cases, but gains clinical significance when primary infection occurs or when there is reactivation of infection in immunosuppressed patients, e.g., AIDS patients. In India, the exact seroprevalence of this infection is not known. The prevalence of toxoplasmosis in pregnant women in India is variably reported to be as low as 5 per cent to as high as 80 per cent1234. Since testing for toxoplasmosis is not a routine activity in our country, information on this zoonotic infection, diagnosis and interpretation of test results is lacking. In Tamil Nadu, various studies have been done in northern districts56 but so far not reported in southern districts except our earlier report on seroprevalence of toxoplasmosis by in house IgG IFAT7. Therefore, a serological survey was carried out to detect antibodies to Toxoplasma gondii using in-house IgG based tests viz., enzyme linked immunosorbent assay (ELISA) and modified direct agglutination test (MAT) in the different groups of patients in southern districts of Tamil Nadu, India.
The present study was conducted at Tirunelveli Medical College Hospital, Palayamkottai, Tirunelveli District, Tamil Nadu, from May 2006 to October 2007. After obtaining an approval from the institutional ethical committee and an informed consent from the inpatients and outpatients, a total of 350 peripheral blood samples were collected from 175 immunodeficient (HIV and cancer patients) and 175 immunocompetent patients (pregnant women, ocular chorioretinitis cases and patients with lymphadenopathy). HIV-infected patients with CD4 T-cell counts <200/μl and diagnosis confirmed cancer patients were included in immunodeficient group. Exclusion criteria for immunodeficient group were: transplant recipients under immunosuppressive therapy or haemodialysis patients with chronic renal failure. Asymptomatic pregnant women, pregnant women with bad obstetrics history (BOH), ocular chorioretinitis cases, and those with lymphadenopathy were included in immunocompetent group and patients with myocarditis and pericarditis were excluded. All serum samples (n=350) were subjected into IgG based assays viz., MAT and ELISA for toxoplasmosis. The results of these assays were compared with our previous results on IFAT.
The T. gondii (RH strain) tachyzoite antigen was prepared by in vivo propagation in mice and in vitro cell culture system in MDCK fibroblast cell line. The tachyzoites count was 1 × 107 and 1 × 109 per ml of antigen by in vivo and in vitro methods, respectively. Formalin-killed T. gondii tachyzoite antigen was prepared for IFAT and MAT. The tachyzoite soluble antigen (TSA) with protein concentration of 0.63 mg/ml was prepared and used for ELISA. The antigen concentration was standardized at 10 μg/ml. The optimum dilutions of serum and conjugate were found to be 1:100 and 1:1,000, respectively, by chequer board titration. In-house IgG micro-ELISA was performed as described by Voller et al8 with minor modifications. The cut-off value was calculated by adding mean OD value of two negative controls and three positive controls and the sum was divided by six. The test serum samples with absorbance values less than the cut-off value were considered non-reactive and those with absorbance values equal or greater than the cut-off value were considered reactive. The minimum detection limit of ELISA was calculated by adding 2 × SD to the mean optical density (OD) obtained with negative human serum.
Modified direct agglutination test (MAT) described by Desmonts and Remington was followed9. Antibody titres of 1:20 or more were taken as significant for reporting positive results. Those test samples positive at 1:20 were further serially two-fold diluted until the end titre was reached. The sensitivity, specificity and positive and negative predictive values of the three assays were calculated using 10 known positive and negative reference serum samples obtained from Toxo Lab, USDA, Maryland, USA, as per the standard procedure described for screening test evaluation formula.
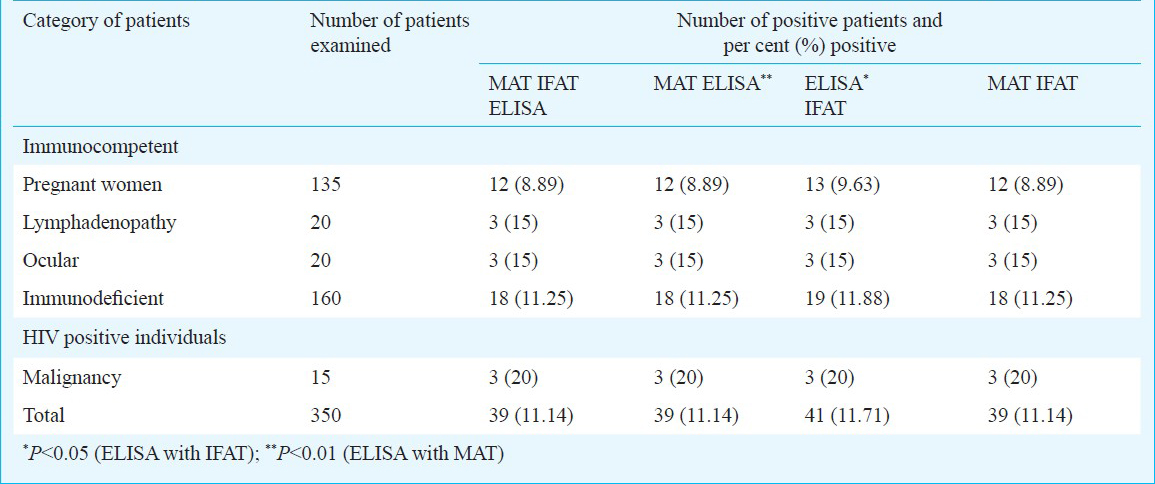
Of the 350 patients tested, 46 (13.14 per cent) and 39 (11.41 per cent) patients were found to be seropositive for toxoplasmosis by ELISA and MAT, respectively whereas 41 (11.71%) were seropositive by IgG IFAT7. The overall seropositivity of toxoplasmosis in and around Tirunelveli District of Tamil Nadu was 13.14 (46/350) per cent based on IgG ELISA. Seropositivity of toxoplasmosis in immunocompromised and immunocompetent patients were of 15.43 (27/175), 12.57 (22/175) and 10.86 (19/175) per cent, 10.29 (18/175) per cent, respectively based on IgG ELISA, and MAT, respectively. It was 12 (21/175) and 10.86 (19/175) per cent by IFAT7. There was no significant difference between the immunocompetent and immunodeficient groups. The seropositivity of toxoplasmosis in pregnant women was found to be 9.63 (13/135) per cent by ELISA which was equal to IFAT whereas it was only 8.89 per cent by MAT. Among the 20 cases of lymphadenopathy, only three (15%) were seropositive with all three tests. Of the 20 ocular cases tested, only 3 (15 per cent) were seropositive with all three tests. The seropositivity of toxoplasmosis in HIV patients was found to be 15 (24/160), and 11.25 (18/160) per cent by ELISA, and MAT, respectively whereas it was 11.88 (19/160) by IFAT. Of the 15 cases of malignancy, only three (20%) were positive with all three tests. The number of samples positive by all the three tests and also in combination of two tests like MAT and ELISA, MAT and IFAT was 39 (11.14%) whereas in combination of two tests like IFAT and ELISA was 41 (11.71%). Among the three assays, IgG ELISA was found to be highly sensitive (90%) and specific (100%) in detecting toxoplasmosis, whereas the IgG IFAT and MAT were equal in sensitivity (80%) and specificity (90%). The efficiency of three assays were compared by McNemar's test and the result revealed that testing efficiency of IgG ELISA in detecting toxoplasmosis was higher (P<0.05) than the other two assays (Table).
The mean CD4+ count of HIV patients of seropositive (male - 111.05 ± 79.96 and female- 79.5 ± 27.36) and seronegative (male - 218.23 ± 169.38 and female - 326.5 ± 259.51) groups of toxoplasmosis was statistically interpreted to assess the risk of development of toxoplasmosis as opportunistic infection. Significant difference between mean CD4+ count in seropositive and seronegative groups of toxoplasmosis was noticed (at P<0.001, OR=2.09, 95 % CI=1.5-2.9). Similarly significant difference was observed in the mean CD8+ count and CD4+/CD8+ ratio in the study groups. Among the 160 HIV positive samples, 24, 19 and 18 samples were found to be positive for anti-Toxoplasma antibody by ELISA, IFAT and MAT, respectively and the association between the presence of anti-Toxoplasma antibody and CD4 cell count was determined. Most of the Toxoplasma seropositive patients 17/24 (71 %), 16/19 (84 %) and 16/18 (88 %) had CD4 cell count <100/μl.
In the present study, the overall seropositivity for toxoplasmosis was 13.14 per cent based on IgG ELISA. Bhatia et al5 reported only 12 per cent seropositivity for toxoplasmosis in south India, whereas, Mittal et al10 revealed an overall seropositivity of only 1 per cent from Delhi. Singh and Nautiyal11 noted a high positivity (75%) of T. gondii in Almora, north India in women of child bearing age. Very high prevalence of 77 per cent has been reported from north India12.
A total of 9.63 per cent pregnant women were seropositive in the current study based on ELISA. Chakraborty et al13 reported 7.1 per cent prevalence of toxoplasmosis in pregnant women from Calcutta (Kolkata). Akoijam et al3 performed a study among primigravid women attending a secondary level hospital in a district of north India using IgG ELISA and reported 41.75 per cent T. gondii seropositivity3. An earlier study reported 7.72 per cent positivity of toxoplasmosis in pregnant women from Delhi14.
Present study revealed 15 per cent seropositivity among the HIV positive individuals tested by IgG ELISA. Meisheri et al15 conducted a study of seroprevalence of toxoplasmosis in general population and in HIV/AIDS patients in Bombay (Mumbai) and reported an overall seroprevalence of 30.9 per cent in the immunocompetent adult (34% in men and 26.2% in women). In HIV infected hosts the seroprevalence was 67.8 per cent15. In the present study, there was no statistical difference in the seropositivity between the immunocompetent and immunodeficient groups which underlines the importance of screening of this parasite in the immunocompetent patients also like in pregnant women.
In our study, most of the Toxoplasma seropositive patients had CD4+ T cell counts <100/μl which was similar to the earlier observations1617.
Of the three assays, IgG ELISA was found to be highly sensitive (90%) and specific (100%) in detecting toxoplasmosis. The sensitivity and specificity were calculated based on 10 known positive and 10 known negative normal healthy individuals reference serum samples obtained from international reference laboratory. However, due to endemicity of many parasitic diseases in this country, it would always be better to include reference samples from other parasitic diseases also. The testing efficiency of IgG ELISA for detecting toxoplasmosis was higher than the other two tests, as observed earlier1819.
In conclusion, among the three assays, seropositivity rate was high in in-house IgG ELISA than in the other two assays for toxoplasmosis in southern districts of Tamil Nadu. The overall seropositivity for T. gondii antigen by IgG-ELISA was 13.14 per cent in and around Tirunalvali district in Tamil Nadu. IFAT showed good agreement with ELISA in detecting positive and negative results in the present study.
Acknowledgment
The first author acknowledges the receipt of financial assistance to MD thesis from Indian Council of Medical Research (ICMR), New Delhi, for pursuing this research work.19
References
- Mother-to-child transmission and diagnosis of Toxoplasma gondii infection during pregnancy. Indian J Med Microbiol. 2003;21:69-76.
- [Google Scholar]
- Incidence and prevalence of toxoplasmosis in Indian pregnant women: a prospective study. Am J Reprod Immunol. 2004;52:276-83.
- [Google Scholar]
- Seroprevalence of Toxoplasma infection among primigravid women attending antenatal clinic at a secondary level hospital in North India. J Indian Med Assoc. 2002;100:591-2.
- [Google Scholar]
- Prevalence of specific IgM due to toxoplasma, rubella, CMV and C. trachomatis infections during pregnancy. Indian J Med Microbiol. 2001;19:52-6.
- [Google Scholar]
- Toxoplasmosis in South India - a serological study. Indian J Med Res. 1974;62:1818-20.
- [Google Scholar]
- Outbreak of ocular toxoplasmosis in Coimbatore, India. Indian J Ophthalmol. 2006;54:129-31.
- [Google Scholar]
- IgG - indirect fluorescent antibody technique to detect seroprevalence of Toxoplasma gondii in immunocompetent and immunodeficient patients in southern districts of Tamil Nadu. Indian J Med Microbiol. 2010;28:354-7.
- [Google Scholar]
- A microplate enzyme-immunoassay for toxoplasma antibody. J Clin Pathol. 1976;9:150-3.
- [Google Scholar]
- Direct agglutination test for diagnosis of Toxoplasma infection: method for increasing sensitivity and specificity. J Clin Microbiol. 1980;11:562-8.
- [Google Scholar]
- Prevalence of toxoplasma antibodies among women with BOH and general population in Delhi. J Commun Dis. 1990;22:223-6.
- [Google Scholar]
- Seroprevalence of toxoplasmosis in Kumaon region of Uttar Pradesh. Indian J Med Res. 1991;93:47-9.
- [Google Scholar]
- Prevalence of TORCH infections in Indian pregnant women. Indian J Med Microbiol. 2002;20:57-8.
- [Google Scholar]
- Toxoplasmosis in women of child bearing age and infant follow up after in utero treatment. Indian J Pediatr. 1997;64:879-82.
- [Google Scholar]
- Prevalence of toxoplasmosis in Indian women of child bearing age. Indian J Pathol Microbiol. 1995;38:143-5.
- [Google Scholar]
- A prospective study of seroprevalence of toxoplasmosis in general population and in HIV/AIDS patients in Bombay, India. J Postgrad Med. 1997;43:93-7.
- [Google Scholar]
- Toxoplasma infection. In: Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL, eds. Harrison's principles of internal medicine Vol II. (16th ed). New York: McGraw-Hill Inc; 2005. p. :1243-8.
- [Google Scholar]
- Natural history of human immunodeficiency virus disease in southern India. Clin Infect Dis. 2003;36:79-85.
- [Google Scholar]
- Determination of IgM and IgG antibodies to Toxoplasma using the IFA test, ELISA and Dot-ELISA procedures. Vet Parasitol. 1986;20:31-42.
- [Google Scholar]
- Comparison of an enzyme-linked immunoassay and a quantitative indirect fluorescent-antibody test with the conventional indirect fluorescent-antibody test for detecting antibodies to Toxoplasma gondii. J Clin Microbiol. 1982;16:341-4.
- [Google Scholar]





