Translate this page into:
Serogroup distribution, antibiogram patterns & prevalence of ESBL production in Escherichia coli
* For correspondence yasht26@yahoo.co.in
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Sir,
Extended spectrum β-lactamases (ESBL) producing Escherichia coli is widely recognized as an emerging threat to the public health1. The prevalence of ESBL producing E. coli is increasing globally and the community-onset infection due to these is major clinical concerns in various countries. There are marked geographical differences in the proportion of ESBL producing E. coli, the highest (61%) being reported from a multicentre study in India2. Global spread of ESBL producing E. coli and the availability of limited therapeutic options for this organism is a matter of concern which may further complicate the present scenario of antimicrobial resistance. Serogrouping of E. coli is important for characterization and also plays an important role in epidemiology to know the prevalent serogroup in the specific geographical area. Hence, in the present study an effort was made to determine and analyse the antibiogram of E. coli isolates received in the laboratory of National Salmonella and Escherichia Centre, Kasauli, India, from various parts of the country and to find out the prevalence of ESBL producers among them.
Three hundred E. coli isolates received from different parts of the country during December 2012 to May 2013 were identified and serogrouped using standard methods34 using an array of antisera (Statens Serum Institute, Copenhagen, Denmark) against various E. coli serogroups. These isolates were further subjected to antimicrobial susceptibility testing as per Clinical and Laboratory Standards Institute (CLSI) guidelines5. The isolates detected as potential ESBL producers on the basis of primary screening method5 using cefotaxime (30 µg) and ceftazidime (30 µg), were further tested for ESBL production using phenotypic confirmatory test5. Phenotypic confirmatory test was performed using ESBL detection kit (Hi-Media, Mumbai). The kit contained two combinations of antibiotic and β-lactamase inhibitor i.e., (i) ceftazidime (30 µg) and ceftazidime+clavulanic acid (30/10 µg); (ii) cefotaxime (30 µg) and cefotaxime+clavulanic acid (30/10 µg). A ≥5 mm increase in zone diameter for either antimicrobial agents tested in combination with clavulanic acid from its zone alone was taken as positive result for ESBL production5.
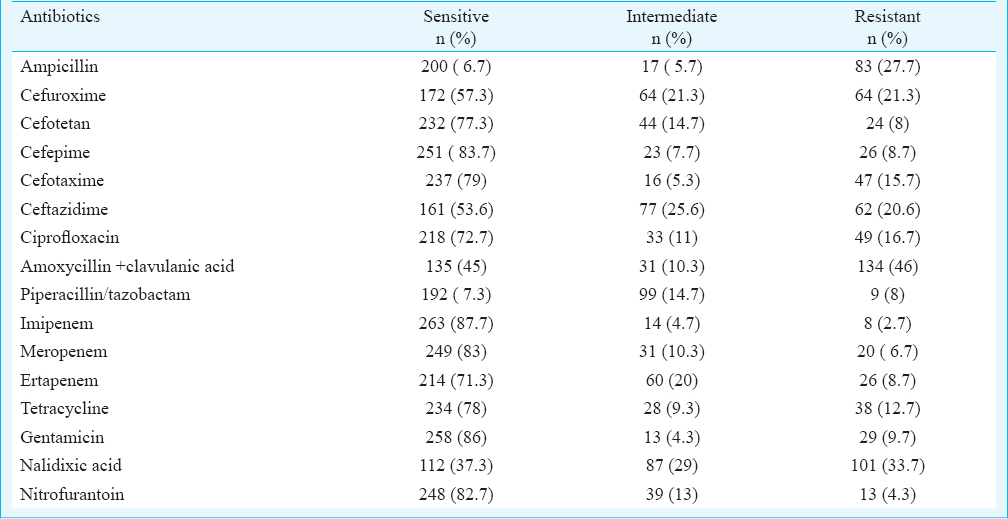
Of the 300 isolates of E. coli, 194 (64.7%) were found to be typeable and belonged to 47 serogroups whereas 106 (35.3%) were found to be untypeable. The major serogroups encountered during the study were O75 (13%) and O8 (9.3%) followed by O2 (7.7%), O29 (7.2%), O1 (6.2%) and O66 (5.2%). All isolates belonging to serogroups O163, O90, O10, O133, O166, O113, O18, O153, O6, O41, O149 and O172 were from human origin whereas none of the isolates of human origin belonged to serogroups O5, O20, O35, O52, O86, O106, O160, O11, O104, O27, O164, O21, O101, O136, O48, O55, O118, O131, O154 and O40 (Fig. 1). ESBL production was detected in 46 (15.3%) of the E. coli isolates (Fig. 2). Among isolates of human origin, 13 (18.3%) and 9 (20.9%) isolates from human stool and human urine, respectively, were detected as ESBL producers. However, ESBL producers among isolates from non-human sources comprised 13.5 per cent (poultry), 16.7 per cent (food) and 8.3 per cent (environment) (Fig. 3). High level of resistance was observed for amoxicillin+clavulanic acid (46%) followed by nalidixic acid (33.7%) and ampicillin (27.7%). However, high proportion of isolates were found to be susceptible to imipenem (87.7%) and gentamicin (86%) followed by cefepime (83.7%), meropenem (83%), nitrofurantoin (82.7%), cefotaxime (79%), tetracycline (78%) and cefotetan (77.3%) (Table).
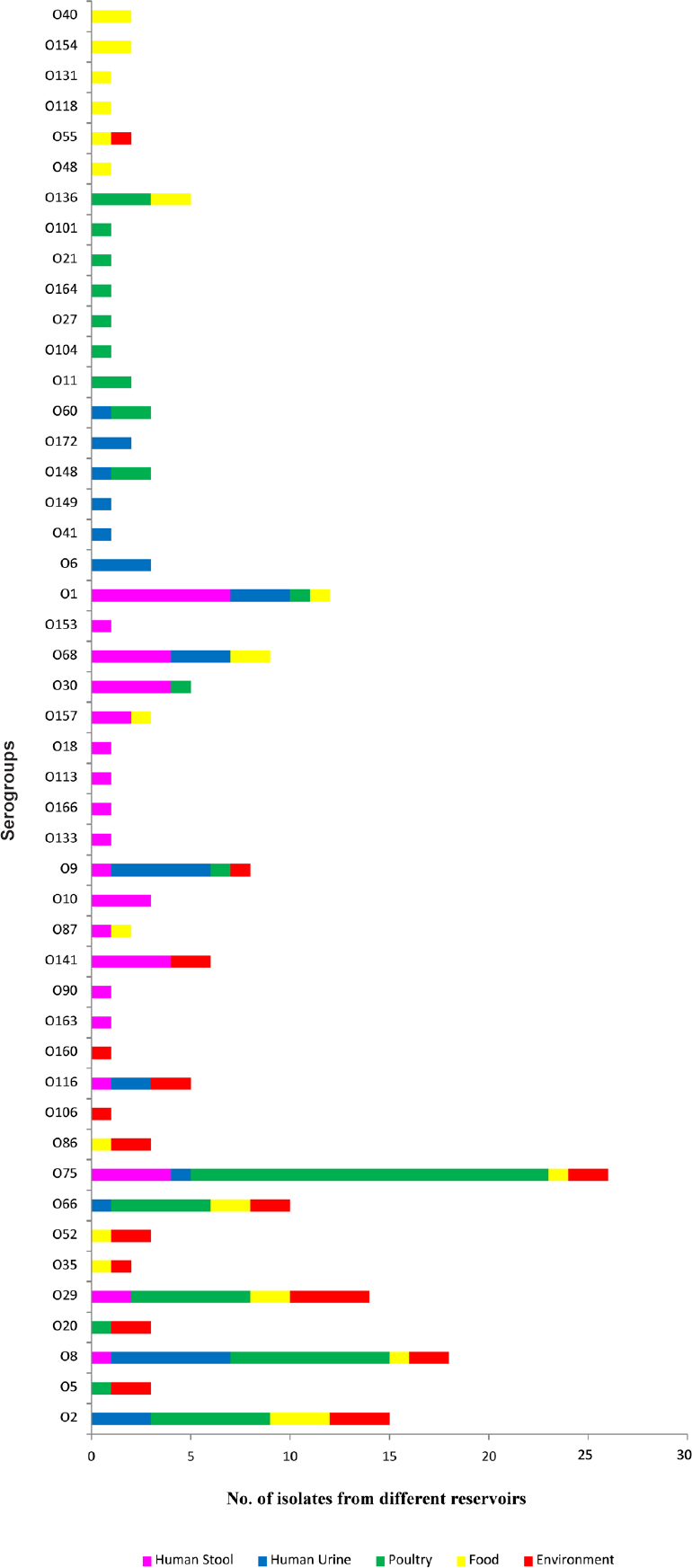
- Distribution of various serogroups among different reservoirs. Serogroups O40, O154, O131, O118 and O48 were isolated only from food; O101, O21, O164, O27, O104 and O11 were isolated only from poultry; O153, O18, O113, O166, O133, O10, O90 and O163 were isolated only from human stool; O149, O41 and O6 were isolated only from human urine; O160 and O106 were isolated only from environment.
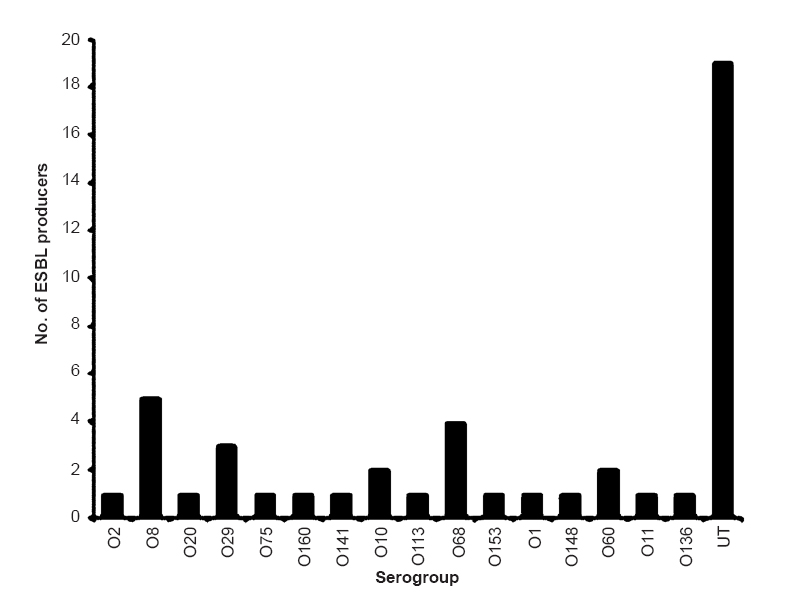
- ESBL production among various serogroups. Maximum number of ESBL producers were found to be O-untypable followed by O8, O68, O29, O10, O60, O2, O20, O75, O160, O141, O113, O68, O153, O148, O11 and O136. No ESBL producer was observed among other serogroups.
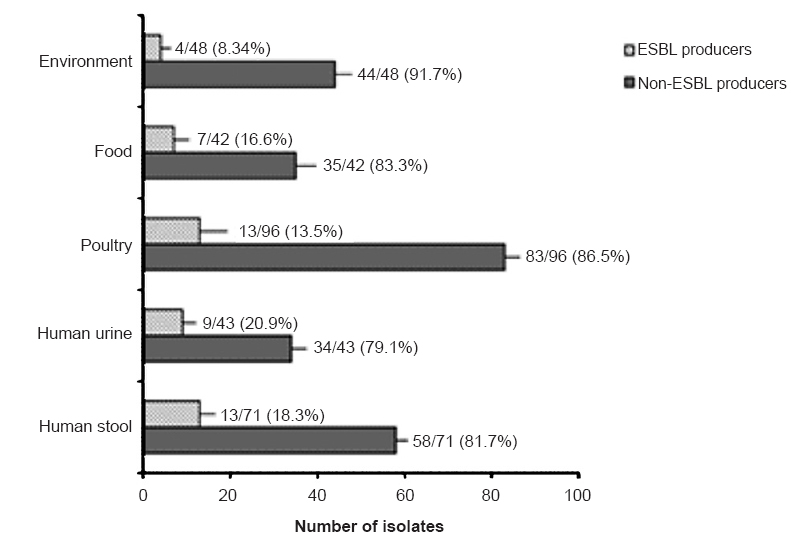
- Distribution of ESBL producers among different reservoirs. Maximum proportion of ESBL producers was observed among human urine (20.9%) followed by human stool (18.3%), food (16.7%) and environment (8.3%).
Among typeable isolates, 27 (13.9%) were found to be capable of producing ESBLs while 19 (17.9%) were found to be ESBL producers among untypeable isolates. This reveals high level of ESBL production prevailing among E. coli. Moreover, substantial amount of ESBL production among untypeable E. coli isolates (O untypeable, OUT) is a matter of concern due to their potential to give rise to foodborne and waterborne outbreaks of gastrointestinal illness6 including bloody diarrhoea7. Expression of various virulence factors in OUT E. coli68 in conjunction with possible production of ESBLs9 may further hinder effective chemotherapy for E. coli infections. High proportion of OUT also emphasizes the need of development, standardization and validation of molecular methods for further characterization of the strains, untypeable using conventional methodologies.
Although, classification of E. coli into different pathotypes can be accurately done by determining different virulence factors expressed by the isolate, epidemiological data also provide information about association of a particular serogroup to a pathotype. In India, there is a paucity of information on prevalence of various E. coli pathotypes; however, a recent study reported 35 per cent enteropathogenic E. coli (EPEC), 3.5 per cent enterotoxigenic E. coli (ETEC) and 9.2 per cent shiga toxin producing E. coli (STEC) in diarrhoeal samples from Hyderabad, India10. Another study from Odisha reported EPEC, ETEC and enteroaggregative (EAEC) as 40.6, 52.7 and 40.6 per cent, respectively11. EAEC (55%) was reported as the main cause of diarrhoeal epidemic in South India12.
Isolation of these serogroups from a wide range of reservoirs may possibly predispose human population to infections with these antimicrobial resistant pathotypes due to exposure to contaminated poultry, food and environment. Highest level of ESBL production was observed among isolates from humans (19.3%) followed by food (16.7%), poultry (13.5%) and environment (8.3%) which corroborated with a study from Chennai13. This may be due to the higher and inappropriate exposure of humans to antimicrobial drugs in the present scenario. Moreover, considerable proportion of ESBL producing E. coli in human stool and urine may further add to the repertoire of ESBL producing E. coli present in the environment.
High susceptibility was found for carbapenem group of drugs viz. Imipenem, meropenem and ertapenem. Similar findings have been reported from the country1314. These findings show that carbapenems may still be considered for the treatment of infections with drug resistant E. coli. High level of susceptibility to nitrofurantoin highlights its importance in the treatment of urinary tract infections due to E. coli. As observed (Table), high proportion of isolates with intermediate susceptibilities may further result in treatment failure and hence, antimicrobial susceptibility testing should always be accompanied with determination of minimum inhibitory concentrations to avoid treatment failures.
Considering high level of ESBL production among various serogroups of E. coli, appropriate steps need to be taken to formulate effective policies to cease inappropriate use of antimicrobial drugs. Execution of efficient surveillance programmes should also be emphasized to monitor ever shifting antibiogram patterns.
Acknowledgment
The authors thank the heads of all the laboratories that referred E. coli isolates to the National Salmonella and Escherichia Centre (National Reference Laboratory), Central Research Institute, Kasauli, India. The technical assistance of Shriyut Khushnihal Kaushal and Yoginder Singh is also acknowledged. Authors thank Shri Devanand for supplying media and biochemicals for biotyping.
Conflicts of Interest: None.
References
- Predictors of hospital surface contamination with extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae: patient and organism factors. Antimicrob Resist Infect Control. 2014;3:5-11.
- [Google Scholar]
- Evaluation of the in vitro activity of six broad-spectrum beta-lactam antimicrobial agents tested against recent clinical isolates from India: a survey of ten medical center laboratories. Diagn Microbiol Infect Dis. 2002;44:367-77.
- [Google Scholar]
- Group 5: Facultative anaerobic Gram negative rods. In: Holt JG, ed. Bergey's manual of determinative bacteriology (9th ed). Baltimore: Williams and Wilkins; 1994. p. :175-201.
- [Google Scholar]
- Serotyping of Escherichia coli. In: Bergon T., ed. Methods in microbiology. Vol xiv. London: Academic Press; 1984. p. :43-122.
- [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility tests. CLSI document M100-S24. CLSI: Wayne, PA; 2014.
- [Google Scholar]
- Association of untypeable enteropathogenic Escherichia coli (EPEC) strains with persistent diarrhea in children from the region of lower Silesia in Poland. Pol J Microbiol. 2013;62:461-4.
- [Google Scholar]
- Phenotypic and molecular characterization of clinically isolated Escherichia coli. Indian J Pathol Microbiol. 2010;53:503-8.
- [Google Scholar]
- Characterization of Shiga toxin-producing Escherichia coli isolated from healthy pigs in China. BMC Microbiol. 2014;14:5.
- [Google Scholar]
- Molecular epidemiology of extended-spectrum β-lactamases and Escherichia coli isolated from retail foods including chicken meat in Japan. Foodborne Pathog Dis. 2014;11:104-10.
- [Google Scholar]
- E. coli pathotypes and their antibiotic resistance in young children with diarrhea in Hyderabad, India. Int J Curr Microbiol App Sci. 2014;3:647-54.
- [Google Scholar]
- Incidence of bacterial enteropathogens among hospitalized diarrhea patients from Orissa, India. Jpn J Infect Dis. 2008;61:350-5.
- [Google Scholar]
- An epidemic of diarrhea in south India caused by enteroaggregative Escherichia coli. Indian J Med Res. 1997;106:7-12.
- [Google Scholar]
- Antibiotic sensitivity and phenotypic detection of ESBL producing E. coli strains causing urinary tract infection in a community hospital, Chennai, Tamil Nadu, India. 2012. Available from: https://www.webmedcentral.com/article_view/3840
- [Google Scholar]
- Antimicrobial susceptibility profile of extended spectrum β-lactamase (ESBL) producing Escherichia coli from various clinical samples. Infect Dis (Auckl). 2014;7:1-8.
- [Google Scholar]





