Translate this page into:
Seroepidemiology of avian influenza H5N1, H9N2 & Newcastle disease viruses during 1954 to 1981 in India
*For correspondence: pawarshailesh@hotmail.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution NonCommercial ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Sir,
Avian influenza (AI) viruses are some of the most important viruses prevalent in water birds1. The higher (~15%) AI infection rates have been reported in waterfowl, particularly in families Anatidae, Gruidae, Phalacrocoracidae and Pelecanidae as compared to terrestrial species (~2%)2. Influenza viruses belong to the family Orthomyxoviridae and are divided in 18 haemagglutinin (HA) and 11 neuraminidase (NA) subtypes based on genetic and antigenic properties. At least 103 of the possible 198 Type A influenza virus, HA-NA combinations have been found in wild birds3. Influenza pandemics occurred in 1918, 1957, 1968, 1977 and 2009 due to the major antigenic variation of the Type A influenza viruses4. AI viruses are broadly classified as low pathogenic AI and highly pathogenic AI (HPAI) viruses, based on their pathogenicity5. The H5N1 influenza virus was first isolated in 1996 from geese in the Guangdong province of China6. In India, the outbreaks of HPAI H5N1 virus were first reported in February 2006 in poultry at Navapur, Maharashtra and then 104 outbreaks have been reported in poultry and in wild and migratory birds in India from 2006 to 201678.
The National Institute of Virology (NIV), Pune, India, conducted avian surveys in India during the years 1954 to 1981 to study the role of wild and migratory birds in transmission of arboviruses in different States of India. During these surveys serum samples were collected from various bird species. These serum samples were stored at −20°C at the repository of NIV, for storage of archived samples. In view of the emergence of influenza viruses globally, the present exploratory study was undertaken at the NIV, Pune, during July 2013-March 2015, to study retrospective seroprevalence of AI H5N1 and H9N2 viruses in India using the archived avian serum samples collected earlier (1954-1981).
For determination of sample size, an assumption of less than five per cent antibody prevalence of H5N1 and H9N2 and Newcastle disease virus (NDV) was made. The sample size calculations were performed using online (OpenEpi) software, Centers for Disease Control and Prevention, USA9. The estimated sample size was 500 assuming five per cent prevalence, 95 per cent confidence interval and precision of 0.02 per cent by two-sided test with finite population correction with a population size of 5000 for the study area. A total of 557 representatives archived avian serum samples from 41 species from 15 avian families of wild, migratory and resident; water birds, water frequenting and terrestrial birds were selected for the study. These samples were from seven States, namely, Maharashtra, Karnataka, West Bengal, Jharkhand (formerly Bihar), Andhra Pradesh, Tamil Nadu and Rajasthan (Table).
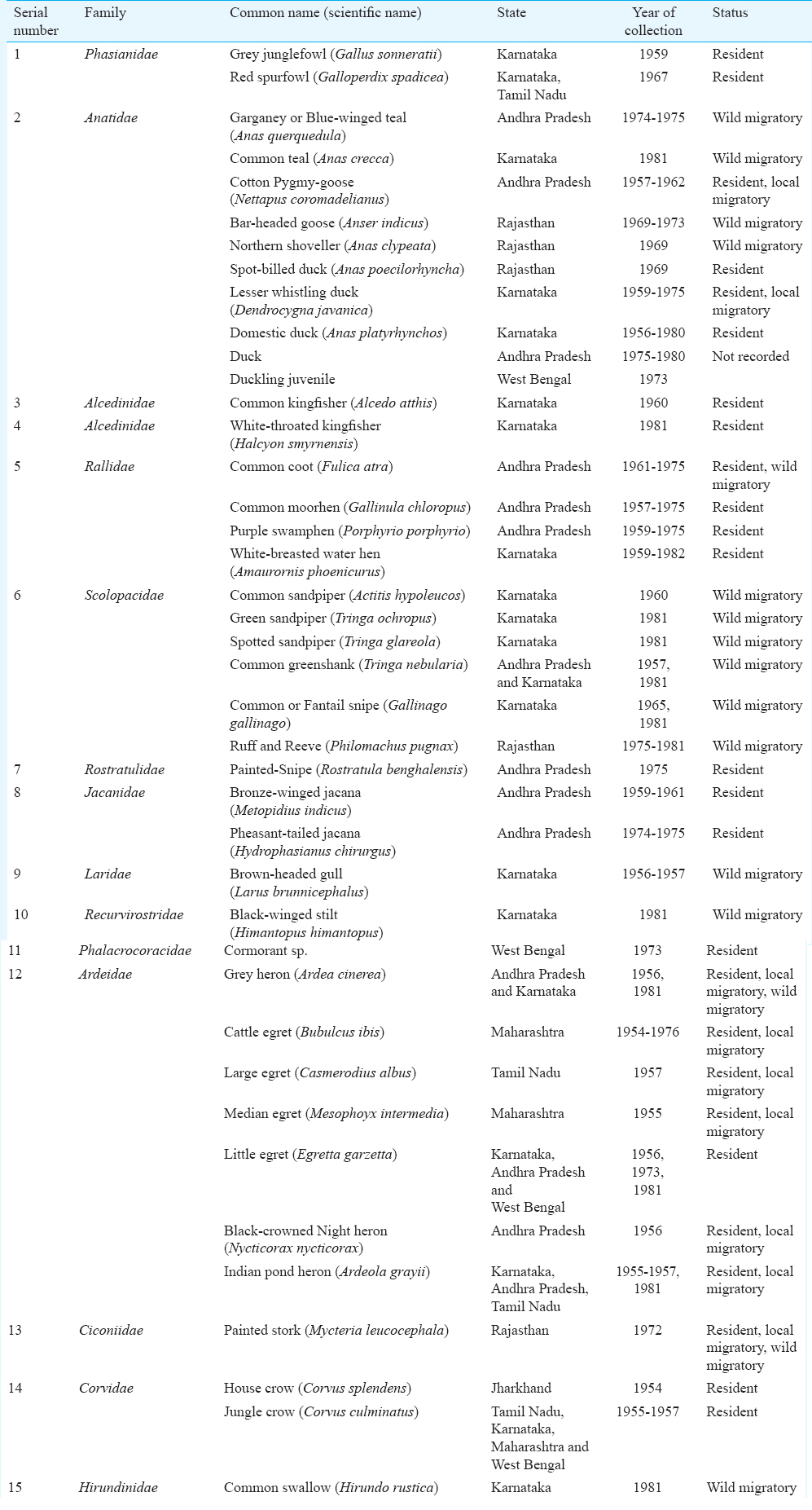
These samples were tested against A/chicken/India/NIV33487/06-RG-2008 (H5N1), A/Turkey/Wisconsin/66 (H9N2) and NDV antigens obtained from the OIE/FAO National Reference Laboratory for AI and Newcastle disease obtained from Legnaro, Italy. The AI H5N1 and H9N2 viruses have been reported to be prevalent in avian species and poultry and resident birds in India710.
These samples were tested by haemagglutination inhibition (HI) and microneutralization (MN) assays for detection of antibodies against AI H5N1 and H9N2 viruses1112. Only HI assay was employed for the detection of antibodies against NDV. For HI assay, all serum samples were treated with receptor-destroying enzyme (RDE) (Denka Seiken, Japan) to remove non-specific inhibitors. Serum samples showing the presence of agglutinins were treated with horse and turkey red blood cells (RBCs) to remove non-specific agglutinins. One volume of packed RBCs was mixed with 20 volumes of RDE-treated serum and incubated at 37°C for one hour, centrifuged at 120xg for 10 min. Adsorbed serum was carefully removed without disturbing packed cells and used in the HI assay. HI assays were performed using 0.5 per cent turkey and one per cent horse red blood RBCs. The reference serum samples from OIE were used as positive control in both HI and MN assays.
The MN assay was used to detect the presence of neutralizing antibodies against AI viruses13. The MN assays were performed using Madin–Darby canine kidney (MDCK) cells maintained in Dulbecco's modified Eagles’ medium (DMEM) containing 10 per cent foetal bovine serum (Gibco, USA), 2 mM L-glutamine and the antibiotics penicillin (100 U/ml) and streptomycin (100 µg/ml). The 50 per cent tissue culture infectious dose (TCID50) of H5N1 and H9N2 viruses was determined. Half-log dilutions of virus were carried out in 96-well polystyrene immunoassay plates (Nunc, Denmark) and mixed with 100 µl of 1.5 × 105/ml MDCK cells and incubated for 18-22 h at 37°C and five per cent CO2. Enzyme-linked immunosorbent assay (ELISA) was performed using influenza A-specific anti-nucleoprotein monoclonal antibodies (Millipore, USA). The TCID50 was calculated as per Reed and Muench method12. For MN assay, RDE treated serum samples were serially two-fold diluted and mixed with an equal volume of influenza virus diluted at 100 TCID50/50 µl. After incubating for one hour at 37°C with five per cent CO2, it was mixed with 100 µl of MDCK cells at 1.5 × 105/ml. The plates were incubated for 18-22 h at 37°C and five per cent CO2. The monolayers were washed with phosphate-buffered saline and fixed in cold 80 per cent acetone for 10 min. ELISA was performed as mentioned above. The titres of the antibodies by both HI and MN assays were expressed as reciprocals of the highest antibody dilution showing haemagglutination inhibition and virus neutralization, respectively.
All samples were negative for the presence of antibodies against AI H5N1 and H9N2 viruses. There are reports of AI H5N1 virus since 195914 and AI H9N2 virus was first reported in 1982 from domestic poultry in China15. AI H5N1 and H9N2 were first reported from India during 2006 and 2003, respectively716. The present study indicated that H5N1 and H9N2 AI viruses did not circulate in the studied avian population during the period 1954 to 1981. Thus, the emergence of these viruses in avian population in India may be recent.
Two samples isolated from Jungle crow (Corvus macrorhynchos) from Maharashtra and one sample from wild duck (species not identified) from Andhra Pradesh collected during 1955-1956 and 1975, respectively, were positive for the presence of antibodies against NDV. The antibody titres of three positive samples from crow and duck were 80, 40 and 20 by HI assay. NDV was first isolated during 1927 from England, but it is said to have been prevalent much earlier than that1718. The seropositivity of NDV indicates the prevalence of this virus in the past. In a previous study from the NIV, conducted in 1980-1981, cloacal swabs from birds were negative for AI virus isolation and NDV was isolated from a chicken19.
The HPAI H5N1 viruses cause high mortality in poultry. However, some species of wild birds survive HPAI H5N1 infection and show antibody response2021. Therefore, the possibility of the absence of antibodies in the studied avian species due to quick mortality in infected birds by HPAI H5N1 viruses could be ruled out as the serum samples used in the study were from wild bird species. There have been retrospective studies on viral diseases, wherein archived serum samples have been tested for antibodies22. The samples used in the present study were stored at −20°C for 34-61 yr. The presence of antibodies against NDV in stored serum samples indicates that the long-term storage of samples did not affect the integrity of the antibodies. However, the protein degradation that might have taken place during the long storage could not be assessed in this study. The samples for virus isolation were not available. The findings of this study cannot be generalized, as the studied samples do not represent the complete geographical areas in India.
In conclusion, AI H5N1 and H9N2 viruses did not circulate in the studied avian population during 1954-1981, and emergence of these viruses in birds in India is probably recent. The seropositivity of NDV indicates the prevalence of this virus in the past.
Acknowledgment
Authors thank Dr D.T. Mourya, Director, NIV and Dr A Basu for permission to use archived samples, J Mullick for support, A.A. Deshpande and S.S. Parkhi for their help in laboratory work and Dr. Satish A. Pande for critical review of the manuscript. This work was supported by the intramural funds from the Indian Council of Medical Research, Department of Health Research, Ministry of Health and Family Welfare, Government of India, New Delhi.
Conflicts of Interest: None.
References
- Emerging viral diseases in water birds. In: Boere GC, Galbraith CA, Stroud DA, eds. Water birds around the world. Edinburgh, UK: The Stationary Office; 2006. p. :418-21.
- [Google Scholar]
- A review of avian influenza in different bird species. Vet Microbiol. 2000;74:3-13.
- [Google Scholar]
- Centers for Disease Control and Prevention (CDC), Avian Influenza (Flu). Available from: http://www.cdc.gov/flu/avian/gen-info/flu-viruses.htm
- The development and genetic diversity of H5N1 influenza virus in China, 1996-2006. Virology. 2008;380:243-54.
- [Google Scholar]
- Characterization of the influenza A H5N1 viruses of the 2008-09 outbreaks in India reveals a third introduction and possible endemicity. PLoS One. 2009;4:e7846.
- [Google Scholar]
- OIE update on highly pathogenic avian influenza in animals (H5 and H7) Available from http://www.oie.int/en/animal-health-in-the-world/update-on-avian-influenza/2016
- Open Source Epidemiologic Statistics for Public Health (Open Epi) 2014. Available from: http://www.openepi.com/Menu/OE_Menu.htm
- Avian influenza surveillance reveals presence of low pathogenic avian influenza viruses in poultry during 2009-2011 in the West Bengal State, India. Virol J. 2012;9:151.
- [Google Scholar]
- World Health Organization 2002. WHO Manual on Animal Influenza Diagnosis and Surveillance. Available from: http://apps.who.int/iris/bitstream/10665/68026/1/WHO_CDS_CSR_NCS_2002.5.pdf
- Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J Clin Microbiol. 1999;37:937-43.
- [Google Scholar]
- Serologic evidence of avian influenza H9N2 and paramyxovirus type 1 infection in emus (Dromaius novaehollandiae) in India. Avian Dis. 2012;56:257-60.
- [Google Scholar]
- Influenza A virus A/chicken/Scotland/59(H5N1) N1 gene for neuraminidase, genomic RNA. GenBank: AJ416625.1. Available from: https://www.ncbi.nlm.nih.gov/genbank/
- Avian influenza a viruses of southern China and Hong Kong: Ecological aspects and implications for man. Bull World Health Organ. 1982;60:129-35.
- [Google Scholar]
- Isolation and pathotyping of H9N2 avian influenza viruses in Indian poultry. Vet Microbiol. 2009;133:154-63.
- [Google Scholar]
- Some observations on the epizootiology of Newcastle disease. Can J Comp Med Vet Sci. 1956;20:155-68.
- [Google Scholar]
- A hitherto unrecorded disease of fowls due to a filter-passing virus. J Comp Pathol. 1927;40:144-69.
- [Google Scholar]
- Zoonotic studies on influenza in pigs and birds, India, 1980-81. Int J Zoonoses. 1983;10:40-4.
- [Google Scholar]
- Pathogenicity of Chinese H5N1 highly pathogenic avian influenza viruses in pigeons. Arch Virol. 2008;153:1821-6.
- [Google Scholar]
- Experimental infection of chickens, ducks and quails with the highly pathogenic H5N1 avian influenza virus. J Vet Sci. 2009;10:53-60.
- [Google Scholar]
- Sero-epidemiological studies of respiratory syncytial and adenoviruses in children in Ibadan, Nigeria, 1985-1988. Trans R Soc Trop Med Hyg. 1992;86:294-7.
- [Google Scholar]





