Translate this page into:
Salivary troponin I as an indicator of myocardial infarction
Reprint requests: Dr Iraj Mirzaii-Dizgah, Department of Physiology, School of Medicine, AJA University of Medical Sciences, Tehran, Iran e-mail: emirzaii@razi.tums.ac.ir
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Accurate and rapid diagnosis of acute myocardial infarction (MI) is of major clinical significance. The troponin is the biomarker of choice for detection of cardiac injury. The objective of this study was to identify salivary levels of cardiac troponin I (cTnI) in patients with acute MI.
Methods:
Thirty patients with acute MI and 28 normal healthy individuals were included in the study. cTnI levels were assayed in serum and saliva 12 and 24 h of acute MI by ELISA method.
Results:
In patients with acute MI, the serum and resting (unstimulated) saliva concentrations of cTnI, but not stimulated saliva cTnI, at both 12 and 24 h of onset of MI, were significantly higher than in controls. Resting saliva cTnI concentrations correlated significantly with serum cTnI levels (spearman rho = 0.34 and 0.45 in the total individuals and in the MI patients respectively).
Interpretation & conclusions:
The present results suggest that saliva can be an analytical matrix for measurement of cTnI in patients with acute MI. Further studies may reveal capability of salivary cTnI for being used for developing point-of-care testing for early detection of MI in pre-clinical settings.
Keywords
Acute myocardial infarction
cardiac troponin I
saliva
Myocardial infarction (MI) is a major cause of death and disability worldwide. According to the contemporary universal definition of MI, the criteria for diagnosis of acute MI were recommended as follow: detection of rise and/or fall of cardiac biomarkers (preferably troponin) with at least one value above the 99th percentile of the upper reference limit together with evidence of myocardial ischaemia with at least one of the following: (i) symptoms of ischaemia; (ii) ECG changes indicative of new ischaemia (new ST-T changes or new left bundle branch block); (iii) development of pathological Q waves in the ECG; and (iv) imaging evidence of new loss of viable myocardium or new regional wall motion abnormality1. Measurement of cardiac specific troponin (cTn) is an essential prerequisite for determining acute MI. As a consequence, measurement of troponin I (cTnI) is now considered the ‘gold standard’ test for myocardial necrosis, and the final arbiter for a diagnosis of acute MI. The high priority of troponin measurement is due to the sensitivity and specificity of the test and the evidence base for outcome prediction23.
The troponin complex is located on the thin filament of striated muscle and is composed of three subunits: troponin T, which attaches the troponin complex to tropomyosin; troponin I, which modulates the interaction of actin and myosin by inhibiting actomyosin adenosine triphosphatase activity; and troponin C, the calcium-binding subunit of the troponin complex. While troponin C in cardiomyocytes is identical to striated skeletal muscle troponin C, troponins I and T in the heart (cTnI and T) are genetically and immunologically distinct from the troponin I and T isoforms of skeletal muscle. As a result, cTn has nearly absolute myocardial tissue specificity and high clinical sensitivity45.
The cardiac specific troponins are detectable in the serum within 4 to 12 h after the onset of myocardial necrosis, and depending on the duration of ischaemia and reperfusion status, peak values occur 12 to 48 h from symptom onset. cTns offer a wide temporal diagnostic window (4 h to 14 days); their levels remain elevated in the serum for up to 14 days after myocardial injury, allowing for diagnostic confirmation even when patients delay their presentation to medical care after onset of symptom67.
The management of acute MI, and in broader terms acute coronary syndrome, is believed to be extremely time-critical. Patients with suspected acute MI gain tremendously less benefit from the treatment if therapy is delayed for more than 2 h. Therefore, it has been recommended that the initial assessment of the patient in emergency department, including measurement of biochemical markers, should be accomplished within 30 min (vein to brain time) so that the treatment can be initiated within 60-90 min (door to needle time) after the arrival of patient. Consequently, the development and implementation of point-of-care testing for cardiac biomarkers to be used in emergency setting for diagnosis and management of acute coronary syndrome has been strongly encouraged7.
The central issue in point-of-care testing is to decrease laboratory test turnaround time. Recently, several devices for point-of-care testing have become available for all of the established biomarkers of myocardial necrosis, namely cardiac troponins, creatine kinase MB isoenzyme (CK-MB), and myoglobin, with their turnaround time less than 20 min78. All of these tests employ whole blood, plasma, or serum as the specimen matrix, but a newly designed saliva-based nano-biochip with 21 biomarkers exhibited a significant diagnostic capability for acute MI and seemed promising for screening cardiac events in pre-hospital stages9.
To achieve less turnaround time in point-of-care tests, and to develop progressively simpler and easier-to-use devices for bed-side detection systems for acute MI, saliva-based assays may have crucial place. This study was performed to see if the cardiac necrosis biomarker, cTnI, is detectable in saliva of patients with established acute MI.
Material & Methods
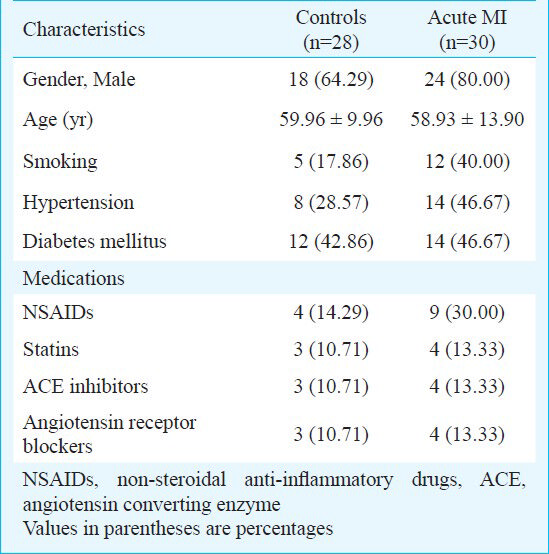
Study design: This study was carried out in Imam Hossein hospital of Shahid Beheshti Medical University, Tehran, Iran during May-October, 2011. Forty consecutive patients, who presented to the emergency department (ED) with angina pectoris, were recruited to this study. Twelve-lead electrocardiography and laboratory analysis of serum biomarkers of cardiac necrosis, including creatine phosphokinase (CPK), creatine kinase MB isoenzyme (CK- MB), and cardiac troponin (cTn) were performed for all patients. Based on these initial evaluations, 10 patients were diagnosed as having unstable angina, whose data were excluded from study, and the remaining 30 were diagnosed as patients with acute ST segment-elevation MI. In addition, 28 age- and sex-matched individuals with no documented heart disease as control group were included in the study. Healthy controls were selected from hospital staff or individuals who accompany patients referred to the hospital. Power of the study was 0.95.
Baseline clinical characteristics were collected for all participants via a questionnaire with a set of inquiries about their demographic information and medical history, including recognized diseases, medical interventions, medications, and other health problems. The selection of study participants was accomplished in a way that at last no subject had the following confounders which can affect cTn levels per se: trauma, congestive heart failure, renal failure, hypothyroidism, inflammatory diseases, severe asthma, sepsis, and burns.
The study protocol was approved by the ethics committee of AJA University of Medical Sciences, Tehran, and written informed consent was obtained from all patients and control subjects.
Saliva and serum sampling: Saliva and blood samples were obtained from patients with acute MI about 12 and 24 h after presentation to ED and from control. They were asked to avoid from eating, drinking, cigarette smoking, and brushing teeth for 1 h before saliva collection10.
For resting saliva sampling, subjects rinsed their mouths with tap water, and 2 min later swallowed all their oral fluid. Thereafter, they expectorated 2-3 ml of their resting whole saliva in a graded plastic tube with no active movement of mouth wall or sucking the oral cavity. Saliva gathering were carried on for 5 min unless 2-3 ml of saliva were collected.
Following resting saliva collection, subjects were asked to chew a piece of natural neutral gum with a given size for two minutes. After that, subjects either expectorated all the oral fluid out or entirely swallowed it, and thereafter they began to collect the stimulated whole saliva into another tube while continuing chewing the gum. The timing of saliva gathering was recorded in order to calculate saliva flow rate (ml/min).
Two ml of venous blood were drawn immediately after saliva sampling. Following sample collection, the specimens were centrifuged at 3800 g for 10 min, and the serum and saliva supernatants were isolated and divided into aliquots. The aliquots were stored in -70°C for analysis of cTnI.
Laboratory measurements: Immunoenzymometric assay was applied to measure the serum and saliva concentrations of cTnI using ELISA kits from Monobind Inc (USA). Determination of cTnI levels was carried out according to the manufacturer's instruction. The absorbance in each well was read at 450 nm in a microplate reader and cTnI concentrations were determined, with the detection limit being 0.05 ng/ml. The 99th percentile of the reference subjects was found to be 0.76 mg/ml in serum samples. The least within-and between-assay coefficients of variation were 2.0 and 5.5 per cent for the cTnI assay.
Statistical analysis: For analyzing goodness of normal fitting, Shapiro-Wilk W test was applied to check if the data had normal distribution. In descriptive statistics, data related to the age were normally distributed, and presented as mean ± standard deviation (SD). Results of other variables had non-normal distributions, and were expressed as median and interquartile range (IQR). Nominal data were shown as frequencies. Since serum and saliva levels of cTnI exhibited non-normal distribution, non-parametric tests (Kruskal-Wallis test followed by Dunn's multiple comparison test) were used to identify statistically significant differences between study groups. Detection of between-groups differences for basic clinical characteristics were carried out with Fisher's exact test or independent samples t-test, as appropriate. Spearman rho test was applied to evaluate correlation between serum and saliva levels of cTnI.
Results
As shown in the Table, age, sex, hypertension, diabetes mellitus, and medication history did not differ among the study groups. However, patients in acute MI group appeared to have marginally higher tendency for cigarette smoking than those in control group. There was no significant difference in baseline unstimulated saliva flow rate between the two groups (0.49 ± 0.06 ml/min in controls vs. 0.47 ± 0.07 ml/min in MI patients), and also in stimulated saliva flow rate (0.99 ± 0.09 ml/min in controls vs. 1.22 ± 0.25 ml/min in MI patients) confirming the integrity of salivary gland function.
Serum levels of cTnI in patients with acute MI, both 12 and 24 h after admission to ED [median (IQR): 6.41 (2.69-11.58) and 4.07 (2.14-8.98) mg/l, respectively] were significantly higher than in control subjects [0.14 (0.08-0.23) mg/l; P<0.0001].
Resting salivary concentration of cTnI proved to be significantly higher in patients with acute MI at both 12 and 24 h [0.40 (0.17-0.62) and 0.71 (0.52-1.07) ng/l, respectively] following the onset of MI compared to those without ischaemic heart diseases [0.19 (0.08-0.27) ng/l, Fig. A]. Regarding stimulated saliva cTnI levels, no significant difference were observed between any of the study groups (Fig. B).
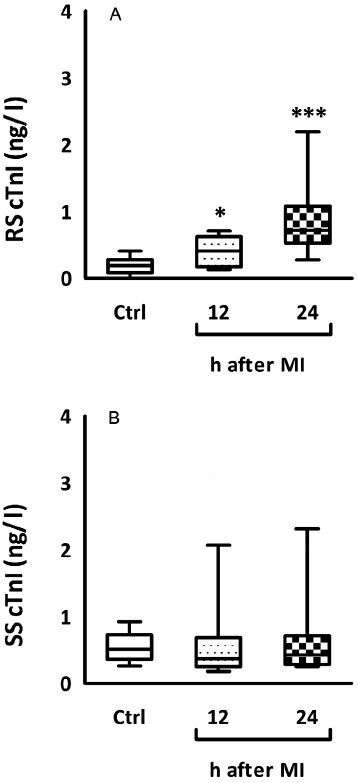
- Variability charts showing the distribution of resting and stimulated saliva cTnI levels. (A), patients with acute MI 12 and 24 h after presentation to ED had higher values of resting whole saliva cTnI levels compared with both healthy subjects. (B), stimulated saliva cTnI levels among study groups (***P<0.001 compared to controls). RS, resting saliva; SS, stimulated saliva.
Serum cTnI levels showed a fairly good correlation with resting saliva cTnI concentrations (spearman rho = 0.34, P=0.004) in the total individuals and (spearman rho =0.45, P=0.001) in the MI group. However, there were no significant correlation between serum and stimulated saliva cTnI levels.
Discussion
In the current study, the patients with acute MI showed significantly higher resting saliva cTnI levels than controls. Additionally, weakly significant positive correlations were found between serum and resting saliva cTnI levels.
Saliva as a diagnostic specimen can give not only the same information as serum testing but also additional information. Saliva is a clear colourless liquid, whereas serum may become milky when lipaemic, red when blood cells are haemolyzed because of trauma, and icteric in the presence of liver disease. Because serum possesses more proteins than saliva, assaying trace amounts of factors may result in a greater risk of non specific interference and a greater chance for hydrostatic interactions between the factors and serum proteins. Therefore assessing biomarkers in saliva for the detection of serious systemic illnesses is of great interest for most researchers11.
Floriano and coworkers suggested that saliva-based tests within lab-on-a-chip systems might provide a convenient and rapid screening method for cardiac events in pre-hospital stages for acute MI patients. From 21 biomarkers used in that study, cTnI was among a small number of markers that did not display any difference between saliva samples of MI patients and healthy controls9. On the contrary, the results obtained from our study showed that cTnI was detectable and elevated in oral fluids of MI patients 12 and 24 h subsequent to necrotic event. This discrepancy might have been raised from the different assays used for cTnI in these two studies.
Several lines of evidence have consistently validated and proposed using saliva-based assays for diagnosing, monitoring, or predicting prognosis of diseases12131415. We have earlier shown that salivary concentrations and outputs of creatine phosphokinase and its MB isoenzyme (CK-MB), as biomarker of cardiac necrosis, were increased in patients with acute MI compared to those with no documented ischaemic heart diseases. Furthermore, the salivary levels and outputs of CK-MB and creatine phosphokinase (CPK) correlated with their serum concentrations, indicating that saliva-based assays of CK-MB and CPK may offer easy-to-use diagnostic tool for point-of-care testing of acute MI1617.
In an attempt to determine if levels of cardiac markers are elevated in salivary secretions of acute MI patients, Miller and coworkers18 used standard immunoassays to measure myoglobin, CK-MB, and cTnI. They found that in comparison with concentrations in non-MI patients, salivary myoglobin levels were significantly higher within 48 h of chest-pain onset in patients with acute MI. However, any report on elevation of CK-MB or cTnI levels in saliva of MI patients was missing in their results. In addition, they found no significant correlation between saliva and serum cTnI levels18. But, results of our study indicated a fairly moderate association between serum and resting whole saliva cTnI.
The saliva components are apparently under the influence of saliva flow out of secretory ducts. When the salivary flow rate is augmented by chewing a piece of gum, the saliva parameters are diluted and thereby their levels changed. This effect is particularly more prominent in patients with higher resting saliva cTnI levels. Our results showed that stimulated whole saliva flow rates were not significantly higher in patients with acute MI than in healthy individuals. This may be the explanation for the observation of the difference in results between stimulated and unstimulated saliva cTnI concentrations in this study.
To establish saliva as an alternative medium to plasma for various biological assays, there must be a high correlation between plasma and saliva levels of measured parameters. As our results indicated, there was a moderate correlation between serum and unstimulated salivary levels of cTnI, we can put forward this idea that saliva-based assays may have the potential to be used as a point-of-care testing to detect acute MI by measuring salivary cTnI.
There were some limitations to this study. Patients with non ST-segment elevation MI (NSTEMI) and unstable angina were not included in the study, thus the salivary levels of cTnI in these patients remained unknown. The changes in salivary levels of cTnI in the early phase of acute MI were not evaluated in this study. Moreover, since only selected and limited number of patients were included, it was not possible to extrapolate the results to the general population and no conclusion could be drawn about the salivary cut-off concentration of cTnI.
Based upon the findings of this study, it can be concluded that subsequent to an acute MI, there is a rise in resting salivary levels of cTnI just as what occurs in serum. Therefore, saliva might be a matrix for measurement of cTnI, but since the most important thing regarding the salivary assay would be the comparability of its diagnostic sensitivity with that of serum assays in the early phase of myocardial infarction, further studies are required particularly to see if cTnI is detectable in the early stages of myocardial infarction.
Acknowledgment
Authors acknowledge the assistance given by the nursing, laboratory and secretarial staff in the Imam Hossein Medical Center, Tehran.
References
- Biomarkers of cardiovascular damage and dysfunction--an overview. Heart Lung Circ. 2007;16(Suppl 3):S71-82.
- [Google Scholar]
- From creatine kinase-MB to troponin: the adoption of a new standard. Anesthesiology. 2010;112:1005-12.
- [Google Scholar]
- New features of troponin testing in different clinical settings. J Intern Med. 2010;268:207-17.
- [Google Scholar]
- Contemporary approach to the diagnosis and management of non-ST-segment elevation acute coronary syndromes. Prog Cardiovasc Dis. 2008;50:311-51.
- [Google Scholar]
- Cardiac markers: a clear cause for point-of-care testing. Anal Bioanal Chem. 2009;393:1453-62.
- [Google Scholar]
- Current trends in diagnostic biomarkers of acute coronary syndrome. Ann Acad Med Singapore. 2010;39:210-5.
- [Google Scholar]
- Use of saliva-based nano-biochip tests for acute myocardial infarction at the point of care: a feasibility study. Clin Chem. 2009;55:1530-8.
- [Google Scholar]
- Saliva specimen: a new laboratory tool for diagnostic and basic investigation. Clin Chim Acta. 2007;383:30-40.
- [Google Scholar]
- Stimulated and unstimulated saliva progesterone in menopausal women with oral dryness feeling. Clin Oral Investig. 2011;15:859-62.
- [Google Scholar]
- A revolution in biomedical assessment: the development of salivary diagnostics. J Dent Educ. 2001;65:1335-9.
- [Google Scholar]
- Salivary diagnostics: enhancing disease detection and making medicine better. Eur J Dent Educ. 2008;12(Suppl 1):22-9.
- [Google Scholar]
- Saliva as a diagnostic medium. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2009;153:103-10.
- [Google Scholar]
- Saliva-based creatine kinase MB measurement as a potential point-of-care testing for detection of myocardial infarction. Clin Oral Investig. 2012;16:775-9.
- [Google Scholar]
- Unstimulated whole saliva creatine phosphokinase in acute myocardial infarction. Oral Dis. 2011;17:597-600.
- [Google Scholar]






